Abstract
Background
Changes in the hypothalamic–pituitary–adrenal (HPA) axis, as evidenced by patterns of cortisol secretion, have been of interest in understanding depression and anxiety disorders across the life span. Previous studies of pediatric depression have pointed to the period around sleep onset as a key time point for observing alterations in cortisol secretion associated with affective disorders. Evidence also indicates that pubertal development may influence the expression of HPA dysregulation. We hypothesized that adolescents with depression and youth with anxiety disorders exhibit elevated peri-sleep-onset cortisol.
Methods
Plasma cortisol was sampled every 20 min around sleep onset from children and adolescents with major depressive disorder (n = 116), anxiety disorders (n = 32), or no history of psychiatric disorder (control; n = 76). Sleep onset was determined by polysomnography. Classification of participants as children or adolescents was based on Tanner staging of pubertal maturation.
Results
Children with anxiety disorders had higher peri-sleep-onset cortisol than children with depression or control children. Adolescents with depression had marginally higher peri-sleep-onset cortisol than control adolescents and significantly higher peri-sleep-onset cortisol than children with depression.
Conclusions
Depression and anxiety are associated with altered cortisol secretion around sleep onset, and these changes appear to be influenced by pubertal maturation.
Keywords: Adolescence, anxiety, childhood, cortisol, depression, sleep
A recent wave of studies in developmental and clinical neuroscience has increased interest in understanding changes in the hypothalamic–pituitary–adrenal (HPA) axis in anxiety and affective disorders. The HPA axis is involved in neuroendocrine and behavioral responses to stress in ways that are shaped by developmental influences. Studies of humans, rodents, and nonhuman primates indicate that HPA functioning is influenced by the enrichment or impoverishment of the early social environment (Higley et al 1991; Lupien et al 2000; Meaney 2001). In animal models, HPA reactivity accompanies both the experience of social stress and the display of abnormal behaviors similar to those in human anxiety and depression (Higley et al 1991). In addition to extensive evidence for early developmental influences on these neurobehavioral systems of stress, there is also evidence that adolescence is a time of plasticity in HPA function. For instance, the effects of early maternal influences can be at least partially modified by experiences during the periadolescent period (Francis et al 2002).
There has also been a long history of clinical research focusing on HPA regulation and affective disorders, with decades of studies showing a tendency for elevated cortisol in adults with depression. Findings in adult depression have consistently reported elevated basal cortisol (Halbreich et al 1985; Rubin et al 1987). Findings in pediatric depression, however, have been less consistent. Studies with children and adolescents for the most part have not found a relation between depression and basal cortisol (Birmaher et al 1992, 1996; Dahl et al 1989; Gispen-de Wied et al 2000; Kutcher et al 1991; Puig-Antich et al 1989).
Depression-related differences in cortisol secretion may be more prominent during the evening (Young et al 1994) or during the period surrounding sleep onset (Dahl et al 1991). Young and colleagues have emphasized the role of mineralocorticoid receptor feedback as potentially significant to these findings (Young et al 2003). Our research group has hypothesized that subtle differences in HPA axis regulation in depression may be most evident when the axis is normally physiologically quiescent because of circadian and sleep influences (Dahl et al 1996).
Pubertal development appears to be another critical factor influencing the relation between depression and cortisol. Studies to date suggest that depressed adolescents tend to have elevated cortisol around sleep onset (Dahl et al 1991; Goodyer et al 1991), whereas depressed children do not (Puig-Antich et al 1989). Furthermore, animal studies indicate that HPA axis function is altered by increasing levels of reproductive hormones (Shansky et al 2003). When extended to human development, animal findings suggest that endocrine changes associated with puberty in humans may modify the HPA response to stress. In addition, the developmental changes in sleep that occur during adolescence may also create greater vulnerability in HPA regulation during the night. More specifically, the physiologic drive toward deep, slow-wave sleep, which is strong in childhood, diminishes during adolescence (Carskadon 2002). Because deep sleep creates a high threshold of arousal and response in children (Busby and Pivik 1985), the maturational decreases in deep sleep during adolescence may render adolescents more vulnerable to the effects of stress in the period near sleep onset. This increased vulnerability to stress could increase cortisol levels during this otherwise quiescent period.
