Abstract
A number of studies have established a role for vascular endothelial growth factor (VEGF) in angiogenesis. Recent reports have shown that VEGF overexpression in the hippocampus improves learning and memory and is associated with enhanced neurogenesis. PTK787/ZK222584 (PTK/ZK) is a reported inhibitor of VEGFR signaling that is currently being tested for its effects on lung and colon cancer. However, the influence of this drug on cognition has not been examined. In the present study, we questioned if post-training administration of PTK/ZK influences hippocampus-dependent memory. When administered to rats immediately following massed training in the Morris water maze, PTK/ZK impaired spatial memory retention tested 48 hours later. This impairment was evidenced by increased latency to the hidden platform and fewer platform crossings. However, this impairment was not associated with a change in neurogenesis during this time frame. PTK/ZK infusion did not reduce VEGFR or AKT phosphorylation, but increased the phosphorylation of ERK. These studies suggest that VEGFR inhibitors such as PTK/ZK may negatively influence cognition.
Keywords: VEGF, hippocampus, long-term memory, water maze, neurogenesis
1. Introduction
VEGF, a hypoxia-inducible protein, has a key role in angiogenesis (Ferrara et al, 2003). VEGF functions by binding to, and thereby activating, two tyrosine kinase receptors: VEGF receptor 1 (VEGFR1, also known as Flt-1), and VEGFR2 (also known as Flk-1 or KDR) (Quinn et al, 1993). Upon ligand binding, VEGF receptors are autophosphorylated making a docking site for intracellular signaling molecules leading to the activation of AKT and ERK (Ferrara et al, 2003). These kinases are thought to mediate many of the growth-related effects of VEGF. As disruption of angiogenesis retards tumor growth, a number of VEGFR inhibitors have been developed for use as anti-cancer agents. Once such compound, PTK787/ZK222584 (1-[4-chloroanilino]-4-[4-pyridylmethyl] phthalazine succinate, PTK/ZK), developed as a joint venture between Novartis Pharma and Schering that is currently being tested for lung and colon cancer (Wood et al, 2000; Drevs et al, 2002), is a selective inhibitor of VEGFR2 tyrosine kinases.
In recent years the function of VEGF and VEGFR has been expanded to include effects on neurons, with both neuroprotective and neurotrophic effects being reported (Schanzer et al, 2004; Storkebaum et al, 2004; McCloskey et al, 2005). For example, enhancing the expression of VEGF increases, while its suppression decreases, neurogenesis in the subgranular zone of the dentate gyrus in the hippocampus. These effects were found to be mediated specifically through the activation of VEGFR2 (Jin et al, 2002; Cao et al, 2004). In addition, enhancing the expression of VEGF in the rodent hippocampus by gene delivery or by chronic antidepressant treatment, not only enhanced neurogenesis, but also resulted in improved behavioral performance (Cao et al, 2004; Warner-Schmidt and Duman, 2007). While these studies demonstrate that chronic manipulations of VEGF signaling can influence neurogenic and behavioral processes, the influence of acute modulation of VEGFR on learning and memory has not been examined.
To examine the consequences of acute manipulation of VEGF receptors in memory formation, we performed post-training, intrahippocampal administration of PTK/ZK. Post-training manipulations of signaling cascades have a number of advantages including eliminating interference with learning or motivational states. We then questioned if the memory impairments seen as a result of transient receptor blockade occur independently, or in conjunction with, influences on neurogenesis. These experiments showed that intrahippocampal administration of PTK/ZK following spatial training impairs long-term memory without having demonstrable effects on neurogenesis.
