Abstract
Numb is an evolutionarily conserved protein that controls the differentiation of neuronal progenitor cells by unknown mechanisms. Here we report that the neural cells expressing Numb isoforms with short phosphotyrosine-binding domain (SPTB) undergo extensive neurite outgrowth, an effect that can be blocked by voltage-gated Ca2+ channels (VGCC) inhibitor or by Ca2+ chelator. In contrast, neither tyrosine kinase inhibitor, genistein, nor selective TrkA inhibitor, K252α affected SPTB Numb-mediated neurite outgrowth. MAP kinase inhibitor, PD98059 partially reduced SPTB Numb-mediated neurite outgrowth. Cells expressing SPTB Numbs exhibit increased whole-cell Ca2+ current densities (ICa) which can be prevented by preincubation of either nifedipine or PD98095. Cells expressing LPTB Numbs expressed little ICa (density) and were not able to grow neurite. Our results indicate that Ca2+ influx through VGCC may be required for SPTB Numb-mediated neurite outgrowth, suggesting that Numb promotes neuronal differentiation by a mechanism involving PTB domain-specific regulation of Ca2+ influx and MAP kinase activation.
Introduction
First identified in Drosophila melanogaster, Numb is a protein which regulates the fate of neural progenitor cells by controlling asymmetric cell divisions of neural progenitor cells: segregating asymmetrically into the daughter cells (Cayouette and Raff, 2002; Shen et al., 2002). The daughter cells with Numb then undergo differentiation, whereas those without Numb continue to proliferate. The mechanisms underlying Numb-mediated cell differentiation are largely unknown. Numb interacts with a cytoplasmic domain of the plasma membrane receptor Notch and inhibits Notch signaling (Artavanis-Tsakonas et al., 1999), suggesting one mechanism whereby Numb regulates cell differentiation (Jarriault et al., 1995). Numb is also important in neuronal fate determination (Petersen et al., 2002). Numb inhibits proliferation and promotes differentiation of neural progenitor cells in the developing nervous system (Dooley et al., 2003). Numb plays roles in endocytic recycling and in intracellular trafficking of transmembrane proteins (Smith et al., 2004). Numb has been shown to mediate endocytosis and recycling of the cell adhesion molecule L1 at the growth cone and therefore regulates axon growth (Nishimura et al., 2003). Numb was also demonstrated to be expressed in postmitotic neurons and to play a role in synaptic formation and function (Nishimura et al., 2006).
Numb contains two protein-protein interaction domains, a phosphotyrosine-binding (PTB) domain and a proline-rich region (PRR). Four different isoforms of Numbs are expressed in humans that differ in their PTB domain (lacking or containing an 11 amino acid insert) and PRR domain (lacking or containing a 48 amino acid insert) (Verdi et al., 1999). Different Numb isoforms differentially regulate cell proliferation and differentiation in neural cells (Verdi et al., 1999). Compared to the pheochromocytoma-12 (PC12) cells expressing long PTB domain (LPTB Numbs), the cells expressing Numb isoforms with short PTB domain (SPTB Numbs) exhibit increased levels of TrkA nerve growth factor (NGF) receptor and of activated p44/p42 MAP kinase that contribute to NGF-enhanced neurite-outgrowth in these cells (Pedersen et al., 2002). Interestingly, SPTB Numbs themselves are capable of growing neurites in the absence of NGF by as yet unknown mechanisms.
Previous studies have shown that Ca2+ plays a fundamental role in regulating neuronal differentiation and neurite outgrowth (Mattson and Kater, 1987). High-threshold voltage-gated calcium channels (VGCC) provide a major route for Ca2+ entry into neurons (Chemin et al., 2002). VGCC can be subdivided into L, N, P/Q, T and R types based upon their channel properties and sensitivity to specific antagonists. VGCC consist of α1, β, α2, γ and δ subunits (Tanabe et al., 1987). α1 subunit is the major unit that forms the channel pore and is essential for channel functions. Molecular cloning has identified five α1 subunits including α1C (cardiac form) and 1D (neuronal form) coding for L-type channels (Bell et al., 2001); α1B for N type (neuron-specific) channels (Dubel et al., 1992); and α1A for P/Q channels (Mori et al., 1991). The α1E subunit underlies at least certain aspects of T-type Ca2+ channel functions and is also involved in high-voltage activated R-type Ca2+ channel. While the α1 subunit is sufficient to produce functional Ca2+ channels (Catterall, 2000), the β subunit affects activation and inactivation kinetics and plays an important role in the transport of the α1 subunit to the plasma membrane (Lacerda et al., 1991).
SPTB Numb-induced neuronal differentiation may involve the changes in Ca2+ homeostasis. We sought to determine whether the effects of Numb on neural cell proliferation and differentiation are mediated by specific actions of Ca2+ influx. Here we show that SPTB Numbs promote neurite outgrowth by a mechanism involving activation of VGCC and Ca2+ influx.
MATERIALS AND METHODS
Cell culture and plasmid transfection
PC12 cells (Black and Greene, 1982) were transfected with the expression vector pcDNA3.1 with or without cDNAs for each of the human Numb isoforms (Verdi et al., 1999). Lipofectamine (Life Technologies, Gaithersburg, MD) was used for the transfection. Clones overexpressing Numb isoforms were selected with G418 (0.8 mg/ml for 4 weeks) as described (Guo et al., 1997). Resulting stable cell lines that exhibited high levels of expression were used for subsequent experiments.
Cultures were maintained in culture flasks at 37°C (5% CO2 atmosphere) in the Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum (FBS) and 0.5 mg/ml G418. In agreement with the slow transit through the cell cycle of PC12 cells (cell doubling every 3–4 days) (Cunningham et al., 2001), PC12 cells were typically subcultured every 3–4 days and the total passage number was about 15–18.
PC12 cells at various passage numbers (<15) were plated in 35 mm, bare plastic Petri dishes in DMEM containing 10% FBS and 44 mM sodium bicarbonate. After 3–4 h, the medium was removed and replaced with serum-free DMEM in the presence or absence of various drugs. The culture medium was replaced with fresh DMEM free of serum every other day and the cultures were maintained for various days (2 to 5 days depending on the treatments). Data was collected normally within five days after treatments and the cultures were trashed. Nifedipine, ω-CgTx GVIA, genistein, PD 98059 and EGTA were purchased from Sigma Chemical Company and stock solutions were prepared in a serum-free DMEM. BAPTA-AM was purchased from Calbiochem and stock solutions were prepared in dimethyl sulfoxide. Murine NGF (2.5S) was purchased from Gibco-BRL (Gaithersburg, MD, USA) as a 100-μg/ml stock. K252α was purchased from Calbiochem (San Diego, CA) and prepared in DMSO as a 100 μM stock solution. DMEM, FBS and horse serum were from Life Technologies (Grand Island, NY).
