Abstract
BACKGROUND
Preliminary evidence suggest that noradrenergic signaling may play a role in mediating alcohol drinking behavior in both humans and rats. Accordingly, we tested the hypothesis that blockade of α1-adrenergic receptors will suppress alcohol drinking in rats selectively bred for alcohol preference (P line).
METHODS
Adult male P rats were given 24-hour access to food and water and scheduled access to a 15% (v/v) alcohol solution for two hours daily. Rats were injected IP with the α1-adrenergic receptor antagonist, prazosin (0, 0.5, 1.0, 1.5 or 2.0 mg/kg BW), once a day at 15 min prior to onset of the daily two hour two-bottle choice, alcohol versus water, access period for two consecutive days and then three weeks later for five consecutive days.
RESULTS
Prazosin significantly reduced (p<0.01) alcohol intake during the initial two daily administrations, and this reduction of alcohol intake was maintained for five consecutive days by daily prazosin treatment in the subsequent more prolonged trial (p<0.05). The prazosin-induced reduction of alcohol intake was not dependent upon drug-induced motor impairment since increases in water drinking (p<0.05) were exhibited during the two hour access periods during both two day and five day prazosin treatment.
CONCLUSIONS
The results indicate that the noradrenergic system plays a role in mediating alcohol drinking in rats of the P line, and suggest that prazosin - a safe, well-characterized and well-tolerated drug - may be an effective pharmacotherapeutic agent for the treatment of alcohol use disorders.
Keywords: noradrenergic, prazosin, alcohol, alcohol-preferring, P rats
INTRODUCTION
Since alcohol can be sympatho-suppressive, anxiolytic and sedating, it has been suggested that anxiety and associated CNS sympathetic activation can lead to excessive use of alcohol as a form of self-medication (Edwards et al., 1972; Kushner et al., 1990; Kushner et al., 1999; Shirao et al., 1988; Koob and LeMoal, 1997). Evidence of shared vulnerability between alcohol abuse and anxiety disorders has led to suggestions that alcohol may exert effects on a physiological mechanism that underlies anxiety (Merikangas et al., 1994; Merikangas et al., 1998; Sinha et al., 1998). The fact that increased sympathetic activation is seen during both alcohol abstinence (Koob and LeMoal, 1997; Ehrenreich et al., 1997) and during periods of increased anxiety (Sullivan et al., 1999) suggests that increased sympathetic activation and excessive noradrenergic signaling may be that mechanism. Furthermore, the fact that rats exhibit both sympathoadrenal activation and increased anxiety-like behavior long after termination of chronic alcohol consumption (Rasmussen et al., 2001; Rasmussen et al., 2006) likewise suggests that excessive sympathetic activation is one of the aversive physiological events that occur during alcohol withdrawal and abstinence, events that are thought to contribute to increased risk of alcohol relapse after cessation of chronic alcohol abuse (Koob and LeMoal, 1997).
Individuals with post-traumatic stress disorder (PTSD) likewise commonly exhibit increased sympathetic activation and increased anxiety (Aston-Jones et al., 1994; Southwick et al., 1999) consistent with the characteristics of abstinent alcoholics (Kushner et al., 1990; Kushner et al., 1999; Koob and LeMoal, 1997), selectively-bred alcohol-preferring (P) rats (Stewart et al., 1993), and alcohol-dependent outbred rats undergoing prolonged abstinence (Rasmussen et al., 2006; Rasmussen et al., 2002). It is especially significant that: a) there is an unusually high comorbidity (50–70% or more, depending on the study and patient population characteristics) between alcohol and/or other drug abuse and PTSD; b) the development of alcohol abuse commonly follows initial development of PTSD (Kushner et al., 1990; Merikangas et al., 1994; Davidson et al., 1990); c) a large increase in alcohol consumption has been reported to parallel progressive severity of PTSD (Kushner et al., 1990); and d) patients report that alcohol attenuates their PTSD symptoms (Kushner et al., 1990), suggesting that patients with PTSD drink alcohol to self-medicate. The selective α1-adrenergic antagonist, prazosin, has been found to alleviate many of the symptoms of PTSD including hyperarousal, sleep disturbances, and overall PTSD severity (Raskind et al., 2003). Interestingly, patients receiving prazosin treatment for PTSD also commonly reported markedly decreased motivation to drink alcohol, and decreased overall alcohol consumption (Raskind, upublished observations).
The association between increased sympathetic signaling and alcohol drinking, together with the clinical observation that administration of prazosin appeared to commonly decrease alcohol consumption in a patient population characterized by increased sympathetic activation and high co-morbidity with alcohol abuse, suggests that a functional relationship may exist between increased sympathetic signaling and alcohol drinking and that attenuation of sympathetic activation may result in decreased alcohol intake. Consequently, we examined the effect of prazosin, a drug which decreases sympathetic signaling by blocking α1-adrenergic receptors, on operant self-administration of alcohol by Wistar rats during acute alcohol withdrawal (Walker et al., 2008). The results demonstrated that prazosin treatment blocked dependence-induced increases in responding for alcohol. In the current study, we further examine the effect of prazosin on alcohol drinking by alcohol-preferring P rats, a well-characterized animal model of excessive voluntary alcohol drinking (Froehlich and Li, 1991; McBride and Li, 1998; Murphy et al., 2002; Waller et al., 1984). We hypothesize that prazosin may be both effective and safe as a pharmacotherapeutic agent for the treatment of alcohol use disorders.
