Abstract
Objective
About a quarter of peripheral vein grafts fail due in part to intimal hyperplasia. The proliferative capacity and response to growth inhibitors of medial smooth muscle cells and adventitial fibroblasts in vitro were studied to test the hypothesis that intrinsic differences in cells of vein grafts are associated with graft failure.
Methods
Cells were grown from explants of the medial and adventitial layers of samples of vein grafts obtained at the time of implantation. Vein graft patency and function were monitored over the first 12 months using ankle pressures and Duplex ultrasound to determine vein graft status. Cells were obtained from veins from 11 patients whose grafts remained patent (non-stenotic) and from 7 patients whose grafts developed stenosis. Smooth muscle cells (SMCs) derived from media and fibroblasts derived from adventitia were growth arrested in serum-free medium and then stimulated with 1 μM sphingosine-1-phosphate (S1P), 10 nM thrombin, 10 ng/ml epidermal growth factor (EGF), 10 ng/ml platelet-derived growth factor-BB (PDGF-BB), PDGF-BB plus S1P, or PDGF-BB plus thrombin for determination of incorporation of [3H]-thymidine into DNA. Cells receiving PDGF-BB or thrombin were also treated with or without 100 μg/ml heparin, which is a growth inhibitor. Cells receiving thrombin were also treated with or without 100 nM AG1478, an EGF receptor kinase inhibitor.
Results
SMCs and fibroblasts from veins of patients that developed stenosis responded more to the growth factors, such as PDGF-BB alone or in combination with thrombin or S1P, than cells from veins of patients that remained patent (P=.012). In addition, while PDGF-BB-mediated proliferation of fibroblasts from grafts that remained patent was inhibited by heparin (P<.03), PDGF-BB-mediated proliferation of fibroblasts from veins that developed stenosis was not (P>.5).
Conclusions
Inherent differences in the proliferative response of vein graft cells to PDGF-BB and heparin may explain, in part, the variability among patients regarding long term patency of vein grafts.
Autogenous saphenous vein remains the preferred conduit for lower extremity arterial bypass. Despite improved surgical techniques and careful postoperative surveillance, hemodynamically significant stenosis continues to affect about one-quarter of all peripheral vein grafts during the first year after implantation1. While a large literature exists on the response of arteries to injury, relatively little is known about the response of veins to implantation in the arterial circulation. Animal models show a rapid loss of cells in the media due to death2 followed by thickening of the intimal and medial layers of the vein as a consequence of cell migration from medial and adventitial layers and the invasion of progenitor cells from the blood3,4 coupled with cell proliferation and deposition of extracellular matrix (review-5). However, these vein grafts in animals do not develop stenosis. In humans, smooth muscle cell (SMC) proliferation6 and deposition of extracellular matrix7 contribute to intimal thickening and luminal stenosis. The advanced vein graft lesions, like the injury-induced lesions in animal arteries, are composed primarily of matrix7,8. Recent observations indicate that both intimal hyperplasia and vessel remodeling play a role in the loss of luminal area9,10, similar to the response of diseased arteries to angioplasty11.
Little is known about why most grafts preserve their lumen while one quarter develop focal or diffuse stenosis. The usual cardiovascular risk factors fail to predict peripheral vein graft failure, although smoking has sometimes been reported to be a risk factor for peripheral vein graft stenosis12–14. Vein morphology preimplantation does not predict outcome, although early flow disturbances detected by duplex scanning may identify sites of predilection12. It is possible that there are either systemic (e.g. blood borne) or local factors that control the vein SMC response to injury15. In addition, there is some evidence that certain patients are predisposed to vein graft stenosis. For example, stenosis in a graft in one leg predicts stenosis in a graft placed in the opposite leg16. Differences among mouse strains in the response to wire injury and vein grafting also support the possibility of genetic differences in susceptibility to vein graft hyperplasia17. Of particular interest are the studies of Chan and colleagues on human vein SMCs that provide an intriguing link between heparin responsiveness and vein graft patency 18,19. Vein graft stenosis is correlated with a loss of heparin sensitivity. Aortic SMCs, and possibly vein graft cells, utilize the heparin binding growth factors, fibroblast growth factor2 (FGF2) and heparin-binding EGF-like growth factor (HB-EGF), in an autocrine/paracrine manner for platelet-derived growth factor (PDGF)-BB and thrombin growth stimulation20,21. Together these data suggest a possible difference in the utilization of heparin-binding growth factors by SMCs from stenotic patients. It is clear that endogenous heparan sulfate proteoglycans can retard as well as promote SMC growth in vivo 22–25. Therefore, we think that variability in heparin responsiveness might be accounted for by variable use of the heparin-sensitive co-stimulatory pathways.