In contrast to research on pediatric depression, hypotheses about cortisol hypersecretion during the period around sleep onset have not been examined systematically across the range of childhood and adolescent anxiety disorders. The exception has been research on posttraumatic stress disorder (PTSD), which has revealed that children with PTSD exhibit elevated basal cortisol (Carrion et al 2002) and 24-hour cortisol (De Bellis et al 1999). Aside from PTSD, few studies have addressed anxiety disorders more generally, and others have tended to focus on anxiety symptoms rather than diagnosed disorders. One recent article reported that prepubertal children with anxiety disorders had lower overall cortisol secretion and an unusual pattern of cortisol increase during the night (Feder et al 2004). The cortisol secretion patterns in that study were not aligned by sleep onset, however, because polysomnography was only conducted with a subsample of participants. The study also did not consider the role of pubertal development because its sample was limited to children. It is not clear whether puberty influences sleep-onset cortisol secretion in anxiety disorders. Thus, it is important to examine whether depression and anxiety, in combination with puberty, involve abnormally high sleep-onset cortisol.
This examined plasma cortisol during the 2 hours before and 2 hours after sleep onset in children and adolescents who had major depressive disorder (MDD), children and adolescents who had anxiety disorders, and children and adolescents who had no history of psychiatric disorder. Participants were part of a longitudinal, multiproject study of the neurobehavioral characteristics of childhood depression. Thus, it was possible to compare findings from the current sample with previous findings from other samples in the same study. Notably, in samples related to the same larger study but independent of the current sample, our group has found cortisol hypersecretion around sleep onset in depressed adolescents (Dahl et al 1991) but not depressed children (Puig-Antich et al 1989). A recent reanalysis of data from a sample originally studied by Puig-Antich et al in New York (Birmaher et al 1996; Puig-Antich et al 1989) also found reduced cortisol secretion during sleep in anxious children (Feder et al 2004). To date, we have not examined the influence of pubertal development directly. Furthermore, adolescents’ high cortisol in previous samples was only evident between 8 PM and midnight (Dahl et al 1991). Our main hypothesis was that adolescents with depression but not children with depression would have higher levels of peri-sleep-onset cortisol (i.e., cortisol in the period surrounding sleep onset) than would control participants. We examined whether the same was true for participants with anxiety disorders, but we did not have a specific hypothesis about puberty-related peri-sleep-onset cortisol differences in this group.
Methods and Materials
Participants
Participants were 138 children and 86 adolescents involved in a multiproject study of neurobehavioral characteristics of pediatric affective disorders. Of all participants, 116 had MDD (76 children), 32 had anxiety disorders (18 children), and 76 were healthy (control) participants with no history of psychiatric disorder (44 children). Participants in the depression and anxiety groups were recruited from the Child and Adolescent Depression Program at Western Psychiatric Institute and Clinic in Pittsburgh, Pennsylvania, and from radio and newspaper advertisements. Participants in the control group were recruited from advertisements. The demographic characteristics of the sample are presented in Table 1.
Table 1.
Mean (SD) of Participant Characteristics
| Control |
Depression |
Anxiety |
|||||
|---|---|---|---|---|---|---|---|
| Children | Adolescents | Children | Adolescents | Children | Adolescents | ||
| n | 44 | 32 | 76 | 40 | 18 | 14 | |
| Age (years) | 10.36 (1.43) | 13.44 (1.61) | 10.49 (1.42) | 13.98 (1.36) | 10.49 (1.38) | 13.38 (1.99) | |
| Sex (female), % | 3 | 9 | 4 | 4 | 3 | 2 6 | |
| Race, % | |||||||
| European American | 90.9 | 90.6 | 76.3 | 84.6 | 100 | 58.3 | |
| African American | 4.5 | 3.1 | 22.4 | 10.3 | 0 | 33.3 | |
| Other | 4.5 | 6.3 | 1.3 | 5.1 | 0 | 8.3 | |
| SES | 46.05 (11.22) | 46.87 (11.66) | 38.12 (14.28) | 39.36 (12.14) | 39.97 (10.32) | 42.42 (14.43) | |
| BMI | 17.79 (2.99) | 21.65 (4.49) | 18.87 (3.39) | 22.31 (3.62) | 18.93 (4.42) | 22.09 (4.24) | |
| Medication (n) | 0 | 0 | 6 | 8 | 0 | 2 | |
| Baseline cortisol | 4.18 (2.65) | 5.20 (3.22) | 4.84 (2.77) | 6.06 (3.62) | 4.65 (3.44) | 6.06 (4.00) | |
| Depressive symptoms | 13.69 (4.87) | 15.71 (6.15) | |||||
SES, socioeconomic status. BMI, body mass index. Adolescent, Tanner stage 3 or above. The “Other” race category includes Asian and biracial. SES is reported in units from the Hollingshead index (1975). Depressive symptoms, available for the participants with depression only, were computed as an extracted score of the K-SADS-PL (Kaufman et al 1997) and are equivalent to Hamilton Depression Rating Scale (Hamilton 1960) scores.