Recordings of voltage-gated Ca2+ currents
Ca2+ currents were recorded on PC12 cells overexpressing either vector or various Numb isoforms after 4–5 day culture in serum-free DMEM. The recordings were performed at room temperature using a whole-cell recording configuration with a patch clamp amplifier (Axopatch-1D) as described previously (Lu et al., 2002). Data were filtered at 2 kHz and digitized at 5–10 kHz using a Digidata 1320A interface (Axon Instruments). Data analysis was performed using pClamp8 software. Glass pipettes were pulled with a Flaming-Brown horizontal puller (Sutter Instruments, Novato, CA). Electrodes were coated with Sylgard (Dow Corning, Midland, MI) and had an average resistance of 2–4 MΩ The ionic composition of the external solution was (in mM) NaCl 153, CaCl2 5, KCl 2.4, D-glucose 10, HEPES 10, TEA 10 and tetrodotoxin 0.0003 (pH: 7.4, adjusted with NaOH, 330 mOsm, adjusted with sucrose). The internal solution consisted of (in mM): CsCl 120, HEPES 20, MgCl2 1, EGTA 10, Mg2ATP 3, Na-GTP 0.3 (pH: 7.2 adjusted with CsOH). The current amplitude was normalized by the cell capacitance to yield the current density. The drugs were applied to neurons by using a rapid switch system of a six-channel valve controller apparatus (Warner Instrument Corporation).
Immunoblotting
The methods employed for immunoblotting analyses were similar to those described previously (Guo et al., 1998). Briefly, samples of 25–50 μg of total protein lysate were separated by electrophoresis in a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred to a nitrocellulose sheet, and incubated for 2 h at room temperature with antibodies recognizing VGCC subunits, α-1D, α-1B Ca2+channel subunits (rabbit affinity isolated antibody; 1:200–500 dilution; Sigma), or with an antibody recognizing all Numb isoforms (mouse monoclonal IgG1 at a final concentration of 0.25 μg/ml; Transduction Laboratories, Lexington, KY USA). The nitrocellulose sheet was further processed using horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA). Bands were visualized with a chemiluminescence detection method (Amersham, Piscataway, NJ, USA). The membranes were then incubated in a Ponceau S solution (Sigma; diluted 1:10 with dH20) and reprobed with a β-actin antibody (rabbit polyclonal IgG at a final concentration of 1:5000 dilution in the blocking solution; Santa Cruz Biotechnology). Ponceau S staining was used to visualize the proteins and confirm the equal loading of proteins onto the gels.
Assessments of neurite outgrowth
Parameters of neurite outgrowth were quantified from phase-contrast images of cells acquired using a 40X microscope objective lens and a Hamamatsu camera. The length of individual neuritis was measured using National Institute of Health (NIH) IMAGE software. A neurite was defined as a process that is equal to or greater than one cell body in length. The percentage of cells with neurites was calculated by the equation: (the number of the cells with neurite/total number of the cells)*100. The average neurite length is the summation of all neurite length divided by cell numbers.
RESULTS
SPTB Numb-induced neurite outgrowth requires Ca2+ influx through L type Ca2+ channels
PC12 cells were transfected with expression plasmids containing one of the four Numb isoforms (SPTB/SPRR, SPTB/LPRR, LPTB/SPRR or LPTB/LPRR; S, short; L, long; PTB, phosphotyrosine binding domain; PRR, proline-rich region) (Pedersen et al., 2002). Cells expressing the LPTB Numb isoforms remained in a proliferative state, whereas cells expressing the SPTB Numb isoforms exhibited extensive neurite outgrowth. To study the mechanisms of SPTB Numb-induced neurite outgrowth, we used the same clones for SPTB/SPRR and SPTB/LPRR as those described by Pedersen et al. (2002).
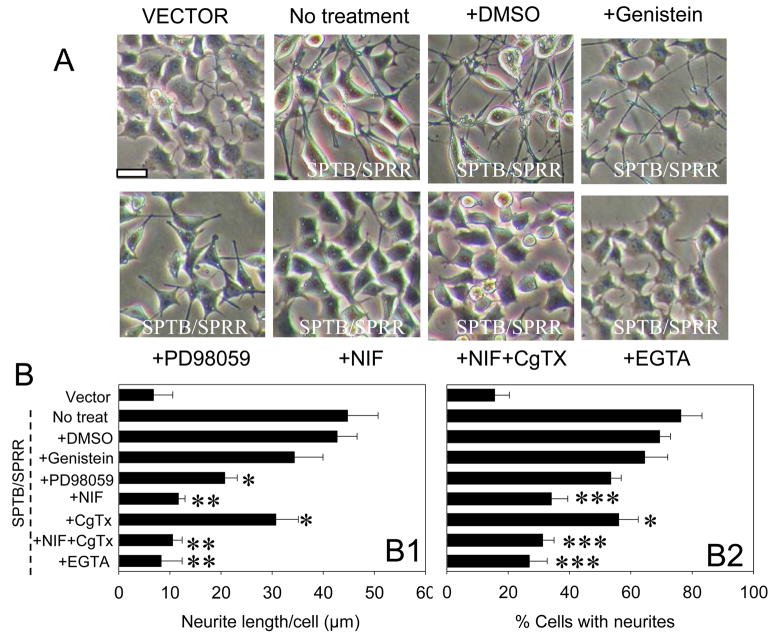
Previous studies have shown that changes in intracellular Ca2+ levels are associated with neuronal differentiation and neurite outgrowth (Mattson et al., 1995). We therefore treated cells expressing SPTB/SPRR Numb with Ca2+ channel blockers and assessed the length of the neurites to determine whether SPTB Numb-induced neurite outgrowth is mediated by Ca2+ influx. As shown in Fig. 1, the L-type Ca2+ channel blocker nifedipine (10 μM), markedly reduced SPTB/SPRR Numb-induced neurite outgrowth both in length (Fig. 1B1) and in the percentage of cells with neurites (Fig. 1B2). However, N-type Ca2+ channel blocker, ω-CgTx (1 μM) had little effect on SPTB Numb-induced neurite outgrowth. These results suggest that Ca2+ influx through L-type Ca2+ channel is required for SPTB Numb-induced neurite outgrowth. It was previously reported that the Ca2+ chelator EGTA inhibited depolarization- (Solem et al., 1995) or forskolin-induced neurite outgrowth (Manivannan and Terakawa, 1994; Obara et al., 2002) and that BAPTA/AM inhibits L1-dependent neurite outgrowth (Williams et al., 1992) in PC12 cells. We thus treated cells expressing SPTB/SPRR Numb with these chelators, EGTA (0.5 mM) treatment greatly reduced SPTB/SPRR Numb-induced neurite outgrowth (Fig. 1B). Similar results were observed when cells were treated with BAPTA/AM (10 μM) for a short time period (less than 36 hr) as BAPTA appeared to be toxic to the cells beyond this time point (data not shown). These results indicate that Ca2+ influx and intracellular actions are required for SPTB Numb-induced neurite outgrowth.