2. Results
Intrahippocampal infusion of PTK/ZK impairs performance in a long-term spatial memory task
To test the influence of PTK/ZK on spatial memory, animals were trained in the Morris water maze task, then immediately after reaching criteria (3 consecutive trials under 15 sec), bilaterally infused with either of 5 μM PTK/ZK (1 μL/hippocampus, n=10) of an equal volume of vehicle (n=9). Assuming a hippocampal volume of 200 μL, it is estimated that this amount of drug would result in an equilibrium concentration of 25 nM, although the drug concentration is likely to be higher proximal to the infusion site. Forty-eight hours following the completion of training, memory was tested by a probe trial. Figure 1A shows that animals receiving post-training infusions of PTK/ZK had significantly longer latencies to cross the previous location of the hidden platform compare to their vehicle-infused counterparts (Latency: vehicle: 17.73 ± 4.51 sec; PTK/ZK: 40.43 ± 6.86 sec, p<.05). The representative probe trial traces for a vehicle-and PTK-infused animal shown in figure 1B show that the path taken by the PTK/ZK-infused animal is less direct than that taken by the representative vehicle-infused animal. Consistent with this, the PTK/ZK-infused animals had significantly longer distances traveled to the first platform crossing as compared to the vehicle-infused animals (Distance traveled: vehicle: 424.48 ± 90.29 cm; PTK/ZK: 916.54 ± 188.03 cm; p<.05). These difference in probe trial performance were not due to an effect of the drug on swimming speed (Swimming speed: vehicle: 28.42 ± 1.47 cm/sec; PTK/ZK: 27.30 ± 2.70 cm/sec, ns), indicating that intrahippocampal infusion of PTK/ZK did not result in any motivation or motor problems.
Figure 1. Intrahippocampal PTK/ZK administration impairs long-term spatial memory.
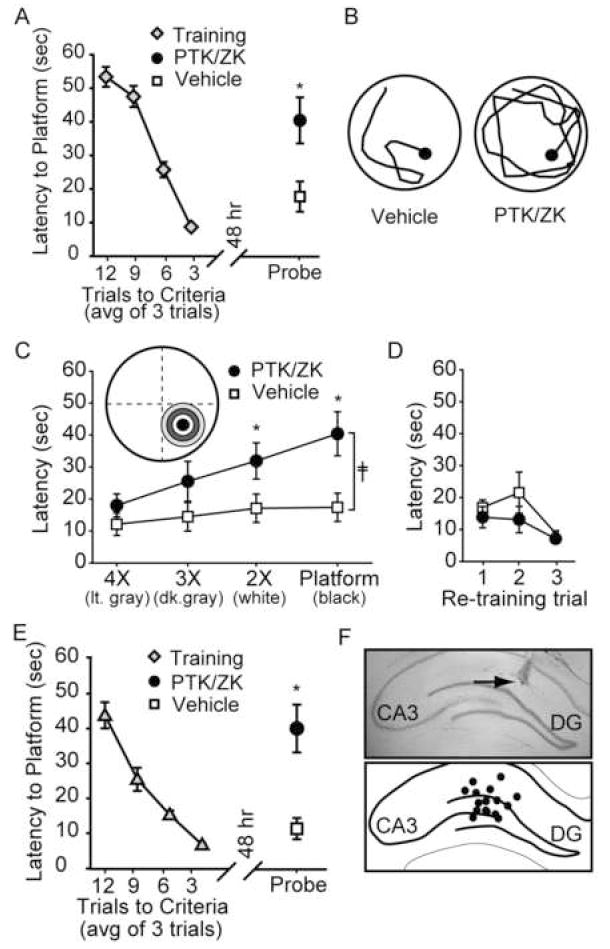
A) Training curve and probe trial latency of vehicle-infused (n=9) and PTK/ZK (5μM)-infused animals (n=11). *, P ≤ 0.05 by Student’s t-test. B) Representative probe trial traces of a vehicle- and PTK/ZK-infused animals during retention testing showing the path taken until the first platform crossing. C) Latencies to enter counter areas of decreasing diameters (4X, 3X, and 2X platform diameter) during the probe trial. ╪, group main effect by two-way ANOVA. *, P≤0.05 by post-hoc analysis. D) Latency to the hidden platform during retraining. E) Training curve and probe trial latency of vehicle-infused (n=9) and PTK/ZK (2.5μM)-infused animals (n=9). *, P ≤ 0.05 by Student’s t-test. F) Representative photomicrograph and schematic representation showing intrahippocampal infusion sites (black dots). Arrow indicates the terminus of the needle tract. DG: dentate gyrus, CA3: Cornu Ammonis field 3. Data are mean ± SEM.