Diagnoses were determined through administration of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL; Kaufman et al 1997). Each participant and a parent (or guardian) were interviewed separately by a bachelor’s-level research specialist trained to administer the K-SADS-PL according to diagnostic reliability standards at Western Psychiatric Institute and Clinic. Reliability for depression and anxiety disorders was >90%, and reliability was maintained through monthly diagnostic review meetings. The results of the interview were then presented at a consensus case conference with a child psychiatrist, who reviewed the findings and diagnosis and provided a best-estimate diagnosis. Participants in the depression and anxiety groups were required to be in a current episode based on DSM-III-R criteria (American Psychiatric Association 1987). Participants in the anxiety group received diagnoses of generalized anxiety disorder (n = 23), overanxious disorder (n = 4), panic disorder (n = 2), separation anxiety disorder (n = 1), social phobia (n = 1), and both generalized anxiety disorder and separation anxiety disorder (n = 1).
Participants in the control group were at low familial risk for depression and were free of any lifetime episode of any major psychiatric disorder. Low familial risk was defined as the absence of lifetime affective disorder in all first-degree relatives; the absence of lifetime mania, schizoaffective disorder, or schizophrenia in second-degree relatives; and lifetime MDD in fewer than 20% of second-degree relatives. First- and second-degree relatives were interviewed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E; Orvaschel et al 1982).
Participants were excluded for the following reasons: use of medication with central nervous system or hypothalamic–pituitary effects within the previous 2 weeks; use of fluoxetine; significant medical illness; extreme obesity (weight > 150% of ideal body weight); IQ < 70; eating disorder, developmental disorder, or schizophrenia; phobia of intravenous (IV) needles; and learning disabilities. For participants who had taken medication during the current episode, medication was tapered under the guidance of the participant’s psychiatrist before entry into the study. Use of nicotine, drugs, or alcohol was also an exclusionary criterion.
Pubertal development was determined through physical examination by a physician or nurse practitioner trained in the assessment of pubertal development. Sexual maturity staging criteria and definitions representing the five stages of breast, genital, and pubic hair development were determined by visual inspection according to the criteria described by Marshall and Tanner (1968). Percentage agreement for exact Tanner stage classification in the longitudinal study has been ≥ 90%. To be consistent with previous publications from our research group, participants were categorized as children if they were Tanner stage 1 or 2 on physical exam at the time of the study and as adolescents if they were Tanner stage 3, 4, or 5.
Procedure
The study protocol was approved by the University of Pitts-burgh Institutional Review Board. Upon entry into the study, participants’ parents or guardians were told about the procedures of the study and signed an informed consent form. Participants who were 14–16 years old also provided consent. Participants younger than 14 years old were present when procedures were described to parents, and all provided verbal assent to participate. Participants were admitted to the Child and Adolescent Sleep and Neuroendocrine Laboratory at Western Psychiatric Institute and Clinic for a neurobiological assessment that included three consecutive nights of polysomnography. The first night was considered an adaptation night. Cortisol and sleep-related electroencephalogram (EEG) activity were measured during the second night of the visit.
The IV catheter was inserted in an antecubital vein, which was then kept open by a slow drip of heparinized saline with a mobile system, which allowed a free range of activities. The IV insertion occurred during the afternoon of the first day, under low stress conditions and soon after participants arrived at the lab. During the second night, participants maintained their usual sleep schedule, as determined by the mean bedtime and waking time reported in sleep logs that participants had completed during the previous week. Before bedtime, participants were encouraged to relax and were allowed to read, watch quiet movies, or talk with laboratory staff.
Plasma sampled at 5 PM on the second day as part of a corticotrophin releasing hormone (CRH) challenge was used to establish a baseline level for the current study. Because at least 24 hours had elapsed between IV insertion and CRH challenge, we consider the pre-CRH challenge cortisol measurement an appropriate baseline. Baseline cortisol was sampled at three points: 40 min, 20 min, and immediately before the CRH challenge. During the night, plasma was sampled every 20 min, beginning 2 hr before the participant’s usual bedtime.
Sleep was assessed through standard polysomnographic (PSG) procedures (see Dahl et al 1996). Electrooculogram, EEG, and electromyography data were collected throughout the night to measure eye movements, brain electrical activity, and muscle tone, respectively. A scorer trained in PSG reviewed these data to determine periods of wakefulness, rapid eye movement (REM) sleep, and non-REM sleep (i.e., stages 1–4).
Cortisol Assays
The assay procedure used was the Diagnostic Products (Los Angeles, California) solid phase 125I radioimmunoassay for cortisol. This method is sensitive to 13.79 nmol/L (.5 mg/dL) of cortisol. Cortisol levels were determined from 25-mL samples assayed in duplicate and added with labeled antigen into tubes coated with a highly specific cortisol antibody. The tubes were incubated for 45 min at 37°C, decanted, and washed to decrease nonspecific binding. The remaining percentage of bound antigen was then determined on a gamma spectrometer. Patient duplicates exceeding a 5 % coefficient of variation (CV) were retested. For the larger study, the intraassay CV has ranged from 1.3% to 2.7%, with a mean of 1.9%. The interassay CV ranged from 11.7% at 103.7 nmol/L (3.76 mg/dL) to 7.0% at 839.0 nmol/L (30.4 mg/dL).