In this study we investigated the hypothesis that luminal narrowing in vein grafts may be explained by the vein graft cells themselves, which may be hyper responsive to growth stimulants or may lack pathways designed to shut off growth. In particular, we have studied the response to the growth factors PDGF, thrombin and sphingosine-1-phosphate (S1P), which are known to play critical roles in the response to vascular injury and atherosclerosis26–28, and to inhibitors of the heparin-binding growth factors, FGF2 and HB-EGF.
Materials and Methods
Materials
Platelet-derived growth factor-BB (PDGF-BB) was purchased from R&D Systems. Thrombin was from American Diagnostica and sphingosine-1-phosphate (S1P) was from Cayman Chemical. AG 1478 was obtained from Calbiochem and 3H-thymidine was obtained from Perkin-Elmer Life Sciences. Epidermal growth factor (EGF) and heparin (porcine intestinal) were from Sigma-Aldrich. All cell culture solutions were purchased from Invitrogen Corporation.
Cell culture and Proliferation
Segments of human saphenous vein were harvested at the time of bypass under a protocol approved by the University of Washington and Puget Sound Veterans Administration Human Subjects review board. The endothelium was wiped off and the media and adventitia were separated. Explants of media and adventitia were cultured in Dulbecco’s minimal essential media supplemented with 20% fetal bovine serum (DMEM 20%). Cells began migrating out of explants after 2–4 days and were first passaged between 2 and 4 weeks. After the first passage medial cells were maintained in 20% serum, while adventitial cells were maintained in 15% serum to maintain approximately equal doubling times. Cells between passages 5 and 9 were used for experiments. Cells were seeded at 20,000 cm2 overnight in DMEM with 20% serum (medial cells) or DMEM with 15% serum (adventitial cells) and then switched to serum-free DMEM for 24 hours. Medium was then changed again with serum-free medium and after another 48 hours agents were added directly as follows: PDGF-BB 10 ng/ml ± 100 μg/ml heparin; 10 nM Thrombin± 100 μg/ml heparin; 10 nM Thrombin + 0.1 μM AG1478; 1 μM S1P; PDGF-BB 10 ng/ml + 10 nM Thrombin ; PDGF-BB 10 ng/ml + 1 μM S1P; and 10 ng EGF ± 0.1 μM AG1478. DMSO was used as a control for AG1478. After 20 hours, 0.5 μCi/ml 3H-thymidine was added and incubated for another 8 hours at which time cells were harvested and incorporation of 3H-thymidine into DNA was determined as previously described29.
Patient Population and Postoperative Surveillance
Male and female patients scheduled for lower extremity bypass using vein grafts were eligible for this study. Patients were excluded if they were unable to return for follow-up examinations, unable to give informed consent, or if they developed acute vein graft failure (less than one month). All patients received saphenous vein grafts except for one patient each in the stenotic and non-stenotic groups that received arm vein-saphenous vein composite grafts (the stenosis occurred in the saphenous vein portion of the composite graft). Other patient demographics for the 11 non-stenotic and 7 stenotic patients are listed in table 1. Not all patients’cells were used in all experiments.
Table 1.