The massed training protocol used in the present study does not give rise to strong preference for the target quadrant. Therefore, to assess localization and search perseverance, the probe trial traces were analyzed by assessing the latency of each animal to enter counter areas (concentric circles of increasing diameters centered on the platform) that are 2X, 3X, and 4X the diameter of the platform. Figure 1C shows that while both the vehicle- and drug-treated animals enter the general location of the platform with similar latencies (light gray, 4X ring), the latencies to enter the rings corresponding to the specific location of the platform (white and black rings) were significantly increased (F(1,3)=14.28, p <0.001 by two-way ANOVA). This suggests that the vehicle treated animals, once in the general location of the platform, proceed to the specific location with minimal delay. In contrast, the PTK/ZK-infused animals enter the general location of the platform, but appear to have an incomplete memory for the exact location of the platform. The number of platform crossings, a measure of search perseverance, was also significantly less in the PTK/ZK-infused animals than in the vehicle-treated rats (Platform crossings: vehicle: 2.00 ± 0.29 crossings; PTK/ZK: 0.90 ± 0.31 crossings, p<.05). VEGFR2 inhibition did not cause complete amnesia, however, as both groups performed equally well when re-exposed to the platform and then given three retraining trials (Figure 1D).
In order to test the dose dependency on the above mentioned memory impairment, a lower concentration of PTK/ZK (2.5μM) was utilized. Figure 1E shows that, similar to the effects seen following 5 μM PTK/ZK infusion, memory was also impaired as a result of 2.5 μM PTK/ZK (n=9) administration. This impairment was indicated by significantly longer latencies to the platform location (Latency: vehicle: 11.28 ± 3.06 sec; PTK/ZK: 40.04 ± 6.86 sec, p<.05), significantly longer distances traveled (Distance traveled: vehicle: 261.35 ± 90.84 cm; PTK/ZK: 846.61 ± 151.10 cm, p<.05), and significantly fewer platform crossings (Platform crossings: vehicle: 2.33 + 0.29 crossings; PTK/ZK: 1.33 ± 0.37 crossings, p<.05) compared to the vehicle-infused controls (n=9). As before, these results were not a result of differences in swimming speed between the groups (Swimming speed: vehicle: 21.59 ± 1.16 cm/sec; PTK/ZK: 23.16 ± 2.68 cm/sec, n.s.). Following behavioral testing, representative animals from each group were killed and brains were stained with cresyl violet (Figure 1F) to determine infusion site accuracy. All animals examined had infusion sites (black circles) that terminated in the dorsal hippocampus (Figure 1F). Only novel infusion sites are represented.
Intrahippocampal administration of PTK/ZK has no effect on neurogenesis
As VEGF has been previously reported to alter hippocampal neurogenesis, and neurogenesis is thought to play a role in hippocampal-dependent memory formation, we next examined if the memory deficits observed following PTK/ZK administration are associated with a decrease in neurogenesis. Groups of animals were injected with BrdU then immediately infused with PTK/ZK (1 μl of 5 μM) into one hippocampus while an equal volume of vehicle was simultaneously infused contralaterally. Neurogenesis was examined in the dorsal hippocampus, using BrdU incorporation and doublecortin immunoreactivity, 48 hours following the infusions. This time point was chosen as it represents the time span from PTK/ZK infusion (immediately following training) to testing (48hr post-training) that was used in our behavioral studies. Figures 2A and 2D show representative images of cells in the dentate gyrus stained using the DNA stain bisbenzimide (Hoechst 33258). The dividing cells in the sub-granular zone were identified using anti-BrdU (red) immunoreactivity (Figure 2B). The BrdU-positive cells can be found imbedded in, and/or immediately adjacent to, the inner (iDG) and outer (oDG) blades of the dentate gyrus. Cell counts revealed that a single intra-hippocampal infusion of PTK/ZK was not sufficient to alter the number of BrdU-positive cells within the granule layer of the dentate gyrus (BrdU+ cells: vehicle: 11.60 ± 1.46 cells/DG/section; PTK/ZK: 11.51 ± 1.43 cells/DG/section, ns) (Figure 2C). In addition to BrdU immunostaining, we examined doublecortin immunoreactivity to assess the influence of PTK/ZK on neurogenesis. Doublecortin is a microtubule-associated protein expressed in immature neurons. Consistent with the results obtained using BrdU, the number of doublecortin-positive cells (representative image shown in Figure 2E) was not altered by PTK/ZK treatment (Doublecortin+ cells: vehicle: 15.71 ± 1.07 cells/mm/section; PTK/ZK: 16.59 ± 1.00 cells/mm/section, ns) (Figure 2F).