Data Reduction
Based on our previous study (Dahl et al 1991), peri-sleep-onset cortisol was defined as the mean of cortisol values during the period ± 2 hours around sleep onset (this included six presleep measurements and six postsleep measurements). Because peri-sleep-onset cortisol values were nonnormally distributed, a natural log transformation was applied to the values before analyses. Sleep onset was computed as the first minute of 10 consecutive minutes of stage 2 sleep. In addition, several sleep variables were computed to assess potential confounds. Total time awake was computed as wakefulness after sleep onset and before waking time. Total slow-wave sleep was computed as time in stage 3 and stage 4 sleep. Total REM sleep was computed as time in REM sleep. Sleep latency was computed as the time difference between bedtime and sleep onset. The EEG data during sleep (i.e., after the peri-sleep-onset period) were missing for 11 participants. Baseline cortisol was computed as the mean of the three pre-CRH values.
Data Analyses
Group differences were tested using an analysis of variance (ANOVA) approach, with diagnostic group (depression, anxiety, or control), development (child or adolescent pubertal status), and the diagnosis × development interaction as between-subjects factors. The primary dependent variable was peri-sleep-onset cortisol. Dependent variables for secondary ANOVAs were baseline cortisol, mean cortisol during sleep, and peak cortisol during sleep. Repeated-measures ANOVAs were then conducted to further examine group differences in cortisol secretion over time as a function of diagnosis and development. Partial eta squared (η2), which reflects the proportion of variance in the dependent variable accounted for by an independent variable, is reported as a measure of effect size for significant effects. Analyses for group differences in mean and peak sleep cortisol secretion included only the depression and control groups because cortisol data for the entire night were available for only a small subset of the anxiety group (n = 6).
Preliminary analyses indicated that age, gender, race, and body mass index did not differ by diagnostic group and were unrelated to peri-sleep-onset cortisol. Additional ANOVAs with per-sleep-onset cortisol indicated no significant interactions of these variables with diagnostic group. Within the depression group, participants who had taken medication during the current episode did not differ in peri-sleep-onset cortisol from those who had not taken medication. Socioeconomic status (SES) differed by diagnosis [F(2,216) = 8.84, p < .001]. Post hoc tests indicated that the control group had higher SES than did the depressed group (p < .001), and SES was therefore included as a covariate in the main analyses. An ANOVA conducted to test group differences in baseline cortisol indicated that, as expected, the diagnostic groups did not differ in baseline cortisol. There was a development main effect for baseline cortisol, with children exhibiting lower levels of cortisol than adolescents [see Table 1; F(1,218) = 6.22, p < .05]. The diagnosis × development interaction for baseline cortisol was nonsignificant.
Results
Peri-Sleep-Onset Cortisol
An ANOVA for peri-sleep-onset cortisol revealed a significant diagnosis × development interaction [F(2,188) = 5.58, p < .005, η2 = .06]. As depicted in Figure 1, follow-up ANOVAs indicated a significant diagnosis effect within the child group [F(2,118) = 8.52, p < .001, η2 = .13]. Children with anxiety had significantly higher peri-sleep-onset cortisol than did either children with depression [F(1,78) = 19.33, p < .001, η2 = .20] or control children [F(1,51) = 6.78, p < .05, η2 = .12]. Although there was no diagnosis effect within the adolescent group, there was a statistical trend for higher peri-sleep-onset cortisol in adolescents with depression than in control adolescents [F(1,59) = 3.20, p = .08, η2 = .05]. Furthermore, there was a development effect within the depression group. Adolescents with depression had significantly higher peri-sleep-onset cortisol than did children with depression [F(1,101) = 13.72, p < .001, η2 = .12]. There were no significant development effects within the anxiety and control groups.
Figure 1.

Cortisol ±2 hours of sleep onset in children and adolescents with depression, anxiety, or no history of psychiatric disorder (control). Group differences are indicated by a dagger (p < .10) or an asterisk (p < .05). Error bars represent one standard error of the mean. Raw data are depicted; natural log-transformed data were included in data analyses.