Patient demographics
| Non Stenotic (11) | Stenotic (7) | |
|---|---|---|
| Male/Female | 11/0 | 6/1 |
| Age | 65.0 ± 2.6 | 71.7 ± 3.2 |
| Race | ||
| White | 10 | 7 |
| Afro-American | 1 | 0 |
| Comorbidities | ||
| Hypertension | 10 | 6 |
| Diabetes | 4 | 4 |
| Renal Insufficiency | 3 | 0 |
| Tobacco use | ||
| current | 8 | 1 |
| ex | 2 | 5 |
| never | 1 | 1 |
| Dyslipidemia | 6 | 5 |
| CAD | 6 | 3 |
| CVD | 0 | 1 |
| Clinical Indication | ||
| gangrene | 3 | 1 |
| ulcer | 3 | 2 |
| Resting pain | 2 | 1 |
| claudication | 3 | 3 |
| Anatomic configuration | ||
| Femoro-popliteal | 5 | 5 |
| Femoro-tibial | 6 | 2 |
| Prior vein graft stenosis | 1 | 2 |
CAD: coronary artery disease
CVD: cerebrovascular disease
All patients were followed for 12 months with ankle pressures and Duplex ultrasound. Examinations were carried out during surgery, at 6 weeks and at 3, 6, 9, and 12 months after surgery. Vein graft stenosis was defined as significant if the peak systolic velocity was greater than 300 cm/s and the ratio of the peak systolic velocities in adjacent segments exceeded 3.0. Additional duplex examinations were performed if a drop in the ankle-arm index of at least 0.15 was noted or symptoms returned. At 12 months after the surgery, the graft status of each patient was disclosed to the investigators performing the in vitro experiments.
Statistical Analysis
Experiments for each cell line were performed in triplicate and were repeated 2–6 times. All combined data were analyzed by analysis of variance using the SPSS 8.0 GLM General Factorial method with growth factor treatment, cell type, and vein graft status as factors; age of the patient as a covariate; and the experiment number as a weighted least squares variable. Comparisons of two groups were performed using the Mann-Whitney or Wilcoxon’s signed rank tests or the paired t-test as appropriate.
Results
By multivariate analysis DNA synthesis in response to growth factors was significantly increased in cells from patients that developed vein graft stenosis compared to those that did not develop stenosis (P≤.0005). In addition, fibroblasts were more responsive than SMCs (P≤.0005). Neither age (P=.128) nor sex (P=.252) significantly affected DNA synthesis. The proliferative response of cultured medial and adventitial cells to specific growth factors is presented in figure 1 in which proliferation is expressed as fold of serum-starved control for cells from veins that go on to stenosis compared to those that do not. Basal DNA synthesis of serum-starved cells was not significantly different among the groups (1048 ± 349, 787 ± 183, 850 ± 172, and 667 ± 128 cpm for non-stenotic SMCs, stenotic SMCs, non-stenotic fibroblasts, and stenotic fibroblasts, respectively; mean ± SEM; N=10, 6, 9, and 6, respectively). Both SMCs (figure 1A) and fibroblasts (figure 1B) responded more to PDGF-BB than to EGF, S-1-P, or thrombin, but the combination of PDGF-BB with either thrombin or S-1-P gave an additive effect. Comparing SMCs and fibroblasts from the same patient, PDGF-BB increased fibroblast and SMC proliferation by 21.4 ± 3.7 and 12.5 ± 1.8 fold of control, respectively (mean ± SEM; 12 paired cell lines; P=.0148). There was no significant correlation between DNA synthesis and cell passage number (data not shown). Finally, we did not find any difference between first passage times of cells after explanting tissue from veins of patients with patent grafts compared to those with stenotic grafts (19.9 ± 1.2 vs. 21.2 ± 2.4 days, respectively; mean ± SEM; P=.639) nor did we find any significant correlation between DNA synthesis and time to first passage (data not shown).
Figure 1.