Figure 1.
SPTB Numb-induced neurite outgrowth requires Ca2+ influx and MAP kinase activation. A. Phase-contrast images of cells expressing SPTB/SPRR Numb that had been incubated for five days in the presence of 0.2% dimethylsulfoxide, 10 μM nifedipine (Nif), 0.5 μM ω-contoxin (CgTx), 10 μM genistein, 5 μM PD98095, 0.5 mM EGTA or in the absence of any treatment (untreated control) and image of cells expressing vector. Drugs were added into the culture medium (DMEM without serum) of 35 mm Petri dishes 3–4 hrs after cells were plated into the dishes. B. Quantification of neurite length (B1) and the percentage of cells with neuritis (B2) in SPTB/SPRR cells that had been exposed for five days to the indicated treatments. Values are the mean ± SEM of the measurements made in four cultures in different days per treatment condition (100–150 cells were evaluated per treatment condition). *p<0.05, **p<0.01, ***p<0.001 compared to either no-treated or DMSO treated group in SPTB/SPRR Numb, ANOVA followed by Holm-Sidak methods for multiple comparison. Horizontal bar: 10 μm.
Cells expressing SPTB Numbs exhibit increased levels of the membrane receptor tyrosine kinase (TrkA) and p42/44 MAP kinase (Pedersen et al., 2002). Therefore, the effects of the tyrosine kinase inhibitors genistein and the MAP kinase inhibitor PD98059 on Numb-induced neurite outgrowth were tested. PD98059 significantly reduced SPTB/SPRR-induced neurite outgrowth both in length (Fig. 1B1) and in the percentage of cells with neurites (Fig. 1B2), but genistein had little effect on SPTB/SPRR-induced neurite outgrowth (Fig. 1B).
NGF-induced neurite outgrowth
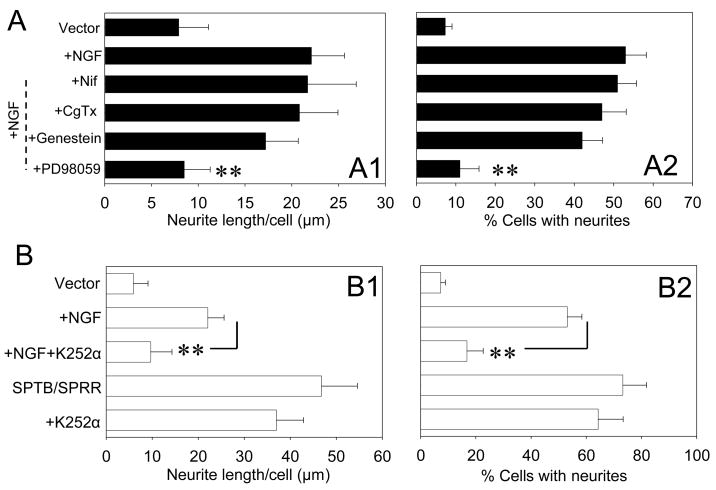
We determined the effect of NGF on neurite outgrowth in cells transfected with vectors. The neurite length of the cells expressing vectors was increased by 180 % following a four-day exposure to NGF (50 ng/ml). Neither the L-type Ca2+ channel blocker nifedipine nor the N-type Ca2+ channel blocker ω-CgTx affected NGF-induced neurite outgrowth. The tyrosine kianse inhibitor genistein had little effect on NGF-induced neurite outgrowth (Fig. 2). However, the MAP kinase inhibitor PD98059 markedly reduced NGF-induced neurite outgrowth. The lack of effect of genistein on SPTB Numb- or NGF- induced neurite outgrowth is indicative that tyrosine kinases related to genistein, may be not involved in neurite outgrowth induced by either condition. This is in agreement with previous observations that showed genistein neither targeted TrkA/NGF receptor (Miller et al., 1993) nor inhibited neurite outgrowth (Hung et al., 2005).
Figure 2.
NGF-induced neurite outgrowth is calcium independent. A. Quantification of neurite length (A1) and the percentage of cells with neuritis (A2) in cells expressing vector in the presence or absence of NGF with or without the indicated treatments after four day culture. NGF (50 ng/ml) and other drugs were added at the same time in the culture medium 3–4 hr after the cells were plated into 35 mm Petri dishes. Values are the mean ± SEM of the measurements made in four cultures in different days per treatment condition (100–150 cells were evaluated per treatment condition). *p<0.05, **p<0.01 compared to NGF- treated group, ANOVA followed by Holm-Sidak methods for multiple comparison. B. Quantification of neurite length (B1) and the percentage of cells with neuritis (B2) in cells expressing either vector or SPTB/SPRR Numb in the presence or absence of NGF in combination with or without K252α. Values are the mean ± SEM of the determinations made in four cultures in different days per treatment condition (100–150 cells were evaluated per treatment condition). *p<0.05, **p<0.01 compared to the NGF treated group, ANOVA followed by Holm-Sidak methods for multiple comparison.
The activation of TrkA is essential in NGF-induced neurite outgrowth (Rogers et al., 1994). As expected, the selective TrkA receptor inhibitor, K252α (0.1 μM) significantly reduced NGF-induced neurite outgrowth in cells transfected with vector. However, K252α had no effect on SPTB/SPRR-induced neurite outgrowth.