MATERIALS AND METHODS
Subjects
Fifty-five alcohol-naive male rats from the 60th generation of selective breeding for alcohol preference were used in this study. The alcohol-preferring (P) rats were 63 – 96 days of age and weighed 298 – 501 g at the start of the study. The rats were individually housed in stainless steel hanging cages in an isolated vivarium with controlled temperature (21±1 °C) and a 12 h light/dark cycle (lights off at 1000 h). Standard rodent chow (Laboratory Rodent Diet #7001, Harlan Teklad, Madison, WI) and water were available ad libitum throughout the study. All experimental procedures were approved by the Indiana University Institutional Animal Care and Use Committee and conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol and Water Intake
Free-choice between alcohol and water was introduced as described below. The alcohol solution was prepared by diluting 95% alcohol (ethanol) with distilled, deionized water to make a 15% (v/v) solution. Alcohol (15 % v/v) and water were presented in calibrated glass drinking tubes, with positions of the tubes alternated daily to control for potential side preferences. Daily fluid intakes were recorded to the nearest ml. Alcohol intake was converted from ml alcohol/kg BW to g alcohol/kg BW. All subjects were acclimated to individual housing for two weeks prior to introduction of a free-choice between alcohol and water.
Alcohol Drinking Induction Phase
All rats were initially provided with 24-hour access to food, water and 15% (v/v) alcohol for three weeks. Fluid intakes and body weights were recorded every other day. Access to alcohol was then reduced to daily scheduled access times using a “step down” procedure. Twenty four hour access to alcohol was first reduced from seven days/week to five days/week (Monday–Friday) for one week. Then 24-hour access to alcohol was reduced to four hours/day for five days/week for two weeks and then to two hours/day for five days/week for the remainder of the experiment. Alcohol was available from 1100 h (one hour after lights off) to 1300 h, five days/week for seven weeks prior to administration of prazosin. All rats were always given ad libitum access to food and water. Alcohol intake, water intake and body weight were recorded daily.
Drugs
Prazosin hydrochloride (Fluka Chemical Corp., Milwaukee, WI) was dissolved in water and then diluted 1:1 with 0.9% (w/v) NaCl to achieve a 0.45% (w/v) saline solution. Prazosin (0.5 mg/ml) was injected intraperitoneally (IP) in a dose of 0.5, 1.0, 1.5, or 2.0 mg/kg body weight (BW). Due to limited solubility of prazosin in saline, the increasing doses of prazosin were administered in increasing volumes of 0.45% saline (1–4 ml/kg BW); the control group received 3 ml/kg BW of 0.45% NaCl vehicle.
Treatments
During the week prior to onset of prazosin administration, baseline alcohol intake was calculated for each rat over four consecutive days, and the rats were ranked in descending order in terms of average daily alcohol consumption. Rats were assigned to drug treatment groups in a manner that ensured that the groups did not differ in baseline alcohol intake prior to prazosin administration. Specifically, the top five alcohol drinkers were randomly assigned to the vehicle or one of four prazosin treatment groups (0.5, 1.0, 1.5 or 2.0 g prazosin/kg BW), followed by the next five highest alcohol drinkers likewise randomly assigned, etc. Two Day Treatment: In order to reduce the stress associated with IP drug administration, all rats were handled as if they were going to receive an IP injection for five consecutive days prior to initial drug treatment, and all rats received an IP injection of vehicle on the day preceding initial drug treatment. Prazosin, in doses of 0.5, 1.0, 1.5 or 2.0 mg/kg BW, or an equivalent volume of vehicle, was administered 15 minutes prior to the daily two hour alcohol access period on each of two consecutive days. Each treatment group (11 rats/group) received a single dose of prazosin or vehicle. Five Day Treatment: After completion of the initial Two Day treatment, rats continued to receive two hour daily access to alcohol for five days/week for three weeks prior to initiating five days of treatment. In order to reduce the stress associated with IP drug administration, all rats were again handled as if they were going to receive an IP injection for 5 consecutive days prior to the Five Day drug treatment, and all rats received an IP injection of vehicle on the final day preceding drug treatment. Average daily alcohol intake over four days was determined and the rats were again ranked and randomly assigned to treatment groups following the same procedure as described above for the previous Two Day trial. Prazosin, in doses of 0.5, 1.0, 1.5 or 2.0 mg/kg BW, or an equivalent volume of vehicle, was then administered 15 minutes prior to the daily two hour alcohol access period on each of five consecutive days. These drug treatments were initiated on a Monday; since ethanol access was not provided on weekends, this trial followed immediately after the weekly two day alcohol deprivation.