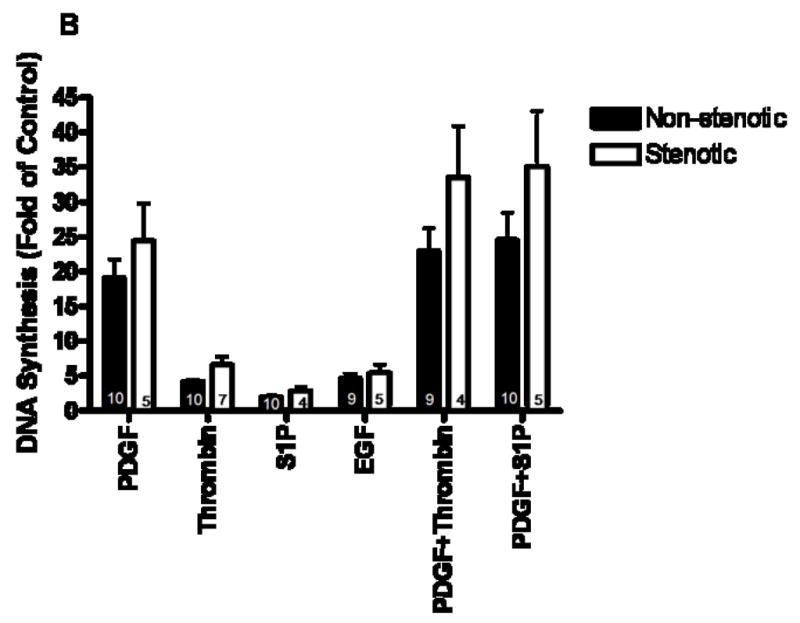
DNA synthesis in reponse to growth factors by saphenous vein smooth muscle cells (A) and fibroblasts (B) from veins that developed stenosis (open bars) compared to. veins that remained patent (closed bars). Values are the mean ± SEM of fold of control values of 3H-thymidine incorporation after treatment with the indicated growth factors. Values for N are indicated within the bars.
Heparin significantly inhibited PDGF-BB-mediated proliferation of venous SMCs and fibroblasts from veins of non-stenotic patients and of SMCs from veins that became stenotic by 21, 10, and 17%, respectively (P=.036, .036, and .011, respectively; figure 2A). In contrast, fibroblasts from veins that became stenotic were not significantly inhibited by heparin when stimulated by PDGF-BB (P=.238; figure 2A). There was no correlation between heparin sensitivity and age of cell donor (P=.974). Thrombin-induced proliferation of all types of cells was inhibited by more than 40% by heparin (P<.05; figure 2B). SMCs tended to have greater sensitivity to heparin than fibroblasts (P=.027 for PDGF and P=.0785 for thrombin with 14 paired SMCs and fibroblasts). Finally, regarding the inhibitory effect of the EGF receptor kinase inhibitor AG1478 on thrombin-mediated proliferation, there were no significant differences between SMCs from veins that became stenotic compared to those from veins that remained patent (56.4 ± 11.5% and 58.1 ± 9.5% inhibition, respectively; mean ± SEM, N=8–10; P>.1) or fibroblasts from non-stenotic and stenotic grafts (44.0 ± 5.9% and 53.5 ± 5.9% inhibition, respectively; N=5–10; P>.1).
Figure 2.
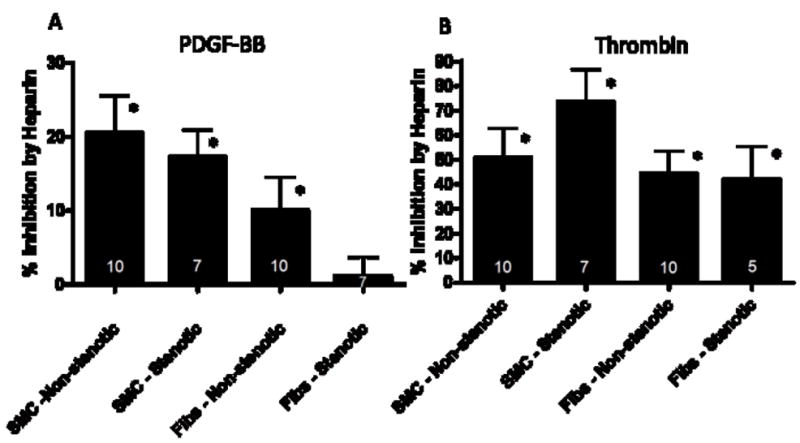
Effect of heparin (100 μg/ml) on PDGF-BB- mediated 3H-thymidine incorporation (A; *-P<.05 comparing PDGF-BB plus heparin vs. PDGF-BB alone) and on thrombin-mediated 3H-thymidine incorporation (B; *- P<.05 comparing thrombin plus heparin vs. thrombin alone). SMC: smooth muscle cells; Fibs: fibroblasts; values for N are indicated in the bars.