These results indicate that the mechanisms for SPTB Numb- and NGF-induced neurite outgrowth differ: SPTB Numb-induced neurite outgrowth requires Ca2+ influx but not TrkA activation, while NGF-induced neurite outgrowth requires TrkA activation but not Ca2+ influx. SPTB Numb and NGF-induced neurite outgrowth appears to share a pathway involving MAP kinase activation.
SPTB Numbs increase Ca2+ current densities
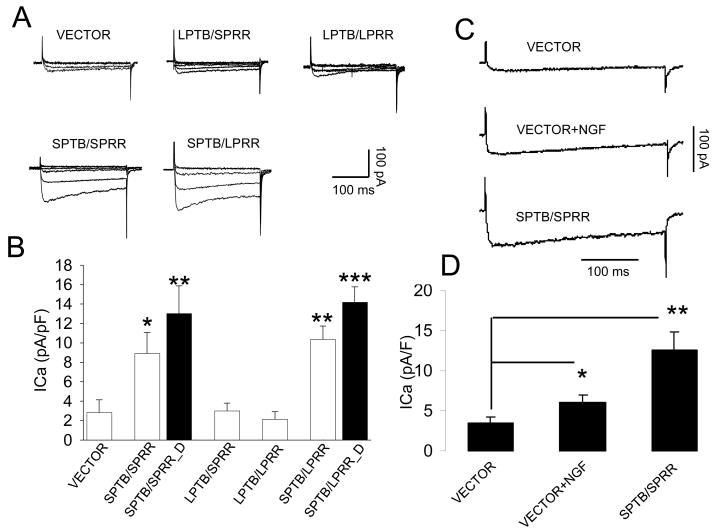
SPTB Numb-induced neurite outgrowth appears to be Ca2+ dependent. We therefore recorded voltage-gated Ca2+ current (ICa) using whole-cell patch-clamp recordings to determine whether there is an alteration of functional expression of Ca2+ channels. ICa density was significantly larger in cells expressing SPTB Numb isoforms, compared to cells expressing LPTB Numbs and to cells transfected with an empty vector (Fig. 3A, B). On average, the ICa densities were 2.8 ± 0.72 pA/pF (n = 12), 3.0 ± 0.8 pA/pF (n =5) and 2.1 ± 0.78 pA/pF (n = 9) for the cells transfected with vector, LPTB/SPRR and LPTB/LPRR, respectively. These cells were mostly undifferentiated (>97%). However, the majority of the cells expressing SPTB Numbs were differentiated (~78–82% for cells expressing SPTB/SPRR and SPTB/LPRR, n=4, data not shown), we therefore recorded ICa in both differentiated and undifferentiated cells expressing SPTB Numbs and plotted the results of ICa density in Fig. 3B. The ICa densities in cells expressing SPTB Numbs were 3–4 fold greater than those observed in cells transfected with an empty vector (control). The differentiated cells had a larger ICa density compared with undifferentiated cells (Fig. 3B). The mean ICa current densities for the cells expressing SPTB/SPRR Numb, the ICa densities were 13.0 ± 2.8 pA/pF (n=14) and 8.9 ± 2.2 pA/pF (n=10) for the differentiated and undifferentiated cells, respectively. Cells expressing SPTB/LPRR Numb had ICa densities of 14.8 ± 3.4 pA/pF (n=12) and 10.4 ± 2.4 pA/pF (n=11) for the differentiated and undifferentiated cells, respectively. These results indicate that the functional Ca2+ channels were largely increased in the cells expressing SPTB Numbs and that the increase of Ca2+ channels in these cells occurred prior to and during cell differentiation.
Figure 3.
Whole-cell Ca2+ current recordings in cells expressing the indicated Numb isoforms, cultured for 4–5 days after cell plating. A. Representative traces of Ca2+ currents (ICa) recorded from cells transfected with vector, or the indicated Numbs in response to the test potentials from −60 mV to 50 mV from a holding potential of −70 mV. B. Quantification of the mean Ca2+ current densities in cells expressing vector or the indicated Numb isoforms. Values are the mean ± SEM of the determinations made in 10–14 cells expressing each Numb isoform from 5 independent cultures in different days. *p<0.05, **p<0.01, ***p<0.001 compared to values of ICa density from cells transfected with vector and LPTB Numb, ANOVA followed by Holm-Sidak methods for multiple comparison. SPTB/SPRR-D: differentiated cells expressing SPTB/SPRR, SPTB/SPRR: undifferentiated cells expressing SPTB/SPRR Numb. C. Representative traces of Ca2+ currents recorded from cells transfected with vector in the presence or absence of NGF, or with SPTB/SPRR Numb in response to the test potential of 10 mV from a holding potential of −70 mV. D. The average changes in ICa density recorded in the cells expressing vector in the presence (n=6) or absence (n=6) of NGF and in the cells expressing SPTB/SPRR numb (n=7). Values are the mean ± SEM. *p<0.05, **p<0.01compared to the value of ICa density from the cells expressing vector, Student t-test.
To test which type Ca2+ channel contributes to the increased ICa density in the cells expressing SPTB Numb, nifedipine (10 μM) and ω-CgTx (1 μM) were added sequentially after stable ICa recording was established (Usowicz et al., 1990; Breustedt et al., 2003). Sequential application of nifidepine and CgTx caused a 72 ± 6.1% reduction and a further 18 ± 8.4% reduction in ICa density of cells expressing vector (n=6), but in cells expressing SPTB/SPRR numb, the reduction in ICa density was 61 ± 5.9 % and 32 ± 7.4 %, respectively (n=5). These results indicate that L-type Ca2+ channels are the major contributors to ICa density for the cells expressing SPTB Numb and vector. The reduction in current observed in cells treated with an empty vector are consistent with those seen in a previous study from PC12 cells (Plummer et al., 1989).
The effect of NGF on ICa density
NGF-induced neurite outgrowth appears to be Ca2+ independent. To determine whether NGF alters ICa density, we recorded ICa in cells expressing vector in the presence and absence of NGF for 4 to 5 days and compared with ICa recorded in the cells expressing SPTB Numb. As shown in Fig. 3C, NGF treatment increased ICa density, though it was only about 50% of ICa density from SPTB Numb. On average, ICa density was 3.4 ± 0.4 pA/pF (n=6), 6.0 ± 0.7 pA/pF (n=6) and 12.6 ± 2.2 pA/pF (n= 7) for cells expressing with vector (control), vector in the presence of NGF and SPTB/SPRR Numb, respectively. There was a statistically significant difference in ICa density between the cells expressing with vector (control) and the vector in the presence of NGF (Student t-test).