Repeated-measures ANOVAs were also conducted with time series data from cortisol assessments during the peri-sleep-onset period. Figure 2 depicts mean cortisol levels for diagnostic and developmental groups. There was a diagnosis × development × time interaction [F(2,40) = 6.61, p < .005, η2 = .25]. Follow-up repeated-measures ANOVAs indicated a diagnosis × development interaction effect for cortisol secretion across the 2-hour period before sleep onset [F(2,54) = 3.38, p < .05, η2 = .11] but no difference for cortisol secretion across the 2-hour period after sleep onset. Within the 2-hour period before sleep onset, repeated-measures ANOVAs indicated a diagnosis effect within the child group [F(2,24) = 4.17, p < .05, η2 = .20] but not within the adolescent group (p = .18). Post hoc Tukey tests revealed that children with anxiety had significantly higher cortisol in the 2-hour period preceding sleep onset than did children with depression (ps < .05) and marginally higher cortisol than did control children (p = .08).
Figure 2.
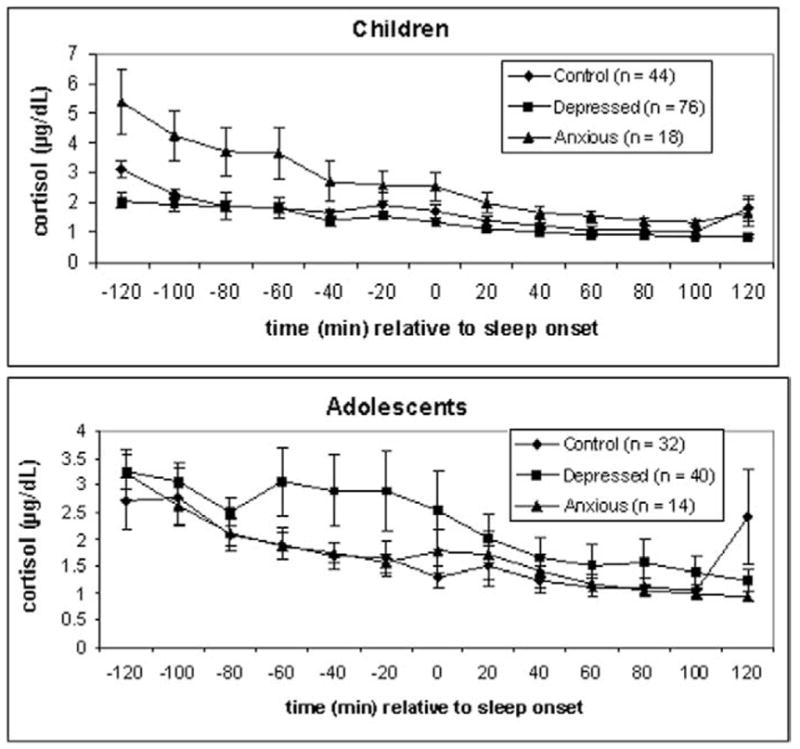
Cortisol at each 20-min sampling interval ±2 hours of sleep onset, by diagnosis. Children and adolescents are depicted separately. Sleep onset = 0. Error bars represent one standard error of the mean.
Peri-Sleep-Onset Cortisol in Depression Subgroups
Because previous studies have reported that peri-sleep-onset cortisol in depressed adolescents varies with the presence of suicidal ideation or the severity of depressive episode (Dahl et al 1991), we tested whether these characteristics were related to mean peri-sleep-onset cortisol. Following a strategy used previously in our group (see Williamson et al 1992), severity was computed using an estimate of the Hamilton Depression Rating Scale (Hamilton 1960) extracted from the K-SADS depression score. The results reported above did not differ when the depression group was divided into suicidal ideation subgroups (present or absent) or severity subgroups (depression score above or below the sample mean). Because high peri-sleep-onset cortisol is associated with recurrent clinical course in early-onset depression (Rao et al 1996), we also examined whether lifetime history of anxiety disorder or duration of current depressive episode was related to peri-sleep-onset cortisol in the depression group. Comorbid lifetime anxiety disorder (present or absent) was not related to differences in cortisol secretion in the depression group. Duration of current episode was correlated with mean peri-sleep-onset cortisol within the depression group (r = .23, p < .05), but including it in models of group differences did not change the original results. Adolescents with depression who had a history of childhood-onset depression did not differ in peri-sleep-onset cortisol from those who did not. Finally, the children and adolescents with depression did not differ in the duration of the current depressive episode or lifetime duration of MDD.
Sleep Cortisol and Sleep EEG
Analyses of variance indicated that the depression and control groups did not differ in mean cortisol during sleep or peak cortisol secreted during sleep (insufficient data for the anxiety group prevented us from including the group in these analyses). In addition, ANOVAs with EEG data indicated that the depression, anxiety, and control groups did not differ in total time awake, total slow-wave sleep, total REM sleep, or sleep latency. Thus, group differences in cortisol secretion were limited to the peri-sleep-onset period and were not confounded by group differences in sleep.