Discussion
We have observed that cells obtained from the adventitia and media of saphenous veins from patients whose femoro-popliteal or femoro-tibial grafts go on to stenose proliferate faster than those obtained from the veins of patients with non-stenotic grafts. This property of the cells may partially explain stenosis of the vein grafts of these patients. Because SMCs from restenotic arterial lesions have been shown to proliferate faster than those from primary arterial lesions30, it should be emphasized that the cells used in our experiments were obtained from normal venous tissue obtained at the time of grafting, not from stenotic or non-stenotic arterialized vein grafts. Aortocoronary grafts fashioned from internal mammary artery or radial artery are much more durable than saphenous vein grafts31, and it is of note that SMCs derived from internal mammary artery proliferate less than SMCs from saphenous vein32. Thus, the proliferative capacity of SMCs in culture may be predictive of graft stenosis. Although there are a number of possible explanations, differences in SMC function and gene expression might account for the differences in graft performance. For example, it is known that the internal mammary artery is more resistant to atherosclerosis than the coronary artery, and this difference is reflected in the molecular phenotype of the cultured SMCs 33. In organ culture, veins exhibit increased synthesis of extracellular matrix proteins, such as versican, compared to arteries34. SMCs from aorta express the protein, regulator of G protein signaling 5 (RGS5), while SMCs from vena cava, coronary artery, and intimal hyperplastic lesions in cynomolgous monkey grafts do not 35–37. RGS5 is an inhibitor of Gαi and Gαq activation and therefore may play a role in modulating the extent of SMC activation. Finally, SMCs obtained from saphenous vein have higher levels of PDGF receptor β and proliferate about two fold faster in response to PDGF than do those from internal mammary artery38. These differences are comparable to the differences we have observed between strains of cells from vein grafts that remain patent or eventually develop stenosis. While these may appear to be modest differences in growth rate, they may be sufficient given that lesion growth in vivo in vein grafts appears to take months39,40. Whether differences in PDGF receptor levels exist between SMCs of veins from patients that develop graft stenosis and SMCs from veins of patients with patent grafts is not known. In this regard, the observations of Conte and colleagues that C-reactive protein increases PDGF receptor β levels in cultured saphenous vein SMCs and that C-reactive protein levels may be correlated with graft outcome in vivo are intriguing15,41.
We have also demonstrated that PDGF-mediated proliferation of fibroblasts from vein grafts that do not develop stenosis are sensitive to heparin, while fibroblasts from grafts that develop stenosis are resistant. These data support the work by Chan and colleagues, who showed that venous SMCs from grafts that develop stenosis are less responsive to the growth inhibitory effects of heparin18,19. There are two important aspects of our data regarding heparin. First, there are some differences between the present work and that of Chan and coworkers. In the present study only adventitial fibroblasts were less responsive to heparin. Chan and colleagues found that medial SMCs from the stenotic grafts were less sensitive, but they did not study adventitial cell responsiveness. The potential importance of our observation with fibroblasts is underlined by differences between fibroblasts and SMCs. Adventitial fibroblasts proliferate faster than the SMCs. This is important given reports that in rat and pig vein grafts medial cells die in response to exposure to arterial pressure and migrating adventitial cells repopulate the medial and intimal layers4,46. It is possible that human vein grafts heal in the same way. It may be of great relevance to vein graft failure that fibroblasts have a greater proliferative response and that fibroblasts from stenotic patients are insensitive to heparin. A second point regarding heparin relates to possible mechanisms for the inhibitory effect of heparin. There is a clear inverse relationship between the extent of proliferation and heparin sensitivity, which suggests the possibility that the high level of proliferation in some of these cell lines that are insensitive to heparin comes about from the autocrine release of heparin insensitive, EGF receptor-binding growth factors, such as epiregulin or betacellulin47. Our results show that thrombin-mediated proliferation is inhibited by the EGF receptor kinase inhibitor, AG1478, in these heparin insensitive cell lines (data not presented). We have shown that FGF2, a heparin binding growth factor, is released by PDGF-BB and thrombin and acts as an autocrine co-stimulator of human arterial SMC proliferation20,21. Heparin binding EGF-like growth factor (HB-EGF) is released by thrombin to increase migration of rat arterial SMCs48. In each case heparin blocks either SMC proliferation or migration by interfering with the released heparin binding growth factor. Our studies suggest that heparin-sensitive human venous cells may release heparin-binding growth factors in response to PDGF-BB and thrombin.