To determine which type Ca2+ channel contributes to the NGF-induced increase in ICa density, we applied the same strategy as used in the cells expressing SPTB Numb or vector based on the sensitivity of ICa to nifedipine and ω-CgTx. After establishing a stable recording in ICa in NGF treated cells, nifedipine was applied and caused 45 ± 6.4 % (n=6) reduction on ICa density. ω-CgTx had an additional 41 ± 6.6 % (n=6) reduction on ICa density. These results indicate the ratio of N-type Ca2+ channel vs. L-type Ca2+ channel was increased after NGF treatment compared with that in vector control. This suggests that the NGF-induced increase in ICa is largely contributed by N-type Ca2+ channels. This is in agreement with previous reports that showed NGF increased mainly the expression of N-type Ca2+ channel (Colston et al., 1998; Tully and Treistman, 2004). However, as calcium channel blockers had no effect upon NGF induced neurite outgrowth the increased N-type Ca 2+ current observed in NGF treated cells is probably merely a consequence of neuronal differentiation.
The effects of SPTB Numbs on Ca2+ channel kinetics
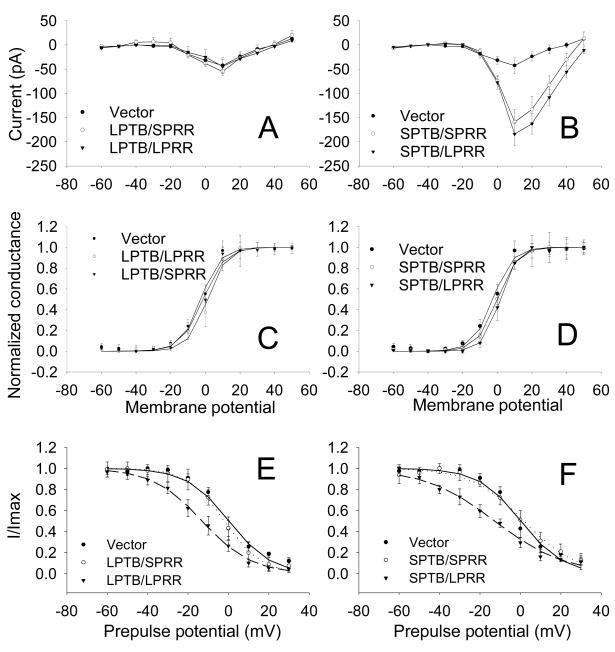
Based on the above data, it seems that PTB (short) domains but not PRR domains of Numb are critically involved in neurite outgrowth and in the increase in ICa density. To determine whether the PTB and or PRR domains of Numbs affect Ca2+ channel properties such as channel activation and inactivation, the voltage dependence of steady-state activation (SSA) and steady-state inactivation (SSI) were evaluated in cells expressing the different Numb isoforms. The current-voltage relationships were not different between different Numb isoforms (Fig. 4A, B). The steady-state activation curves of Ca2+ currents are shown in Fig. 4C, D. The voltage of half-maximal activation (V1/2) did not differ between vector control and various Numb groups. On average, the V1/2 was: −2.6 ± 0.21 mV (n=6), 1.7 ± 0.24 mV (n=5), −1.3 ± 0.18 mV (n=5), −0.3 ± 0.12 mV) and 1.6 ± 0.23 mV (n=7) for cells expressing vector, LPTB/LPRR, LPTB/SPRR, SPTB/SPRR and SPTB/LPRR, respectively.
Figure 4.
Ca2+ channel steady state activation (SSA) and steady-state inactivation (SSI). A. Current-voltage curves of Ca2+ currents in cells expressing vector or SPTB/LPRR, SPTB/SPRR, LPTB/LPRR or LPTB/SPRR Numbs, constructed by plotting the normalized current amplitude at various membrane potentials. B. SSA curve of Ca2+ channels in cells expressing vector and various Numb isoforms. The steady-state conductance (G) and voltage (V) data were transformed from the I−V data shown in A. The curves show data that were fit with the Boltzmann equation of the following form: G/Gmax = 1/(1 + exp (V−V1/2)/S), where Gmax is maximum conductance, V1/2 is half-maximal voltage, and S is the slope. C. Plot of I/Imax for cells transfected with vector (filled circle, n=7), SPTB/SPRR (empty circle, n=6) and SPTB/LPRR (filled triangle, n=6). Current amplitude (I) from the inactivation protocol (#), normalized to the maximum (Imax), was plotted as a function of prepulse membrane potentials and best fitted with a Boltzmann function: I/Imax=1/(1+exp (V1/2−V)/S). V1/2: the pooled half-maximal voltages. # to determine the SSI, a standard +10 mV test pulse for 40 ms was elicited from a holding potential of −80 mV, preceded by a 5-second (steady-state) incremental depolarization from −70 mV to +30 mV every 20 seconds. D. Plot of I/Imax for vector (filled circle, n=7), LPTB/SPRR (empty circle, n=6) and LPTB/LPRR (filled triangle, n=5). Values are the mean ± SEM.
The inactivation curves in Fig. 4E, F showed a shift towards hyperpolarization in cells expressing Numb isoforms with a LPRR regardless of the composition of the PTB domains. On average, the voltage of half-maximal inactivation (V1/2) was: 0.3 ± 0.46 mV (n=7), −13 ± 0.43 mV (n=5), 1.9 ± 0.51 mV (n=6), 1.7 ± 1.1 mV (n=6) and −13 ± 1.4 mV (n=6,) for the cells expressing vector, LPTB/LPRR, LPTB/SPRR, SPTB/SPRR and SPTB/LPRR, respectively. There was a statistically significant difference in V1/2 between LPTB/LPRR and vector or between SPTB/LPRR and vector (p<0.001, ANOVA followed by Holm-Sidak method). These results suggest Ca2+ channel kinetics are not influenced by PTB domains of Numbs. Although PRR (long) domain of Numb may affect channel inactivation, such a role of LPRR domain had no impact on neurite outgrowth. The functional significance of this role remains to be further studied.