Data analyses
Alcohol intake during drug treatment trials, including one pre- and one post-treatment day, were analyzed by two way analysis of variance (ANOVA) with repeated measures (Dose X Day with repeated measures on Day) followed, only when justified by determination of significant differences or interactions in the ANOVA, by pairwise multiple comparisons using Fisher’s least significant difference (LSD) tests. Secondary analyses of alcohol intake were also conducted with more conservative Tukey HSD multiple comparisons. As discussed in the Methods section, Kruskal-Wallis one way ANOVA on ranks followed by Dunn’s multiple comparisons procedure was used to analyze water intake. All analyses were conducted using the SigmaStat 3.5 program (Systat Software, Inc., Chicago, IL) with significance accepted at p<0.05. Data are presented as mean±SE.
RESULTS
Initial Two Day Treatment
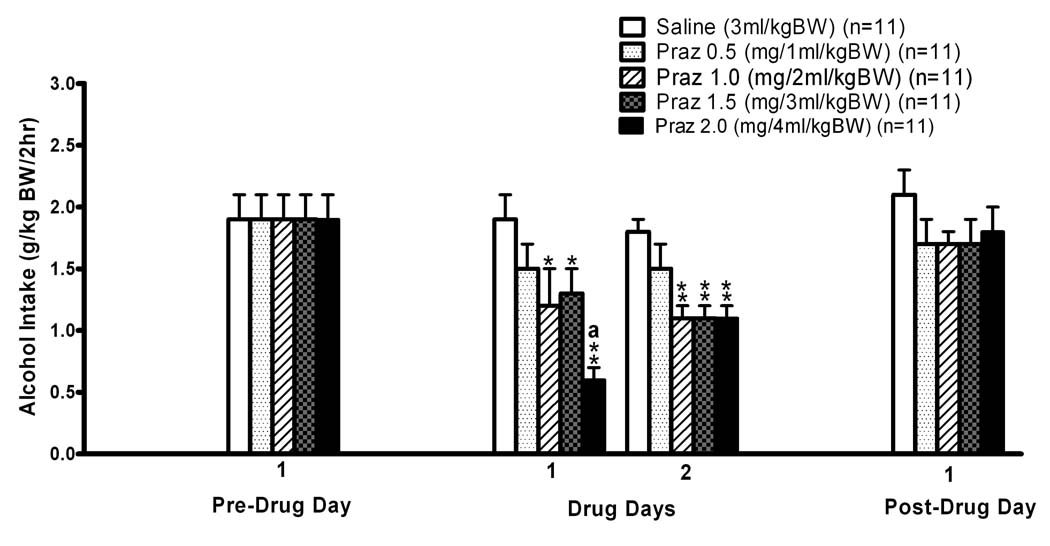
The effects of prazosin treatment on alcohol intake during daily two hour alcohol access periods on two consecutive days are presented in Fig. 1. Two way repeated measures ANOVA (Dose X Day, with repeated measures on Day) over all four consecutive days (one pre-treatment day, two drug treatment days, and one post-treatment day) revealed significant main effects of Dose [F (4, 50) = 3.34, p<0.05), Day [F (3, 150) = 26.47, p<0.001] and Dose X Day interaction [F (12,150 = 2.83, p<0.01], indicating differences between the treatment groups (Dose) which were dependent upon day (Day) of the trial. Further pairwise comparisons using the Fisher’s LSD test revealed that alcohol intake was not different between treatment groups (Dose) on the Pre-Drug Day or on the Post-Drug Day, and that alcohol intakes on the Post-Drug Day were not different from those on the Pre-Drug Day. Pairwise comparisons of Day within Dose revealed that alcohol intake by the saline (0 mg prazosin/kg BW Dose) vehicle-treated control rats was not different among the four days of the trial (i.e., Pre-Drug Day, Drug Days 1 and 2, and Post-Drug Day); in contrast, alcohol intake by the rats receiving 0.5 mg/kg BW prazosin was less on both Drug Day 1 and 2 than on the Pre-Drug Day (p<0.05), and alcohol drinking by the rats receiving 1.0, 1.5 or 2.0 mg/kg BW were all less on both Drug Day 1 (p<0.05, p<0.01 and p<0.01, respectively) and 2 (p<0.05, p<0.05, and p<0.01, respectively) than on both the Pre-Drug and Post-Drug days. On Drug Day 1, administration of 1.0 and 1.5 mg/kg BW doses of prazosin decreased alcohol intake by 37 and 32%, respectively, relative to alcohol intake by saline-treated control rats (p<0.05 for each), 2.0 mg prazosin/kg BW decreased alcohol intake by 68% relative to alcohol intake by saline-treated control rats (p<0.01), and the suppression by 2.0 mg prazosin/kg BW was significantly greater than the suppression by both 1.0 and 1.5 mg prazosin/kg BW (p<0.05 and 0.01, respectively). On Drug Day 2, administration of 1.0, 1.5 and 2.0 mg prazosin/kg BW all suppressed (p<0.01 for each) alcohol intake by approximately 39%. Pairwise comparisons of Day within Dose revealed that the suppression of ethanol intake by 2.0 mg prazosin/kg BW was significantly greater on the first drug administration day than on the second (p<0.01).
Figure 1. INITIAL TWO DAY TREATMENT.