The role of PDGF in vein graft stenosis is not clear, but there are several reasons to suggest it is important. First, we observed that there is a differential response to PDGF-BB in cells from stenotic compared to non-stenotic vein grafts. PDGF-BB has been found in normal human saphenous veins49, the levels of PDGF correlate with intimal hyperplasia in a rodent vein graft model50,51, and blockade of PDGF prevents intima formation in human saphenous vein organ culture52. Of particular interest, levels of PDGF decline in arterialized vein grafts undergoing atrophy after re-implantation in the venous circulation50. We have shown that blockade of the PDGF receptors α and β causes neointimal regression in synthetic arterial grafts without affecting the adjacent normal arteries53. The tyrosine kinase inhibitor Gleevec, which inhibits PDGF receptor kinase activity, causes regression of pulmonary artery wall thickening in animals with pulmonary hypertension and was reported to normalize symptoms of idiopathic pulmonary hypertension in two human case studies54–56. In summary, the results of these studies support the conclusion that PDGF is required not only to drive growth of the intima, but also to prevent regression of established thickening. If this conclusion is correct, then pharmacological blockade of the PDGF pathway might prevent stenosis or restore an adequate lumen by inducing intimal atrophy60. Additional studies are required to determine if specific components of the PDGFR signaling pathway are different in cells from patients that will develop graft stenosis compared to those that do not. If this is found to be the case, it is possible that screening of venous tissue at the time of grafting might identify those patients requiring more stringent follow up care.
Limitations of this study are primarily related to small sample size. Because we did not critically limit the time between removal of the vein from the patient and explant culture, it is possible that ex vivo injury affected outcome of the in vitro experiments. However, we did not find any correlation between response to growth factors and the time to first passage of cells in culture.
Acknowledgments
We thank Suzanne Justice and Amanda Barg for technical assistance. This work was supported by HL30946.
Supported by National Institutes of Health HL30946
Footnotes
Presented at the Western Vascular Society Meeting, Sept 13-16, 2008, Napa, CA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS, PREVENT III. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–750. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez E, Lambert EH, Magno MG, Mannion JD. Contractile smooth muscle cell apoptosis early after saphenous vein grafting. Ann Thorac Surg. 2000;70:1145–1152. doi: 10.1016/s0003-4975(00)01768-9. [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13–e20. doi: 10.1161/01.res.0000037090.34760.ee. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, O’Brien JE, Jr, Mannion JD, Morrison RC, Chung WS, Fard A, Zalewski A. Remodeling of autologous saphenous vein grafts - The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 5.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofstra L, Tordoir JHM, Kitslaar PJEHM, Hoeks APG, Daemen MJAP. Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts - Does it correlate with flow parameters? Circulation. 1996;94:1283–1290. doi: 10.1161/01.cir.94.6.1283. [DOI] [PubMed] [Google Scholar]
- 7.Gentile AT, Mills JL, Westerband A, Gooden MA, Berman SS, Boswell CA, Williams SK. Characterization of cellular density and determination of neointimal extracellular matrix constituents in human lower extremity vein graft stenoses. Cardiovasc Surg. 1999;7:464–469. doi: 10.1016/s0967-2109(98)00093-3. [DOI] [PubMed] [Google Scholar]
- 8.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 9.Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, Lowe HC, Freedman BS, Kritharides L. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:I435–I440. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]