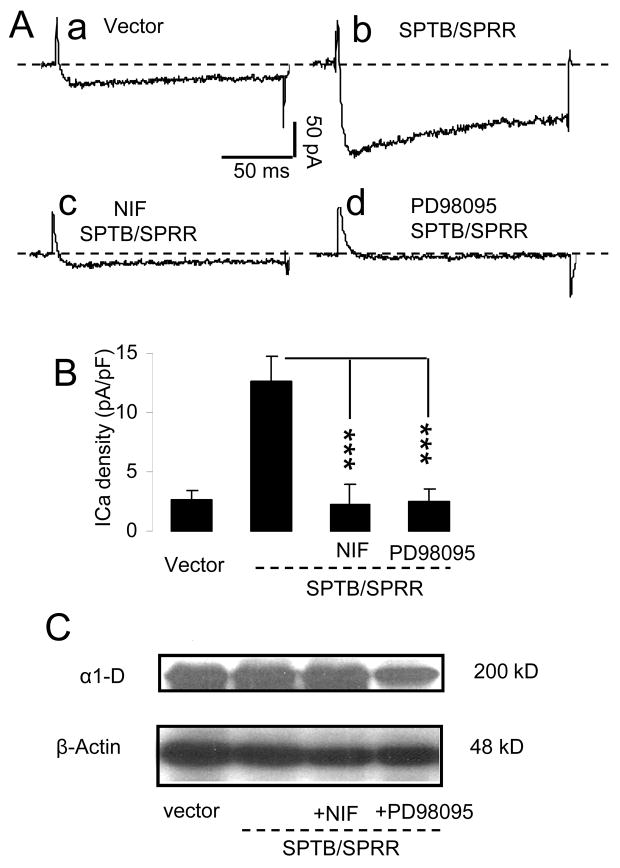
Pretreatment of Nifedipine or PD98095 prevents SPTB Numb-induced increase on ICa density
In order to determine whether L-type Ca2+ channel activity or the activity of MAP kinase is a consequence of neurite outgrowth or is required for the growth of neuritis, cells were preincubated with nifedipine or PD989095. We recorded ICa from the cells expressing SPTB/SPRR numb after 4–5 days in culture in the presence or absence of L-type Ca2+ blocker, nifedipine or MAP kinase inhibitor, PD98095 and compared with ICa density from the cells expressing vector. As shown in Fig. 5 the preincubation of either nifedipine (10 μM) or PD98095 (5 μM) prevented SPTB Numb-induced increase in ICa density. On average, the ICa density was 2.67 ± 0.75 pA/pF, 12.6 ± 2.2 pA/pF, 2.3 ± 1.7 pA/pF and 2.5 ± 1.0 pA/pF for the cells expressing vector (control), SPTB/SPRR numb (no treatment), SPTB/SPRR + nifedipine and SPTB/SPRR +PD98095, respectively. There was no difference in ICa density between vector (n=6) and SPTB/SPRR + nifedipine (n=6) or between vector and SPTB/SPRR + PD98095 (n=6) (One way ANOVA followed by Holm-Sidak method). The reduction in SPTB Numb-induced neurite outgrowth in the presence of these drugs may be associated with the functional inhibition of calcium channels or with the reduced channel expression. To test the latter possibility, we performed the western blot analyses on Ca2+ channel expression in whole cell lysates from cells expressing SPTB in the absence or presence of nifedipine (10 μM) or PD98095 (5 μM) and from the cells expressing vector. The expression level of α1-1D subunit of Ca2+ channel did not differ between these groups (n=3, Fig. 5C). These results indicate that inhibition of functional Ca2+ channel by nifedipine did not alter expression level of the channel and that the profound reduction in ICa density induced by PD98095 may be associated with an alteration in channel phosphorylation or reduced membrane expression of the channel subunit.
Figure 5.
The effects of preincubation of nifedipine and PD98095 on the ICa density. A: Representative traces of Ca2+ currents recorded from cells transfected with vector, SPTB/SPRR numb in the absence and presence of nifedipine or PD98095 in response to the test potential of 10 mV from a holding potential of −70 mV. B. The average changes in ICa density recorded in the cells expressing vector, SPTB/SPRR numb in the absence and presence of nifedipine or PD98095. Values are the mean ± SEM of the determinations made in 6 cells in each group except for SPTB/SPRR (n=7). ***p<0.001compared to values of ICa from the cells expressing SPTB/SPRR in the absence of treatment, Student t-test. C: Immunoblots of proteins in whole cell lysates from the cells expressing vector, SPTB/SPRR numb in the absence and presence of nifedipine or PD98095, probed with antibodies against α1-D subunits of Ca2+ channels. The immnuoblotting was repeated three times.
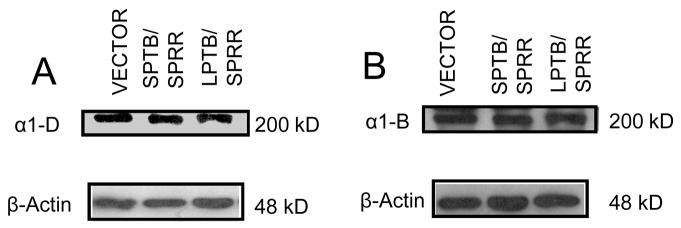
SPTB Numb did not alter expression level in Ca2+ channel subunits in the whole cell lysate
We also performed western blot analyses to show the total protein levels of α1D and α1B Ca2+ channel subunits from whole cell lysate were similar among the cells expressing vector and different Numb isoforms (Fig. 6). The normalized density (normalized to β-actin) of Ca2+ channels averaged from four blots did not show statistically significant differences between vector, SPTB/SPRR and LPTB/SPRR in Ca2+ channel subunits, α1D and α1B. These data indicate that abundant Ca2+ channels of these two subunits already exist in cells expressing not only SPTB Numb but also LTPB Numb. The latter showed extensive proliferating and little functional Ca2+ channel activity (shown by a small ICa density), suggesting that LPTB Numb and SPTB Numb might act differently on the modulatory pathway of Ca2+ channel such as channel phosphorylation or on subcellular/membrane localization of Ca2+ channels.
Figure 6.
SPTB Numb did not increase expression level of Ca2+ channel subunits in whole cell lysate. Immunoblots of proteins in whole cell lysates from cells transfected with vector and the indicated Numb isoforms probed with antibodies against α1-D (A) and α1-B (B) subunits of Ca2+ channels. The immnuoblotting was repeated four times.