Effects of IP prazosin administration on two hour alcohol (15% v/v) intake by adult male alcohol-preferring P rats. Prazosin was injected on each of two consecutive days, identified as Drug Days 1 and 2, at 15 minutes prior to onset of a two hour, two bottle free-choice between alcohol and water. *p<0.05 and **p<0.01 vs saline control treatment within individual days. a p<0.01 vs Praz 0.5 and Praz 1.5, p<0.05 vs Praz 1.0 treatment. Each bar represents the mean±SE of 11 rats.
The responses to initial two day prazosin administration were also further analyzed with a supplemental approach focused on only the two days of drug administration (i.e., excluding the Pre-Drug and Post-Drug days) and using the relatively conservative Tukey HSD multiple comparisons test. Two way repeated-measures ANOVA (Dose X Day, with repeated measures on Day) of only Drug Day 1 and Drug Day 2 revealed a significant main effect of Dose [F (4, 50) = 7.824, p<0.01], but no effect of Day [F (1, 50) = 0.144, p = 0.71] and no Dose X Day interaction [F (4, 50) = 1.777, p = 0.148]. Tukey multiple comparisons of Dose, independent of Day, again revealed that 1.0, 1.5 and 2.0 mg/kg BW doses of prazosin all suppressed alcohol intake relative to that of saline vehicle-treated rats (p<0.001 for 2.0 and p<0.01 for 1.0 and 1.5 mg/kg BW).
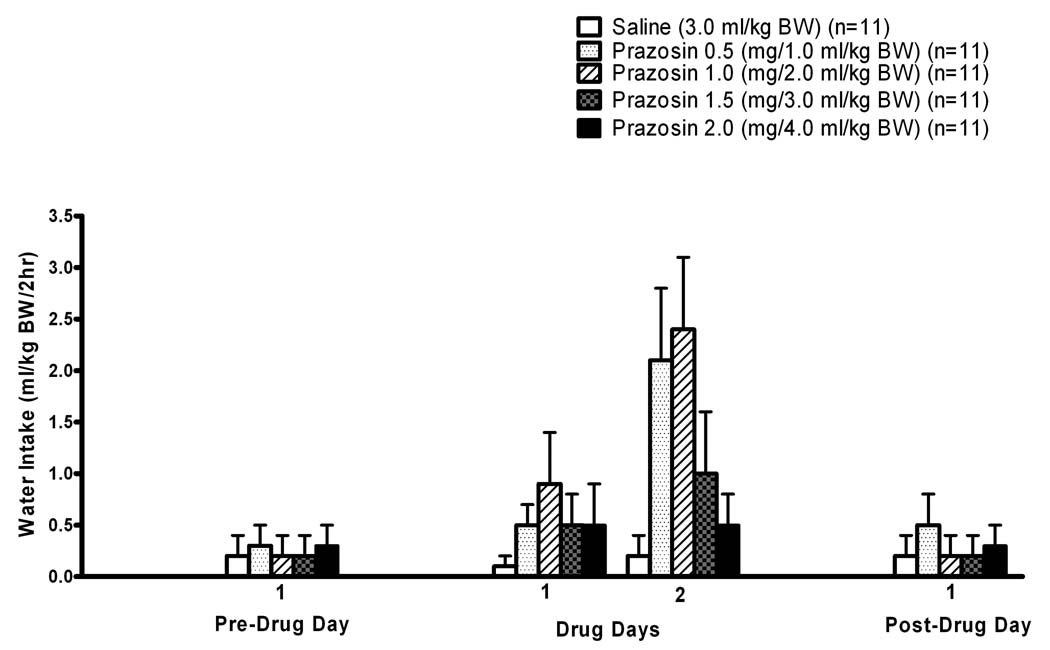
The effects of prazosin treatment on water intake during these daily two hour trials are presented in Fig. 2. In this initial Two Day treatment trial, as well as in the subsequent Five Day treatment trial, no commonly-used data transformations were effective in achieving homogeneity of variance and normal distribution when the Pre-Drug and Post-Drug days were included with the drug treatment days for the ANOVA. Since non-parametric two way ANOVA with repeated measures tests are not available, three separate non-parametric one way ANOVA analyses were conducted – one evaluating water intake on the Pre-Drug Day, the second evaluating average water intake during Drug Days 1 and 2 (i.e., for each rat, intake on Drug Days 1 and 2 were averaged to yield a single value for subsequent analysis), and the third evaluating water intake on the Post-Drug Day. Kruskal-Wallis one way ANOVA on ranks indicated that water intake was not significantly different among the treatment groups (Dose) on either the Pre-Drug Day (H = 0.801, 4 df, p = 0.938) or the Post-Drug Day (H = 1.229, 4 df, p = 0.873). However, the water intakes on Drug Days 1 and 2 were significantly different among the 0, 0.5, 1.0, 1.5 and 2.0 prazosin Doses (H = 13.588 with 4 df, p<0.01). With the Dunn’s multiple comparisons procedure, only the 1 mg prazosin/kg BW Dose induced average water intake which was individually determined to be greater (p<0.05) than that of rats receiving the saline control (0 mg prazosin/kg BW) Dose.
Figure 2. INITIAL TWO DAY TREATMENT.
Effects of IP prazosin administration on two hour water intake by adult male alcohol-preferring P rats. Prazosin was injected on each of two consecutive days, identified as Drug Days 1 and 2, at 15 minutes prior to onset of a two hour, two bottle free-choice between alcohol and water. Each bar represents the mean±SE of 11 rats.