- 10.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, Creager MA, Conte MS. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44:740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong SC, Hong MK, Kovach JA, Leon MB. Arterial remodeling after coronary angioplasty - A serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Gentile AT, Mills JL, Gooden MA, Westerband A, Cui H, Berman SS, Hunter GC, Hughes JD. Identification of predictors for lower extremity vein graft stenosis. Am J Surg. 1997;174:218–221. doi: 10.1016/s0002-9610(97)00087-1. [DOI] [PubMed] [Google Scholar]
- 13.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008;13:63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 14.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, Conte MS. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39:1178–1185. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens CD, Ridker PM, Belkin M, Hamdan AD, Pomposelli F, Logerfo F, Creager MA, Conte MS. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy MJ, Varty K, Naylor AR, London NJ, Bell PR. Bilateral infrainguinal vein grafts and the incidence of vein graft stenosis. Eur J Vasc Endovasc Surg. 1998;15:231–234. doi: 10.1016/s1078-5884(98)80181-0. [DOI] [PubMed] [Google Scholar]
- 17.Cooley BC. Mouse strain differential neointimal response in vein grafts and wire-injured arteries. Circ J. 2007;71:1649–1652. doi: 10.1253/circj.71.1649. [DOI] [PubMed] [Google Scholar]
- 18.Chan P, Patel M, Betteridge L, Munro E, Schachter M, Wolfe J, Sever P. Abnormal growth regulation of vascular smooth muscle cells by heparin in patients with restenosis. Lancet. 1993;341:341–342. doi: 10.1016/0140-6736(93)90139-8. [DOI] [PubMed] [Google Scholar]
- 19.Refson JS, Schachter M, Patel MK, Hughes AD, Munro E, Chan P, Wolfe JHN, Sever PS. Vein graft stenosis and the heparin responsiveness of human vascular smooth muscle cells. Circulation. 1998;97:2506–2510. doi: 10.1161/01.cir.97.25.2506. [DOI] [PubMed] [Google Scholar]
- 20.Rauch BH, Millette E, Kenagy RD, Daum G, Clowes AW. Thrombin- and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circulation Research. 2004;94:340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- 21.Millette E, Rauch BH, Defawe O, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96:172–179. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]
- 22.Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circulation Research. 1999;84:384–391. doi: 10.1161/01.res.84.4.384. [DOI] [PubMed] [Google Scholar]
- 23.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 24.Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circulation Research. 2004;94:550–558. doi: 10.1161/01.RES.0000117772.86853.34. [DOI] [PubMed] [Google Scholar]
- 25.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci USA. 1995;92:8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Gerdes C, Faber-Steinfeld V, Yalkinoglu Ö, Wohlfeil S. Comparison of the effects of the thrombin inhibitor r-hirudin in four animal models of neointima formation after arterial injury. Arterioscler Thromb Vasc Biol. 1996;16:1306–1311. doi: 10.1161/01.atv.16.10.1306. [DOI] [PubMed] [Google Scholar]
- 28.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Daum G, Forough R, Clowes M, Walter U, Clowes AW. Overexpression of human endothelial nitric oxide synthase in rat vascular smooth muscle cells and in balloon-injured carotid artery. Circ Res. 1998;82:862–870. doi: 10.1161/01.res.82.8.862. [DOI] [PubMed] [Google Scholar]
- 30.Dartsch PC, Voisard R, Bauriedel G, Höfling B, Betz E. Growth characteristics and cytoskeletal organization of cultured smooth muscle cells from human primary stenosing and restenosing lesions. Arteriosclerosis. 1990;10:62–75. doi: 10.1161/01.atv.10.1.62. [DOI] [PubMed] [Google Scholar]
- 31.Desai ND, Cohen EA, Naylor CD, Fremes SE. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302–2309. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 32.Frischknecht K, Greutert H, Weisshaupt C, Kaspar M, Yang Z, Luscher TF, Carrel TP, Tanner FC. Different vascular smooth muscle cell apoptosis in the human internal mammary artery and the saphenous vein. Implications for bypass graft disease. J Vasc Res. 2006;43:338–346. doi: 10.1159/000093606. [DOI] [PubMed] [Google Scholar]