DISCUSSION
This study demonstrated that (1) Numb with a short PTB domain can induce neurite outgrowth of PC12 cells; (2) SPTB Numb-, but not NGF-induced neurite outgrowth was mediated by the sustained activation of L-type Ca2+ channel; (3) SPTB Numb-induced neurite outgrowth was dependent on neither receptor tyrosine kinase nor TrkA activation, the later is critical for NGF-induced neurite outgrowth; (4) Both SPTB Numb and NGF-induced neurite outgrowth were mediated by MAP kinase activation. These results suggest SPTB Numb-induced neurite outgrowth potentially involved a distinct mechanism in the upstream signaling but may share MAP kinase signaling with NGF.
Our results are similar to depolarization-induced neurite outgrowth (Solem et al., 1995) that was also dependent on Ca2+ influx and involved in activation of extracellular-regulated kinase (ERK)/mitogen-activated protein kinase pathway (Rusanescu et al., 1995; Bouron et al. 1999) but required no receptor tyrosine kinase activity (Bouron et al., 1999). Our results are also similar to MSH-induced neurite outgrowth (Sakai et al., 1999), in which Ca2+ influx and MAP kinase activation are critically involved in neurite outgrowth.
TrkA activation is vital for NGF-induced neurite outgrowth (Rogers et al., 1994) and contributes to NGF-induce MAP kinase activation (Pang et al., 1995). MAP kinase activation in SPTB Numb (Pederson et al., 2002) is likely to be a secondary effect of Ca2+ influx which is similar to the depolarization- or MSH-induced neurite outgrowth (Bouron et al., 1999).
SPTB Numb-induced neurite outgrowth is dependent Ca2+ influx but not on the Ca2+ release from internal store (ER), because treatment of PC 12 cells with thapsigargin (an inhibitor of internal store Ca2+/ATPase) or dantrolene (blocks Ca2+ release from internal stores) did not affect SPTB Numb-induced neurite outgrowth (Lu and Mattson, unpublished observation), although Ca2+ release from internal stores is involved in the enhancement of NGF-induced neurite outgrowth by GTP (Gysbers et al., 2000).
Associated with neurites extension in cells expressing SPTB Numbs was a large increase in the whole-cell Ca2+ current density which nevertheless contributed to Ca2+ dependent neurite outgrowth. In parallel to the inhibition of SPTB Numb-induced neurite outgrowth, preincubation of either nifidepine or PD98095 dramatically reduced ICa density in cells expressing SPTB Numb. The preincubation of nifedipine leads to chronic inhibition of L-type Ca2+ channel function and contributes to the reduction of SPTB Numb-induce neurite outgrowth. The reduction in ICa density caused by PD98095 suggests that interruption of MAP kinase is associated with functional changes in Ca2+ channel which is likely to be involved in the changes of channel phosphorylation or membrane expression. SPTB Numb-induced neurite outgrowth was not associated with increased Ca2+ channel expression in whole cell lysates supported this hypothesis. However, this remains to be further studied.
Numb mediates endocytosis in axon growth (Nishimura et al., 2003). The PTB domain of Numb is important for plasma membrane localization of Numb protein and for regulation of cell fate in Drosophila (Knoblich et al., 1997; Qin et al., 2004). Combining the data of ICa density and Ca2+ channel expression from the whole cell level, it is possible that LPTB Numbs have strong role on Ca2+ channel endocytosis which keep abundant Ca2+ channel inside the cells, but SPTB Numbs work differently to preferentially localize the Ca2+ channels to the cell membrane. PTB domain may play a role in regulation of the membrane localization of Ca2+ channel subunits by either directly binding to the channel subunits or by indirectly interacting with cytoskeletal proteins. Cytoskeleton protein actin filaments are known to be involved in regulating both the intracellular localization of Numb (Knoblich et al., 1997) and the membrane insertion/removal of several different types of channels including glutamate receptors (Zhou et al., 2001). The proteins containing PTB domain, such as GRS12 have been shown to directly interact with N type Ca2+ channels (Schiff et al., 2000). Therefore, further study to reveal protein-protein interaction between Numb PTB domain and Ca2+ channel subunits would yield valuable information toward Numb’s function.
Increased ICa density occurs before neurite outgrowth in STB Numbs supported that Ca2+ influx through the VGCC channels is a prerequisite for SPTB Numb-induced neurite outgrowth. Interestingly, although activation of the TrkA receptor was not required for neurite outgrowth by SPTB Numbs, it was present also in undifferentiated cells expressing SPTB Numbs (Pedersen et al., 2002). The mechanism by which Numb-induced these changes is unknown, these data suggest that the machinery is in place for Ca2+ and/or TrkA receptor dependent regulation of neurite outgrowth (Ronn et al., 2002). It might be that, in the absence of NGF, there is no TrkA activation and SPTB Numb-induced neurite outgrowth is mainly dependent on a calcium dependent cascade involving MAP kinase activation; while in the presence of NGF, SPTB Numb-induced neurite outgrowth involves activation of both Ca2+ and TrkA dependent pathways that converge on MAP kinase. Indeed, a synergistic role in neurite outgrowth existed when NGF was added in cells overexpressing SPTB Numb (Prederson et al., 2002).
Ca2+ mediates proliferation, differentiation and neurite outgrowth that respond to a variety of signals including growth factors such as basic fibroblast growth factor and brain-derived neurotrophic factor, and neurotransmitters such as glutamate (Deisseroth et al., 2004). Numb isoforms with short and long PTB domains might therefore differentially modify responses of neural progenitor cells and differentiated neurons to such neurotrophic factors and neurotransmitters.