Discussion
Using a large, well-characterized sample of children and adolescents with affective disorders, we found that both diagnosis and development influenced peri-sleep-onset cortisol secretion. Both anxiety and depression were associated with increased peri-sleep-onset cortisol, but the two types of disorders showed different patterns of influence based on development. Children with anxiety disorders had higher cortisol than did children with depression or children with no psychiatric history. When examined in greater detail, this pattern of group differences suggests that cortisol was significantly elevated in children with anxiety during the 2 hours before EEG-determined sleep onset (at a circadian phase when cortisol should be quite low), with levels approaching those of the other two groups of children by sleep onset. Among the adolescents, in contrast, it was the depression group that showed a trend toward elevated peri-sleep-onset cortisol. Elevated cortisol secretion in the depression group appeared to be most prominent in the hour before sleep onset. These findings raise some interesting questions regarding the interaction of pubertal maturation, affective disorders, and cortisol secretion.
These findings contribute to the literature on pediatric affective disorders in several ways. First, the results replicate at a trend level previous depression-related findings by our research group, with a larger and completely independent sample. As indicated in previous findings (Dahl et al 1991; Puig-Antich et al 1989), adolescence appears to be an important period for the emergence of cortisol hypersecretion with depression. Second, the study allowed the direct comparison of cortisol secretion in youth with anxiety, depression, or no history of psychiatric disorder, all studied in the same environment. Third, because the sample included both children and adolescents, the study enabled us to test hypotheses related to development. The statistical trend of elevated cortisol in adolescents with depression represents a partial replication of previous findings in adolescents with depression and supports the hypothesis that changes in HPA regulation associated with depression emerge during adolescence, particularly in the usually quiescent period near sleep onset. Two methodologic strengths are also worth noting. Measuring plasma cortisol allowed us to test hypotheses about sleep-related cortisol, and using PSG rather than clock time allowed us to align cortisol by each participant’s actual sleep onset.
An important question that these findings raise is why childhood but not adolescent anxiety disorders were associated with elevated cortisol in the peri-sleep-onset period. One possibility is that children with anxiety had more difficulty settling in the evening as they prepared for bed or were experiencing greater social stress and anxiety at this time. Therefore, we hypothesize that cortisol levels are correlated with symptoms, arousal, and stress at the time that cortisol is measured. Future studies could address the possibility that psychologic factors influence peri-sleep-onset cortisol by measuring worry, arousal, and other anxiety symptoms at bedtime. It is important to note that our findings suggest some specificity for elevated cortisol during the sleep-onset period. Children with anxiety did not exhibit elevated cortisol levels at baseline, and they did not show disrupted sleep or difficulties falling asleep.
Another consideration is the health consequences of persistently elevated cortisol. That is, adaptation to stress and anxiety could entail a gradual lowering of serum cortisol, so that children with anxiety disorders no longer have elevated peri-sleep-onset cortisol once they reach adolescence. This possible reduction in cortisol may occur because anxiety disorders, which have high continuity (Pine 1999), directly serve as a chronic stressor or are accompanied by chronic stressors. Physiologic adaptation to anxiety disorders may therefore involve increased allostatic load, as occurs with affective disorders generally (McEwen 2003). This view is consistent with the claim that hypocortisolism occurs with chronic stress (Heim et al 2000). The physiologic costs of long-term cortisol hypersecretion are high (Charney 2004), and as a result, adjustments may occur at other points in the HPA axis to regulate cortisol levels. The claim that hypocortisolism results from chronic stress is controversial (see Rasmusson et al 2003), however, and prospective studies of pediatric anxiety disorders are needed to examine long-term changes in cortisol secretion in greater detail.
The difference in cortisol secretion between adolescents with depression and children with depression has implications regarding neurobehavioral changes during puberty that influence or interact with HPA changes that are associated with depression. Models of canalization of development emphasize that vulnerability to abnormal development varies, with protection from abnormality more likely at certain points in development than at others (Grossman et al 2003; Waddington 1940, 1957). It may be that physiologic processes such as sleep and HPA function are protected through childhood but become more vulnerable to disruptions during adolescence. Consequently, childhood depression may not create measurable disruptions in HPA function and sleep (Dahl et al 1996). With pubertal development, however, these systems may become vulnerable to dysregulation, so that adolescent depression exerts an influence on peri-sleep-onset cortisol. Furthermore, the period around sleep onset may therefore be an important period for detecting disruptions to HPA function that become evident during adolescence.
The variability of peri-sleep-onset cortisol within the depression group at the time points before sleep onset was notable (see Figure 2). This variability was not explained by factors such as duration or severity of the current episode, presence of suicidality, or history of anxiety disorder. Thus, the mechanisms of increased cortisol in adolescent depression—and the differences between adolescents who do or do not exhibit increased peri-sleep-onset cortisol—remain to be elucidated. To shed light on these mechanisms, it will be valuable to assess reproductive hormone levels, psychosocial stressors, and affective symptoms in future studies of HPA function in this population.