Figure 2. Intrahippocampal administration of PTK/ZK has no effect on neurogenesis.
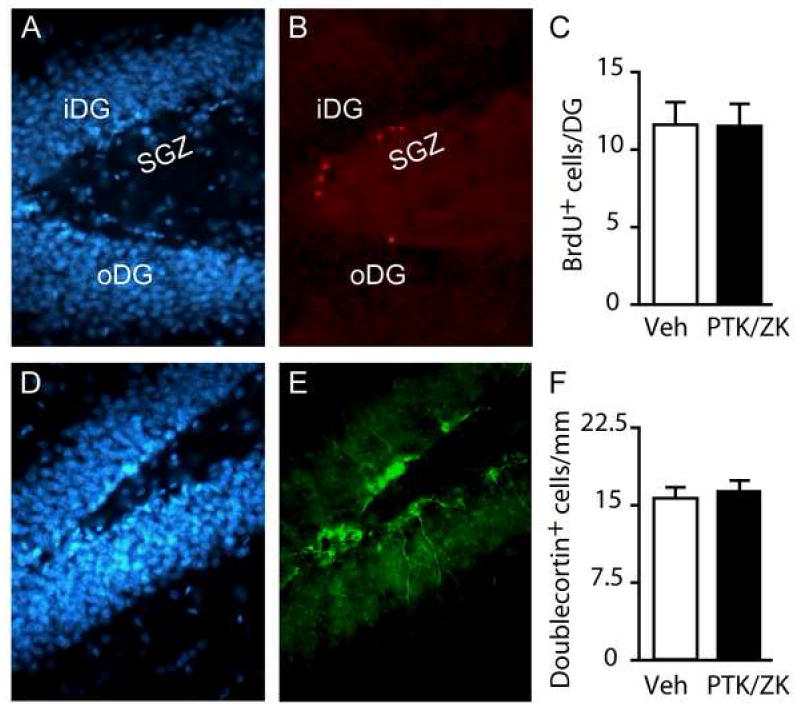
Representative photomicrographs of A) cells in the dentate gyrus stained with bis-benzamide (Hoechst 33258), and B) recently divided cells stained with anti-BrdU. C) Composite image showing that the BrdU-labeled cells (red) are found in close proximity to the bis-benzamide stained granule neurons (blue). D) Summary data showing the average number of BrdU positive cells within the dentate gyrus of vehicle-and PTK/ZK-infused hippocampi. E) Anti-doublecortin stained dorsal hippocampi indicating immature neurons. F) Summary data showing the average number of doublecortin-positive cells/mm from vehicle- and PTK787-infused hippocampi. iDG: inner blade of the dentate gyrus; oDG: outer blade of the dentate gyrus; SGZ: sub-granular zone. Data are presented as mean ± SEM.