Subsequent Five-Day Treatment
The effects of prazosin treatment on alcohol intake in a second trial with daily two hour alcohol access periods on five consecutive days are presented in Fig. 3. Two-way repeated measures ANOVA (Dose X Day, with repeated measures on Day) of intake during all seven consecutive days (Pre-Drug Day, Drug Days 1–5, Post-Drug Day) indicated significant main effects of Day [F (6, 299) = 17.040, p<0.001] and Dose X Day interaction [F (24, 299) = 2.955, p<0.001] but no significant main effect of Dose [F (4, 50) = 2.028, p = 0.10]. Further pairwise comparisons using Fisher’s LSD testing revealed that alcohol intake was not different between treatment groups (Dose) on the Pre-Drug Day, and that alcohol intake by the saline (0 mg prazosin/kg BW) vehicle-treated control rats was not different among the Pre-Drug Day (on Friday, three days before initiation of drug treatments on the following Monday following alcohol deprivation over the weekend) and five Drug Days, but was decreased (p<0.05) on the Post-Drug day (i.e., on the Monday following completion of drug treatments on the previous Friday and alcohol deprivation over the weekend). On Drug Day 1, administration of 1.0, 1.5 and 2.0 mg/kg BW doses of prazosin decreased (p<0.01 for each) alcohol intake by 38, 48, and 67%, respectively, relative to alcohol intake by saline-treated control rats, and the suppression by 2.0 mg prazosin/kg BW was significantly greater than the suppression by both 0.5 and 1.0 mg prazosin/kg BW (p≤0.05). On Drug Day 2, administration of 1.0 or 2.0 mg prazosin/kg BW suppressed (p<0.05 for each) alcohol intake by 40 and 35% respectively. On Drug Day 3, 0.5 and 2.0 mg prazosin/kg BW each suppressed alcohol intake by 30% (p<0.05 for each), and 1.0 mg prazosin/kg BW suppressed alcohol intake by 40% (p<0.01). On Drug Days 4 and 5, 0.5 and 1.0 mg prazosin/kg BW each suppressed (p<0.05 for each) alcohol intake by approximately 35 (Drug Day 4) or 41% (Drug Day 5). Pairwise comparisons of Day within Dose revealed that ethanol intake in response to 2.0 mg prazosin/kg BW was significantly less (p≤0.01) on the first drug administration day than on all remaining drug administration days (i.e., Drug Days 2–5. For 0.5, 1.0, 1.5 and 2.0 mg prazosin/kg BW, ethanol intake on Drug Days 1–5 were all less (p<0.05) than ethanol intake on the Pre-Drug Day. On the Monday following the final drug treatment on the previous Friday (including an intervening two day alcohol deprivation), overall alcohol intake independent of Dose was decreased relative to the Pre-Drug Day (p<0.01), and within-Day alcohol drinking in response to 1.5 mg prazosin/kg BW was increased (p<0.05) relative to intake by the saline-treated control rats.
Figure 3. SUBSEQUENT FIVE DAY TREATMENT.
Effects of IP prazosin administration on two hour alcohol (15% v/v) intake by adult male alcohol-preferring P rats. Prazosin was injected on each of five consecutive days, identified as Drug Days 1–5, at 15 minutes prior to onset of a two hour, two bottle free-choice between alcohol and water. Pre-Drug Day 3 was the Friday preceding Drug Days 1–5 which were conducted on Monday–Friday of the following week, and Post-Drug Day 3 is the following Monday; there were no alcohol vs water two hour choice trials on the intervening weekends before and after Drug Days 1–5. *p<0.05 and **p<0.01 vs saline control treatment within individual days. ap≤0.05 vs Praz 0.5 and Praz 2.0. bp≤0.05 vs Saline. Each bar represents the mean±SE of 11 rats.
The responses to five-day prazosin administration were also further analyzed with a supplemental approach focused on only the five days of drug administration (i.e., excluding the Pre-Drug and Post-Drug days) and using the more conservative Tukey HSD multiple comparisons test. Two-way repeated-measures ANOVA (Dose X Day, with repeated measures on Day) of only Drug Days 1–5 revealed significant main effects of Dose [F (4, 50) = 3.614, p<0.05] and Day [F (4, 199) = 2.706, p <0.05] and a significant Dose X Day interaction [F (16, 199) = 1.942, p < 0.05]. Tukey multiple comparisons of Dose, independent of Day of treatment, indicated that 1.5 and 2.0 mg/kg BW doses of prazosin each suppressed (p<0.05) alcohol intake relative to alcohol intake by the saline vehicle-treated rats, with a trend (p<0.10) for suppression by 0.5 mg prazosin/kg BW. Comparisons of Day within 0 mg/kg BW (saline control) Dose revealed that ethanol intake by the control rats was not different among the five days. Comparing Dose within Days, Drug Day 1 alcohol intake was suppressed by 1.5 and 2.0 mg prazosin/kg BW (p<0.5 and 0.1, respectively), relative to alcohol intake by the saline control-treated rats. With this Tukey HSD analysis, Day 5 was the only other individual day on which prazosin treatment significantly suppressed alcohol intake, in response to 0.5 and 1.0 mg prazosin/kg BW (p<0.05 for each). Comparing Day within Dose, alcohol intake by rats receiving 1.5 or 2.0 mg prazosin/kg BW were each less (p<0.05) on Drug Day 1 than on Drug Days 4 and 5.