The size of the anxiety disorders group created limitations in our statistical power to examine differences among various anxiety disorders. Given that the anxiety disorders vary in the extent to which they predict future depression and involve physiologic factors such as respiratory abnormalities (Pine 1999; Sinha et al 1999), this is an issue worth considering. In addition, it is possible that the adolescents with anxiety disorders may have differed from the other groups in peri-sleep-onset cortisol. Examination of Figure 2 indicates that this is not likely, however. Another issue worthy of investigation is intraindividual change in HPA axis function with pubertal development, an issue we could not examine because of our study’s cross-sectional design. We were not able to test, for instance, whether individuals whose onset of depression was during childhood do not exhibit cortisol hypersecretion until adolescence. Because adolescence is an important period for the emergence of depressive disorders generally and of gender differences in the prevalence of depression (Angold et al 1998; Cohen et al 1993; Kessler and Walters 1998; McGee et al 1992), it will be important to examine whether childhood-onset and adolescent-onset depression differ in HPA function during adolescence.
Because early-onset depression is often preceded by anxiety disorders (Giaconia et al 1994; Kovacs et al 1989; Rohde et al 1991), our findings suggest that there may be continuity in the dysregulation of the HPA axis for individuals who experience childhood anxiety and then adolescent depression. Behavior genetics studies indicate that anxiety and depression may represent different phases of a single disorder (Williamson et al, in press). Accordingly, the adolescents with depression in our sample may be the mid–late pubertal counterparts of the anxious children. Children with anxiety who exhibit hypersecretion of cortisol around sleep onset may develop into adolescents with depression who also exhibit hypersecretion of cortisol during this period. When we examined subgroups of the depressed group, we did not find that history of anxiety disorder was associated with peri-sleep-onset cortisol. This could indicate that many developmental pathways to adolescent depression, not simply prior anxiety, are associated with high cortisol. Indeed, our own study will allow the eventual examination of physiologic characteristics of children who experience anxiety disorders and then develop depressive disorders.
In summary, this study has meaningful implications for the study of pediatric affective disorders. One implication is that the same physiologic pattern may not pertain equally to children with depression and to adolescents with depression or anxiety. Despite the widely replicated finding that childhood depression has high continuity (Costello et al 2002), it is also true that there are many possible trajectories for children with depression. Some of these trajectories, in combination with pubertal development, may lead to adolescent depression and elevated cortisol levels. Early HPA function plays an important role in brain development, social affiliation, and behavioral functioning, and with a longitudinal strategy to elucidate the course of childhood affective disorders, it will be possible to examine the ways that alterations to HPA function occur.
Acknowledgments
We thank Laura Trubnick, Michele Bertocci, and the staff of the Child and Adolescent Sleep and Neurobehavioral Laboratory at the University of Pittsburgh School of Medicine for their invaluable contributions to assessing the participants in this study. We also thank the participants and their families. This research was supported by National Institute of Mental Health (NIMH) Training Grant No. T32 MH018269 (Paul A. Pilkonis and Marsha D. Marcus, principal investigators [PIs]), a Klingenstein Third Generation Foundation Postdoctoral Fellowship (Erika E. Forbes, PI), NIMH Program Project No. P01 MH41712 (Neal D. Ryan, PI, Ronald E. Dahl, Co-PI), and NIMH Research Network Grant No. R24 MH67346 (Ronald E. Dahl, PI).