Intrahippocampal administration of PTK/ZK increases ERK phosphorylation
Previously, it has been demonstrated that PTK/ZK can block VEGF-induced phosphorylation of VEGFR2 (Wood et al, 2000). In order to examine the effect of PTK/ZK on VEGFR signaling, 1 μl of the drug (stock concentration 5 μM) was injected into one hippocampus while an equal volume of vehicle was simultaneously infused into the contralateral hippocampus of the same animal. The phosphorylation of VEGFR2 (Tyr1054/1059), AKT (ser473), and ERK (Thr202Tyr204) were examined by western blots using phosphorylation-specific antibodies. Results presented in figures 3A and 3B indicate that PTK/ZK had no reproducible influence on VEGFR or AKT phosphorylation. However, the phosphorylation of ERK (both p42 and p44) was found to be significantly enhanced in the PTK/ZK-treated hippocampus (phospho-p42 ERK; vehicle: 100.0 ± 23.2%; PTK/ZK: 224.2 ± 34.6%; p<.05; phospho-p44 ERK; vehicle: 100.0 ± 11.8%; PTK/ZK: 164.8 ± 6.9%; p<.05) (Figure 3C and 3D). There was no detectable change in the total level of ERK after PTK/ZK administration (Figure 3D).
Figure 3. PTK/ZK activates ERK in vivo.
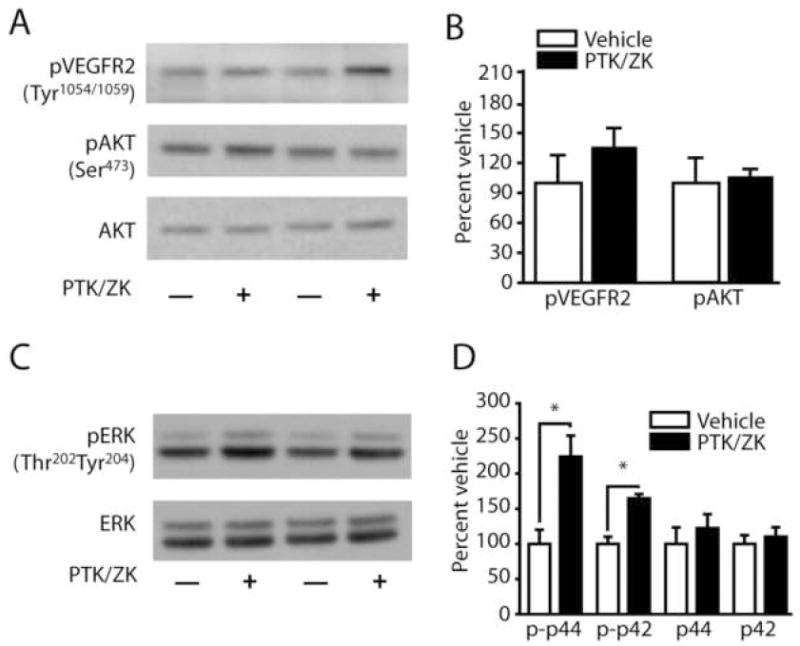
A) Representative western blots showing VEGFR2 (Tyr1054/1059) and AKT (Ser473) phosphorylation following intrahippocampal infusion of 1μl of either 5μM PTK/787 or vehicle. B) Summary results showing that PTK/ZK does not significantly alter VEGF or AKT phosphorylation in vivo. C) Representative western blots showing ERK (Thr202Tyr204) phosphorylation following intrahippocampal infusion of 1μl of either 5μM PTK/787 or vehicle. D) Summary results showing that intrahippocampal infusion of PTK/ZK significantly increases phospho-ERK (both p44 and p42) immunoreactivity compared to the vehicle-infused contralateral hippocampus. No change was observed in total ERK levels. p-p42, phospho-p42 ERK; p-p44, phosphor-p44 ERK; *, P ≤ 0.05 by two-tailed t-test.
3. Discussion
The results presented in this report demonstrate two key findings: 1) PTK/ZK, when infused into the hippocampus immediately after learning, impairs long-term spatial memory, and 2) this impairment was not associated with a reduction in the rate of hippocampal neurogenesis. Taken together, these findings support a role for VEGF-mediated signaling in long-term memory.