Acknowledgments
The work was supported by the Intramural Research Program of the National Institute on Aging. Authors disclose that there is no interest conflict related to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nurnberg B, Dolphin AC. Biophysical properties, pharmacology, and modulation of human, neuronal L-type (alpha(1D), Ca(V)1.3) voltage-dependent calcium currents. J Neurophysiol. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- Black MM, Greene LA. Changes in the colchicine susceptibility of microtubules associated with neurite outgrowth: studies with nerve growth factor-responsive PC12 pheochromocytoma cells. J Cell Biol. 1982;95:379–386. doi: 10.1083/jcb.95.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, Becker C, Porzig H. Functional expression of voltage-gated Na+ and Ca2+ channels during neuronal differentiation of PC12 cells with nerve growth factor or forskolin. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:370–377. doi: 10.1007/pl00005363. [DOI] [PubMed] [Google Scholar]
- Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D. Alpha1E-containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100:12450–12455. doi: 10.1073/pnas.2035117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Raff M. Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5:1265–1269. doi: 10.1038/nn1202-1265. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540:3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston JT, Valdes JJ, Chambers JP. Ca2+ channel alpha 1-subunit transcripts are differentially expressed in rat pheochromocytoma (PC12) cells following nerve growth factor treatment. Int J Dev Neurosci. 1998;16:379–389. doi: 10.1016/s0736-5748(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Cunningham ME, Kitajewski JK, Greene LA. Efficient generation of stable pheochromocytoma (PC12) cell lines using a recombinant retrovirus (LNC) Methods Mol Biol. 2001;169:135–147. doi: 10.1385/1-59259-060-8:135. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dooley CM, James J, Jane MC, Ahmad I. Involvement of numb in vertebrate retinal development: evidence for multiple roles of numb in neural differentiation and maturation. J Neurobiol. 2003;54:313–325. doi: 10.1002/neu.10176. [DOI] [PubMed] [Google Scholar]
- Dubel SJ, Starr TV, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysbers JW, Guarnieri S, Mariggio MA, Pietrangelo T, Fano G, Rathbone MP. Extracellular guanosine 5′ triphosphate enhances nerve growth factor-induced neurite outgrowth via increases in intracellular calcium. Neuroscience. 2000;96:817–824. doi: 10.1016/s0306-4522(99)00588-6. [DOI] [PubMed] [Google Scholar]
- Hung SP, Hsu JR, Lo CP, Huang HJ, Wang JP, Chen ST. Genistein-induced neuronal differentiation is associated with activation of extracellular signal-regulated kinases and upregulation of p21 and N-cadherin. J Cell Biochem. 2005;96:1061–1070. doi: 10.1002/jcb.20626. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc Natl Acad Sci U S A. 1997;94:13005–13010. doi: 10.1073/pnas.94.24.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352:527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Lu C, Chan SL, Fu W, Mattson MP. The lipid peroxidation product 4-hydroxynonenal facilitates opening of voltage-dependent Ca2+ channels in neurons by increasing protein tyrosine phosphorylation. J Biol Chem. 2002;277:24368–24375. doi: 10.1074/jbc.M201924200. [DOI] [PubMed] [Google Scholar]
- Manivannan S, Terakawa S. Rapid sprouting of filopodia in nerve terminals of chromaffin cells, PC12 cells, and dorsal root neurons induced by electrical stimulation. J Neurosci. 1994;14:5917–5928. doi: 10.1523/JNEUROSCI.14-10-05917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. J Neurosci. 1987;7:4034–4043. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Miller DR, Lee GM, Maness PF. Increased neurite outgrowth induced by inhibition of protein tyrosine kinase activity in PC12 pheochromocytoma cells. J Neurochem. 1993;60:2134–2144. doi: 10.1111/j.1471-4159.1993.tb03498.x. [DOI] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Tokunaga A, Hara A, Hamaguchi T, Kato K, Iwamatsu A, Okano H, Kaibuchi K. Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2. Mol Biol Cell. 2006;17:1273–1285. doi: 10.1091/mbc.E05-07-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara Y, Aoki T, Kusano M, Ohizumi Y. Beta-eudesmol induces neurite outgrowth in rat pheochromocytoma cells accompanied by an activation of mitogen-activated protein kinase. J Pharmacol Exp Ther. 2002;301:803–811. doi: 10.1124/jpet.301.3.803. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Zhu H, bdur-Rahman LA, Verdi JM, Mattson MP. Numb isoforms containing a short PTB domain promote neurotrophic factor-induced differentiation and neurotrophic factor withdrawal-induced death of PC12 Cells. J Neurochem. 2002;82:976–986. doi: 10.1046/j.1471-4159.2002.01036.x. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Qin H, Percival-Smith A, Li C, Jia CY, Gloor G, Li SS. A novel transmembrane protein recruits numb to the plasma membrane during asymmetric cell division. J Biol Chem. 2004;279:11304–11312. doi: 10.1074/jbc.M311733200. [DOI] [PubMed] [Google Scholar]
- Rogers MV, Buensuceso C, Montague F, Mahadevan L. Vanadate stimulates differentiation and neurite outgrowth in rat pheochromocytoma PC12 cells and neurite extension in human neuroblastoma SH-SY5Y cells. Neuroscience. 1994;60:479–494. doi: 10.1016/0306-4522(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Ronn LC, Dissing S, Holm A, Berezin V, Bock E. Increased intracellular calcium is required for neurite outgrowth induced by a synthetic peptide ligand of NCAM. FEBS Lett. 2002;518:60–66. doi: 10.1016/s0014-5793(02)02644-3. [DOI] [PubMed] [Google Scholar]
- Sakai T, Furuyama T, Ohoka Y, Miyazaki N, Fujioka S, Sugimoto H, Amasaki M, Hattori S, Matsuya T, Inagaki S. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca(2+) influx. J Biol Chem. 1999;274:29666–29671. doi: 10.1074/jbc.274.42.29666. [DOI] [PubMed] [Google Scholar]
- Schiff ML, Siderovski DP, Jordan JD, Brothers G, Snow B, De VL, Ortiz DF, verse-Pierluissi M. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408:723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Smith CA, Dho SE, Donaldson J, Tepass U, McGlade CJ. The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol Biol Cell. 2004;15:3698–3708. doi: 10.1091/mbc.E04-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem M, McMahon T, Messing RO. Depolarization-induced neurite outgrowth in PC12 cells requires permissive, low level NGF receptor stimulation and activation of calcium/calmodulin-dependent protein kinase. J Neurosci. 1995;15:5966–5975. doi: 10.1523/JNEUROSCI.15-09-05966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tully K, Treistman SN. Distinct intracellular calcium profiles following influx through N- versus L-type calcium channels: role of Ca2+-induced Ca2+ release. J Neurophysiol. 2004;92:135–143. doi: 10.1152/jn.01004.2003. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Bashirullah A, Goldhawk DE, Kubu CJ, Jamali M, Meakin SO, Lipshitz HD. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc Natl Acad Sci U S A. 1999;96:10472–10476. doi: 10.1073/pnas.96.18.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Doherty P, Turner G, Reid RA, Hemperly JJ, Walsh FS. Calcium influx into neurons can solely account for cell contact-dependent neurite outgrowth stimulated by transfected L1. J Cell Biol. 1992;119:883–892. doi: 10.1083/jcb.119.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]