References
- American Psychiatric Association. 3. Washington, DC: American Psychiatric Association; 1987. Diagnostic and Statistical Manual for Mental Disorders (DSM-III-R) [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychol Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Perel J, Williamson DE, Nelson B, Stull S, et al. Corticotropin-releasing hormone challenge in prepubertal major depression. Biol Psychiatry. 1996;39:267–277. doi: 10.1016/0006-3223(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Dahl R, Rabinovich H, Ambrosini P, Williamson DE, et al. Dexamethasone suppression test in children with major depressive disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:291–297. doi: 10.1097/00004583-199203000-00017. [DOI] [PubMed] [Google Scholar]
- Busby K, Pivik RT. Auditory arousal thresholds during sleep in hyperkinetic children. Sleep. 1985;8:332–341. doi: 10.1093/sleep/8.4.332. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Soc Biol Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Adolescent sleep patterns. New York: Cambridge University Press; 2002. [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, et al. An epidemiological study of disorders in late childhood and adolescence—I. Age- and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34:851– 867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529 –542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, et al. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatr Scand. 1989;80:18 –26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Matty MK, Birmaher B, al-Shabbout M, Williamson DE, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry. 1996;39:400 – 410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, et al. 24-hour cortisol measures in adolescents with major depression: A controlled study. Biol Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45:1259 –1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Feder A, Coplan JD, Goetz RR, Mathew SJ, Pine DS, Dahl RE, et al. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorders. Biol Psychiatry. 2004;56:198 –204. doi: 10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840 –7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaconia RM, Reinherz HZ, Silverman AB, Pakiz B, Frost AK, Cohen E. Ages of onset of psychiatric disorders in a community population of older adolescents. J Am Acad Child Adolesc Psychiatry. 1994;33:706–717. doi: 10.1097/00004583-199406000-00012. [DOI] [PubMed] [Google Scholar]
- Gispen-de Wied CC, Jansen LM, Duyz JH, Thijssen JH, van Engeland H. Pituitary-adrenal function in adolescent psychiatric patients: Impact of depressive symptoms. J Affect Disord. 2000;59:71–76. doi: 10.1016/s0165-0327(99)00116-0. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8–16 year olds. Soc Biol Psychiatry. 2001;50:351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: Possible contributions to psychopathology. J Child Psychol Psychiatry. 2003;44:33– 63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in exogenous depression. I. Basal plasma levels. Arch Gen Psychiatry. 1985;42:904 –908. doi: 10.1001/archpsyc.1985.01790320076010. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56 – 62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Sociology Department; 1975. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980 –988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Walters EE. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the national comorbidity survey. Depress Anxiety. 1998;7:3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Gatsonis C, Paulauskas SL, Richards C. Depressive disorders in childhood. IV. A longitudinal study of comorbidity with and risk for anxiety disorders. Arch Gen Psychiatry. 1989;46:776 –782. doi: 10.1001/archpsyc.1989.01810090018003. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Malkin D, Silverberg J, Marton P, Williamson P, Malkin A, et al. Nocturnal cortisol, thyroid stimulating hormone, and growth hormone secretory profiles in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1991;30:407– 414. doi: 10.1097/00004583-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976 –980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200 –207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McGee R, Feehan M, Williams S, Anderson J. DSM-III disorders from age 11 to age 15 years. J Am Acad Child Adolesc Psychiatry. 1992;31:50 –59. doi: 10.1097/00004583-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. The development of individual differences in behavioral and endocrine responses to stress. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J, Chambers W, Tabrizi MA, Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-e. J Am Acad Child Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Pine DS. Pathophysiology of childhood anxiety disorders. Biol Psychiatry. 1999;46:1555–1566. doi: 10.1016/s0006-3223(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Dahl R, Ryan N, Novacenko H, Goetz D, Goetz R, et al. Cortisol secretion in prepubertal children with major depressive disorder. Episode and recovery. Arch Gen Psychiatry. 1989;46:801– 809. doi: 10.1001/archpsyc.1989.01810090043008. [DOI] [PubMed] [Google Scholar]
- Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, et al. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry. 1996;40:474 – 484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vythilingam M, Morgan CA., 3rd The neuroendocrinology of posttraumatic stress disorder: New directions. CNS Spectr. 2003;8:651–656. 665–667. doi: 10.1017/s1092852900008841. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comorbidity of unipolar depression: II. Comorbidity with other mental disorders in adolescents and adults. J Abnorm Psychol. 1991;100:214 –222. [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett AL. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry. 1987;44:328–336. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2003;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Sinha SS, Coplan JD, Pine DS, Martinez JA, Klein DF, Gorman JM. Panic induced by carbon dioxide inhalation and lack of hypothalamic-pituitary-adrenal axis activation. Psychiatry Res. 1999;86:93–98. doi: 10.1016/s0165-1781(99)00029-3. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Organizers and genes. Cambridge: Cambridge University Press; 1940 . [Google Scholar]
- Waddington CH. The strategy of the genes. London: Allen and Unwin; 1957 . [Google Scholar]
- Williamson DE, Forbes EE, Dahl RE, Ryan ND. Examining the relation between depression and anxiety: evidence from a family-genetic study. Child Adolesc Psychiatr Clin N Am. doi: 10.1016/j.chc.2005.05.007. (in press) [DOI] [PubMed] [Google Scholar]
- Williamson DE, Ryan ND, Dahl RE, Jeanette L. Hamilton depression scores can be extracted from the K-SADS-P in adolescents. J Child Adolesc Psychopharmacol. 1992;2:175–181. doi: 10.1089/cap.1992.2.175. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Grunhaus L, Pande A, Weinberg VM, Watson SJ, et al. Increased evening activation of the hypothalamic–pituitary–adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch Gen Psychiatry. 2003;60:24 –28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]