One problem associated with the use of pharmacological inhibitors is specificity. PTK/ZK is a selective VEGFR tyrosine kinase inhibitor, having a more potent influence on VEGF receptor-2 (IC50 = 37nM), than on VEGF receptor-1 (IC50 = 77nM) (Wood et al, 2000), that is currently being investigated in clinical trials for its anti-tumorigenic properties. Although selective for VEGFR1 and VEGFR2 receptors, PTK/ZK has been reported to also inhibit the receptor tyrosine kinase activity of PDGF, c-kit, and c-fms, albeit at 10-fold higher IC50 concentrations (IC50=580nM for PDGF; IC50=730nM for c-kit; IC50=1.4mM for c-kit) (Wood et al, 2000; Drevs et al, 2002). While the exact final concentration of PTK/ZK following intrahippocampal infusion is difficult to determine, it is estimated that the final concentrations of inhibitor (1 μl of 5μM or 2.5μM) in the hippocampus (estimated volume of 200 μl) would be 25nM and 12.5nM, respectively. If the high dose of PTK/ZK used in this study (5 μM) diffused to only 10% of the volume of the hippocampus (20 μl), this would result in a final concentration of only 250nM, well below the reported IC50 levels of these other targets. However, the initial concentration immediately adjacent to the infusion site is likely to be higher. A local high concentration of inhibitor may have led to the enhanced ERK phosphorylation we observed, although the mechanism by which this occurred is uncertain.
Our results demonstrate that intrahippocampal infusion of PTK/ZK results in impaired long-term memory. The infusion paradigm used herein, post-training intrahippocampal infusion, allowed us to evaluate the influence of PTK/ZK without interference from altered learning, or complications from state-dependent effects. The impairment in long-term memory was indicated by a difference in three parameters: 1) latency to find the hidden platform, 2) latency to enter the immediate vicinity of the platform, and 3) number of platform crossings. These effects were not due to differences in swimming speed, nor the result of permanent hippocampal dysfunction since both groups performed equally well in a retraining session given after the memory test. Furthermore, the memory impairments observed in the PTK/ZK animals were not due to suppression of ongoing hippocampal neurogenesis. Our evaluation of BrdU-positive (dividing cells) and doublecortin-positive (newly generated neurons) cells within the dentate gyrus found that a single intrahippocampal infusion of PTK/ZK was not sufficient to influence neurogenesis, even though this treatment dramatically impaired spatial memory. A lack of effect from PTK/ZK on basal neurogenesis is consistent with a previous report using SU5416, a potent VEGF signaling inhibitor, in which hippocampal neurogenesis was unaffected in the absence of exogenous VEGF application (Warner-Schmidt and Duman, 2007).
A number of previous studies have established that the activation of ERK cascade is a necessary step in long-term spatial memory (Atkins et al, 1998; Blum et al, 1999; Ou and Gean, 2006). However, as our western blot results using hippocampal extracts indicated that PTK/ZK results in enhanced ERK phosphorylation, it is possible that the impairment we observed in spatial memory may be related to excessive activation of this signaling cascade. Similar memory impairments have been reported for excessive stimulation of the cAMP cascade (which is known to activate ERK), suggesting that memory requires an optimal level of intracellular signaling stimulation (Byers et al, 1981). Furthermore, since VEGFR2 is also expressed on glial and endothelial cells, activation of this receptor on these cells may also influence memory formation by altering neuron-glial and neuron-vascular signaling. Future studies will need to be performed to determine the mechanism(s) and cell types responsible for the effects we observed.
4. Experimental Procedures
Materials
Male Long Evan rats (250-280g) were purchased from Charles River Laboratories (Wilmington,MA). Human-VEGF was purchased from R&D Systems (Minneapolis,MN). The VEGFR2 tyrosine kinase inhibitor, PTK/ZK, was generously provided by Novartis Pharma AG (Basel, Switzerland). The phospho-VEGFR2 (Tyr1054/1059) and BrdU antibody were purchased from Sigma (St. Louis, MO). Phospho-AKT and AKT antibodies were bought from Cell Signaling Technology (Danvers, MA) and the mouse monoclonal BrdU antibody was purchased from Roche (Indianapolis, IN).