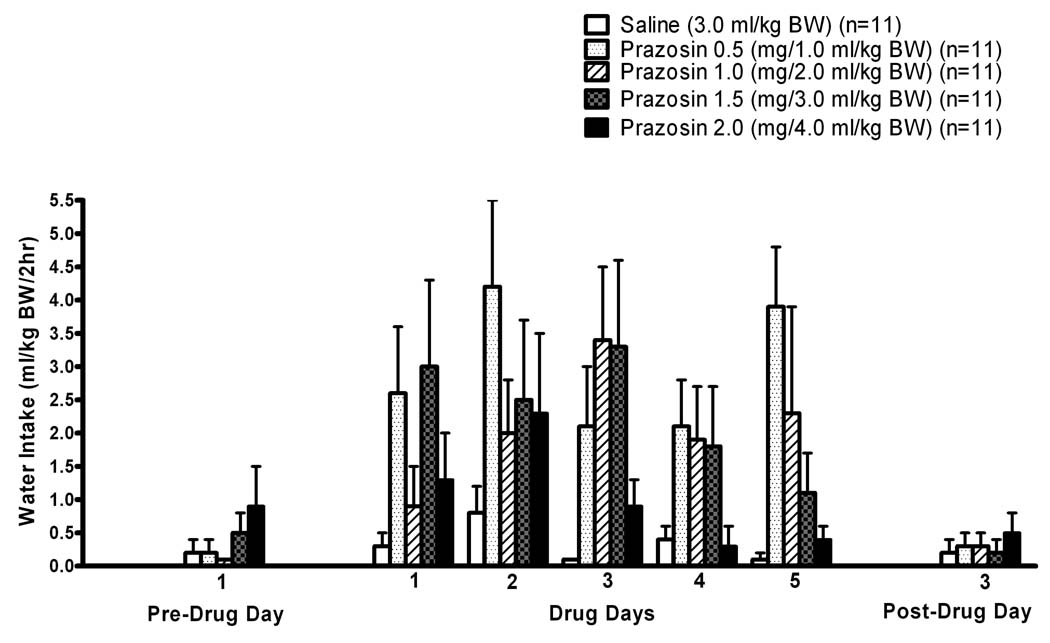
The effects of prazosin treatment on water intake during this Five Day treatment trial are presented in Fig. 4. As in the previous Two Day treatment trial, no commonly-used data transformations were effective in achieving homogeneity of variance and normal distribution when the Pre-Drug and Post-Drug days were included with the drug treatment days for the ANOVA, so three separate non-parametric one-way ANOVA analyses were conducted – one evaluating water intake on the Pre-Drug Day, the second evaluating average water intake during Drug Days 1–5 (i.e., for each rat, intake on Drug Days 1–5 were averaged to yield a single value for subsequent analysis), and the third evaluating water intake on the Post-Drug Day. Kruskal-Wallis one way ANOVA on ranks indicated that water intakes were not significantly different among the treatment groups (i.e., Dose: 0, 0.5, 1.0, 1.5, and 2.0 mg/kg BW) on either the Pre-Drug Day (H = 4.135, 4 df, p = 0.388) or the Post-Drug Day (H = 0.994, 4 df, p = 0.911). However, the average water intakes on Drug Days 1–5 were significantly different (p<0.01) among the 0, 0.5, 1.0, 1.5 and 2.0 prazosin Doses (H = 15.160, 4 df, p<0.01). With the Dunn’s multiple comparisons procedure, the 0.5 and 1.5 mg prazosin/kg BW Doses induced average water intake which was greater than that of rats receiving the saline control (0 mg prazosin/kg BW) Dose.
Figure 4. SUBSEQUENT FIVE-DAY TREATMENT.
Effects of IP prazosin administration on two hour water intake by adult male alcohol-preferring P rats. Prazosin was injected on each of five consecutive days, identified as Drug Days 1–5, at 15 minutes prior to onset of a two hour, two bottle free-choice between alcohol and water. Pre-Drug Day 3 was the Friday preceding Drug Days 1–5 which were conducted on Monday–Friday of the following week, and Post-Drug Day 3 is the following Monday; there were no alcohol vs water two-hour choice trials on the intervening weekends before and after Drug Days 1–5. Each bar represents the mean±SE of 11 rats.
Body weight gain was not significantly altered by the five-day prazosin administration (4.6±1.4, 7.8±4.3, 8.2±4.3, 17.2±4.7 and 5.7±3.1 g weight gain for saline- and 0.5, 1.0, 1.5, and 2.0 mg/kg BW prazosin-treated rats, respectively, during five days of treatment; p>0.05 by Kruskal-Wallis one way analysis of variance on ranks).
DISCUSSION
Male alcohol-preferring P rats established stable alcohol intake during daily two hour alcohol access periods. This alcohol intake was suppressed by administration of the α1-adrenergic receptor antagonist, prazosin, once each day prior to onset of the daily two hour alcohol access period for either two consecutive days or five consecutive days.