Drug Preparation and Intrahippocampal Infusion
All protocols involving the use of animals are in compliance with NIH’s Guide for the Care and Use of Laboratory Animals. Stock solutions of PTK/ZK (50 mM) were prepared by dissolving in DMSO. Prior to infusion, PTK/ZK was diluted in sterile saline to the desired concentration (in 10% DMSO). Previous studies have shown that a 10% DMSO solution does not cause demonstrable effects on learning and memory (Blum et al, 1999). Rats were anesthetized using 4% isoflurane with a 2:1 N2O:O2 mixture and then maintained with a 2% isoflurane/2:1 N2O:O2 mixture via a facemask. Bilateral guide cannulae, aimed at the dorsal hippocampus (AP -3.3 mm, L ± 2.0 mm from bregma and V -2.0 mm from the dura), were implanted. The dorsal hippocampus was targeted for drug infusion because of its well established role in spatial learning and memory (Moser et al, 1995). The rats were then allowed to recover for 10-12 days. After the completion of behavioral training, injection cannulae that extend 1.75 mm beyond the tips of the guides (yielding a total depth of 3.75 mm below the dura) were inserted into the guides for infusion of drugs. All injections (1 μl/hippocampus of either drug or vehicle) were performed in freely moving animals at a rate of 0.25 μl/min using a dual syringe infusion pump (Stoelting).
Sample preparation and Western Blotting
Rats were given targeted infusions (AP -3.3 mm, L ± 2.0 mm from bregma and V -3.75 mm from the dura) of 5μM PTK/ZK into one dorsal hippocampus, while an equal volume of vehicle was simultaneously infused into the contralateral hippocampus of the same animal. Thirty minutes following the infusion, animals were killed and brains were removed and submerged under ice-cold artificial cerebrospinal fluid (10 mM HEPES pH 7.2, 1.3 mM NaH2PO4, 3mM KCl, 124 mM NaCl, 10mM dextrose, 26 mM NaHCO3, and 2mM MgCl2). The hippocampi were quickly removed and snap-frozen on dry ice. The tissue was homogenized in a lysis-buffer containing 10 mM Tris pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.5 μM DTT, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM PMSF, and 0.1 μM okadaic acid.
Protein was quantified using the bicinchoninic acid assay (MicroBCA kit; Pierce, St. Louis, Mo.) and denatured at 70°C for 10 min in 1x NuPage LDS sample buffer (Invitrogen, Carlsbad, Calif.). Equal amounts of protein were loaded, electrophoresed, and transferred using the NOVEX X-Cell II system (Invitrogen) using the buffers and protocol provided by the vendor. Blots were blocked in PBS with 5% nonfat dried milk and probed with primary antibodies to pVEGFR-2 (Tyr1054/1059), phospho-Akt (Ser473), total Akt, phosphor-ERK (Thr202Tyr204) and total ERK (all antibodies at 1μg/mL). Immunoreactivity was detected by species-specific secondary antibodies conjugated to alkaline phosphatase and a chemiluminescence detection system (CDP Star; New England Biolabs). Multiple exposures of each blot were taken to ensure the linearity of the immunoreactive bands. The optical densities of the resultant bands were quantified using Image J (freely available from NIH). The levels of phosphorylation for VEGFR2, AKT and ERK were normalized by reprobing stripped gels with antibodies raised against these proteins independent of their phosphorylation states.
Immunohistochemistry
The influence of PTK/ZK infusions on hippocampal neurogenesis was assessed in a separate group of rats than that used for behavioral testing. Rats were given a single intraperitoneal injection of BrdU (200 mg/kg), then returned to their home cages. Two hours later, animals were anesthetized and infused with 1.0 μl of 5 μM PTK/ZK into one hippocampus, while an equal volume of vehicle was simultaneously infused into the contralateral hippocampus, of the same animal. Forty-eight hours later, rats were anesthetized and perfused with 4% paraformaldehyde. Brains were removed, cryoprotected in 30% sucrose (in PBS) and sectioned into 40 micron thick slices using a cryostat. Free-floating slices were processed for BrdU immunohistochemistry as previously described (Dash et al, 2001). Every twelfth section of the dorsal hippocampus was processed (5 sections/animal). In adjacent sections, doublecortin immunoreactivity was assessed using an antibody from Cell Signaling Technology. A blind counting methodology was employed for determination of BrdU-and doublecortin-positive cell numbers. Positive cells within the granule cell layer of the DG in both the vehicle- and PTK/ZK-infused hippocampi were counted by two independent observers who were blind to the group assignment. Cell counts between the two observers were averaged for each section. The number of cells/DG (for BrdU) and the number of cells/mm (for doublecortin) across sections was averaged for each animal.
Behavioral Training and Testing
All behavioral protocols were performed by an experimenter who was kept blind to the treatment schedule. Cannulated animals were trained in the hidden platform version of the Morris water maze task (Morris et al, 1986) in a single session with an intertrial interval (iti) of 4 min until they could locate the platform three consecutive times under 15 sec (Blum et al, 1999). Animals which failed to reach this criterion by trial 13 were eliminated from the study. Each trial was initiated by placing the animal in one of four randomly chosen locations facing the wall of the tank. Animals were allowed to search for the hidden platform for a period of 60 sec. If an animal failed to find the platform, it was placed there by the experimenter. Animals were allowed to remain on the platform for a period of 30 sec before being returned to a warming cage between trials. Once criterion was reached, animals were bilaterally infused with either drug or vehicle as described above. Following the completion of training, animals were returned to their home cages until retention testing.
Forty-eight hours after training, animals were tested for retention by a probe trial in which the hidden platform was removed from the maze and animals were allowed to search for a period of 60 sec. Movement within the maze was monitored using a video camera linked to tracking software (EthoVision, Noldus). The time to platform was calculated as the latency for each animal to cross the site at which the hidden platform was located during training. In addition, the number of times the animal crossed the previous platform location, and the dwell times and latencies to “counter areas” (concentric circles of increasing diameters centered on the platform) were recorded. Using the tracking software, swimming speed was calculated by dividing the cumulative total distance (cm) traversed in each zone by the cumulative dwell time.
Statistical Analysis
All data was subjected to a Kolmogorov-Smirnov normality and an equal variance test. Statistical significance for behavioral results was determined by either two-tailed Student’s t-test for unpaired variables (latency, platform crossings, etc.) or by two-way analysis of variance (ANOVA), when appropriate. The optical densities of the western blot bands were statistically compared using either a one-way ANOVA or by a two-tailed Student’s t-test. Data were considered significant at P < 0.05. Data are presented as the mean±SEM.
Acknowledgments
The authors would like to thank Melanie Moody for help with the rodent surgery. This work was made possible by grants from NIH.
List of abbreviations
- AP
anterior posterior
- BrdU
bromodeoxyuridine
- DG
dentate gyrus
- DMSO
dimethylsulfoxide
- HUVEC
human umbilical vein endothelial cells
- ITI
intertrial interval
- L
lateral
- MAPK
mitogen activated protein kinase
- PDGF
platelet-derived growth factor
- PI3K
pPhosphoinositide 3-kinase
- PMSF
phenylmethylsulphonyl fluoride
- PTK787/ZK222584
1-[4-chloroanilino]-4-[4-pyridylmethyl] phthalazine succinate
- SGZ
sub-granular zone
- V
vertical
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Drevs J, Muller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, Unger C, Marme D. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci. 2005;25:8889–8897. doi: 10.1523/JNEUROSCI.2577-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Peters KG, de Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]