There was no evidence suggesting that any of the prazosin doses decreased water intake during the two hour limited access periods in either the Two Day or Five Day trials, and indeed this water consumption, although irregular, was significantly increased by several prazosin doses. This lack of suppression of water intake is consistent with the previously reported lack of effect of these same doses and route of prazosin on operant self-administration of water during acute withdrawal of alcohol-dependent rats (Walker et al., 2008), as well as with reports that these same doses and route of prazosin administration did not alter any measures of locomotion in rats (Wellman et al., 2002; Vanderschuren et al., 2003; Darracq et al., 1998). Consequently, it is highly unlikely that the decreases in alcohol intake after prazosin treatment were dependent upon a motor-impairing effect of prazosin that interfered with the ability of the rats to drink. Furthermore, five day prazosin treatment did not alter body weight gain, suggesting that chronic treatment did not make the rats sick.
It is, however, plausible that non-specific adverse effects could have played a role in the response to the highest dose of prazosin on the first day of both the Two Day and the Five Day treatment trials. In both trials, on the first day the highest dose of prazosin induced significantly greater suppression of alcohol intake than did lower doses. Likewise, in both trials this high dose-dependent enhanced suppression in response to the first exposure subsequently diminished on the second day. In clinical practice, prazosin dose is commonly ramped up over an initial several days to avoid uncommon episodes of first-dose hypotension. It is plausible that this or similar transiently adverse side effects could have been responsible for the initial dose-dependence exhibited in response to administration of the highest prazosin dosage on the first day of both trials in the current study. The similarity of the first day response to the highest dose of prazosin, and second day decrease in this response, in both the Two Day and the subsequent Five Day trials illustrates that conducting two sequential treatment trials with re-randomization to treatment groups during the intervening three weeks provided effective intra-study replication of the prazosin suppression of ethanol drinking.
The Five Day study further demonstrated that even the lowest dose of prazosin, which was insufficient to suppress alcohol intake on the first day of the Two Day and Five Day treatment trials, became effective after three daily administrations during Five Day treatment. In contrast, the highest doses of prazosin appeared to exhibit diminished efficacy with repeated administrations. These adaptive responses to repeated administrations, which may reflect changes in receptor or other mechanisms which cannot be determined from these results, remain to be replicated and mechanisms characterized.
In the Five Day trial, the first day of prazosin treatment followed the weekly two day (weekend) alcohol deprivation, suggesting the possibility that results at the start of the Five Day trial could have been confounded by an alcohol deprivation effect. However, alcohol intake by the saline-treated rats on the day following the two day deprivation was equivalent to intake on the day preceding the deprivation, indicating that a deprivation effect was not expressed at that time of the study. In addition, the responses to prazosin administrations in the first day of the Five Day trial were remarkably similar to the responses in the first day of the earlier Two Day trial, which did not immediately follow the weekend two day alcohol deprivation.
The current results complement and extend results of our recent report that prazosin treatment, at similar doses, route and time of administration, decreased dependence-induced operant alcohol self-administration by Wistar rats during acute alcohol withdrawal (Walker et al., 2008). The current results are also consistent with our clinical observation that PTSD patients, who exhibit co-morbid alcohol abuse and increased sympathetic activation, commonly report markedly decreased motivation to drink alcohol, and decreased overall alcohol consumption, during prazosin treatment of PTSD (Raskind, unpublished observations). Finally, these results are also consistent with our recent finding that prazosin treatment promotes abstinence among alcohol-dependent non-PTSD patients, without significant adverse side effects (Simpson et al., 2007; Simpson et al., 2008).
The current results suggest that noradrenergic signaling plays a key role in maintaining alcohol drinking in a genetic animal model of increased alcohol preference and excessive alcohol drinking. Although the mechanism underlying prazosin-induced suppression of alcohol drinking is not yet known, other evidence suggests a role for noradrenergic neurotransmission in maintaining alcohol drinking. For example, blocking norepinephrine biosynthesis has been reported to decrease alcohol self-administration in rodents (Amit et al., 1977; Brown et al., 1977; Davis et al., 1978). Begleiter and Porjesz proposed that hyperexcitability is the key feature of the simplest effective model of the neuronal milieu underlying genetic predisposition to alcoholism (Begleiter and Porjesz, 1999). Consequently, it is significant that hyperexcitability, as reflected in an enhanced startle response, is associated with increased adrenergic activation, is mediated at least in part by brain α1-adrenergic mechanisms (Stevens et al., 1994), and is characteristic of abstinent alcoholics, selectively-bred alcohol-preferring P rats, alcohol-dependent outbred rats experiencing acute alcohol withdrawal, alcohol-dependent outbred rats experiencing prolonged imposed abstinence, and PTSD patients with high alcohol abuse co-morbidity (Rassnick et al., 1992; Chester et al., 2004; Rasmussen et al., 2005; Krystal et al., 1997; Davis et al., 1997).
Prazosin is a selective α1-adrenergic antagonist which passes the blood brain barrier and decreases brain noradrenergic activation by blocking α1-adrenoceptors following peripheral administration (Menkes et al., 1981; Rojawski and Aghajanian, 1982). Consequently, peripheral administration of prazosin can suppress not only central α1-adrenergic-mediated hyperexcitability but also stress-induced anxiety, both of which have been suggested to contribute to development of alcoholism and both of which are mediated at least in part by brain α1-adrenergic mechanisms (Cecchi et al., 2002). Long-term clinical administration of prazosin is associated with a low incidence of side effects (Graham, 1984).
The results of the current study indicate that the noradrenergic system plays a role in mediating alcohol drinking in rats of the P line. The results also strongly suggest that prazosin – a safe, well-characterized, well-tolerated, CNS-active, and inexpensive α1-adrenergic receptor antagonist – may be an effective pharmacotherapeutic agent for the treatment of alcohol use disorders.
Acknowledgments
This material is based upon work supported in part by resources from the VA Puget Sound Health Care System, Seattle, Washington, and by NIH grants AA10567 and AA13881 (DDR) and AA10709 and AA007611 (JCF).
REFERENCES
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol. I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. Locus coeruleus, stress, and PTSD: neurobiological and clinical parallels. In: Murburg MM, editor. Catecholamine Function in Posttraumatic Stress Disorder: Emerging Concepts. Washington, D.C: American Psychiatric Press, Inc; 1994. pp. 17–62. [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Levitan DE, Ogren SO, Sutherland EA. Noradrenergic mediation of the positive reinforcing properties of ethanol. II. Extinction of ethanol-drinking behavior in laboratory rats by inhibition of dopamine-beta-hydroxylase. Implications for treatment procedures in human alcoholics. Arch int Pharmacodyn. 1977;230:76–82. [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine respnses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RT, Kudler HS, Saunders WB, Smith RD. Symptom and morbidity patterns in World War II and Vietnam veterans with post-traumatic stress disorder. Compr Psychiatry. 1990;31:1162–1170. doi: 10.1016/0010-440x(90)90020-s. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacol Biochem Behav. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- Edwards G, Chandler J, Hensman C, Peto J. Drinking in a London suburb. II. Correlates of trouble with drinking among men. Q J Stud Alcohol. 1972;6 Suppl.6:94–119. [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21:1285–1293. [PubMed] [Google Scholar]
- Froehlich JC, Li T. Animal models for the study of alcoholism: Utility of selected lines. J Addict Disord. 1991;10:61–71. doi: 10.1300/J069v10n01_05. [DOI] [PubMed] [Google Scholar]
- Graham RM. Selective alpha 1-adrenergic antagonists: therapeutically relevant antihypertensives. Am J Cardiol. 1984;53:16A–20A. doi: 10.1016/0002-9149(84)90829-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CAI, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorphenylpiperazine (mCPP) Psychopharmacology. 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. Am J Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Erickson DJ. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. Am J Psychiatry. 1999;156:723–732. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by α1-adrenoceptors in brain. Naunyn-Schmiedeberg's Arc Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Risch NJ, Weissman MM. Co-morbidity and co-transmission of alcoholism, anxiety and depression. Psychol Med. 1994;24:69–80. doi: 10.1017/s0033291700026842. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stevens D, Fenton B, O'Malley SS, Woods S, Stolar M, Risch N. Comorbidity and familial transmission of alcoholism and anxiety disorders. Psychol Med. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li T. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter KD, Petrie EC, Radant A, Thompson CE, Dobie DJ, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal: 3. Forebrain proopiomelanocortin gene expression and implications for dependence, relapse and deprivatin effect. Alcohol Clin Exp Res. 2002;26:535–546. [PubMed] [Google Scholar]
- Rasmussen DD, Burke B, Crites NJ. Chronic daily ethanol and withdrawal: melatonin treatment reverses persistently increased acoustic startle response during abstinence. Alcohol Clin Exp Res. 2005;29 Suppl:16A. [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during "abstinence". Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology. 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Rojawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: selective blockade by the α1-adrenoceptor antagonist prazosin. Brain Res. 1982;250:31–39. doi: 10.1016/0006-8993(82)90950-7. [DOI] [PubMed] [Google Scholar]
- Shirao I, Tsuda A, Ida Y, Tsujimaru S, Satoh H, Oguchi M, Tanaka M, Inanaga K. Effect of acute ethanol administration on noradrenaline metabolism in brain regions of stressed and nonstressed rats. Pharmacol Biochem Behav. 1988;30:769–773. doi: 10.1016/0091-3057(88)90097-4. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Meredith CW, Gross CA, Raskind MA, Saxon AJ. Pilot trial of prazosin for treatment of alcohol dependence. Alcohol Clin Exp Res. 2007;31 Suppl:60A. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte C, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot study of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00807.x. (in press). [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S. Stress response dampening: effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology. 1998;137:311–320. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Stevens DR, McCarley RW, Greene RW. The mechanism of noradrenergic alpha-1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994;14:6481–6487. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li T, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Beemster P, Schoffelmeer AN. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology. 2003;169:176–185. doi: 10.1007/s00213-003-1509-8. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, McBrided WJ, Gatto GJ, Lumeng L, Li T. Intragastric self-infusion of ethanol by the P and NP (alcohol-preferring and -nonpreferring) lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J. Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]