- 33.Qin M, Zeng Z, Zheng J, Shah PK, Schwartz SM, Adams LD, Sharifi BG. Suppression subtractive hybridization identifies distinctive expression markers for coronary and internal mammary arteries. Arterioscler Thromb Vasc Biol. 2003;23:425–433. doi: 10.1161/01.ATV.0000059303.94760.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott L, Kerr A, Haydock D, Merrilees M. Subendothelial proteoglycan synthesis and transforming growth factor beta distribution correlate with susceptibility to atherosclerosis. J Vasc Res. 1997;34:365–377. doi: 10.1159/000159245. [DOI] [PubMed] [Google Scholar]
- 35.Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–631. doi: 10.1161/01.res.87.7.623. [DOI] [PubMed] [Google Scholar]
- 36.Geary RL, Wong JM, Rossini A, Schwartz SM, Adams LD. Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2002;22:2010–2016. doi: 10.1161/01.atv.0000038147.93527.35. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Adams LD, Wang X, Pabon L, Schwartz SM, Sane DC, Geary RL. Regulator of G protein signaling 5 marks peripheral arterial smooth muscle cells and is downregulated in atherosclerotic plaque. J Vasc Surg. 2004;40:519–528. doi: 10.1016/j.jvs.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Oemar BS, Carrel T, Kipfer B, Julmy F, Luscher TF. Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels: role of PDGF receptors, mitogen-activated protein kinase, and cyclin-dependent kinase inhibitors. Circulation. 1998;97:181–187. doi: 10.1161/01.cir.97.2.181. [DOI] [PubMed] [Google Scholar]
- 39.Caps MT, Cantwell-Gab K, Bergelin RO, Strandness DE., Jr Vein graft lesions: time of onset and rate of progression. J Vasc Surg. 1995;22:466–474. doi: 10.1016/s0741-5214(95)70016-1. [DOI] [PubMed] [Google Scholar]
- 40.Landry GJ, Moneta GL, Taylor LM, Jr, Edwards JM, Yeager RA, Porter JM. Patency and characteristics of lower extremity vein grafts requiring multiple revisions. J Vasc Surg. 2000;32:23–31. doi: 10.1067/mva.2000.107306. [DOI] [PubMed] [Google Scholar]
- 41.Ho KJ, Owens CD, Longo T, Sui XX, Ifantides C, Conte MS. C-reactive protein and vein graft disease: evidence for a direct effect on smooth muscle cell phenotype via modulation of PDGF receptor-beta. Am J Physiol Heart Circ Physiol. 2008;295:H1132–H1140. doi: 10.1152/ajpheart.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Torres A, Gimeno A, Melón J, Mendez L, Muñoz FJ, Macía M. Age-related loss of proliferative activity of human vascular smooth muscle cells in culture. Mech Ageing Dev. 1999;110:49–55. doi: 10.1016/s0047-6374(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 43.Ihnat DM, Mills JL, Dawson DL, Hughes JD, Hagino RT, DeMaioribus CA, Gentile AT, Westerband A. The correlation of early flow disturbances with the development of infrainguinal graft stenosis: A 10-year study of 341 autogenous vein grafts. J Vasc Surg. 1999;30:8–14. doi: 10.1016/s0741-5214(99)70171-0. [DOI] [PubMed] [Google Scholar]
- 44.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 45.Carty CS, Huribal M, Marsan BU, Ricotta JT, Dryjski M. Nicotine and its metabolite cotinine are mitogenic for human vascular smooth muscle cells. J Vasc Surg. 1997;25:682–688. doi: 10.1016/s0741-5214(97)70295-7. [DOI] [PubMed] [Google Scholar]
- 46.Tomas JJ, Stark VE, Kim JL, Wolff RA, Hullett DA, Warner TF, Hoch JR. Beta-galactosidase-tagged adventitial myofibroblasts tracked to the neointima in healing rat vein grafts. J Vasc Res. 2003;40:266–275. doi: 10.1159/000071890. [DOI] [PubMed] [Google Scholar]
- 47.Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53. doi: 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 48.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 49.Drubaix I, Giakoumakis A, Robert L, Robert AM. Preliminary data on the age-dependent decrease in basic fibroblast growth factor and platelet-derived growth factor in the human vein wall and in their influence on cell proliferation. Gerontology. 1998;44:9–14. doi: 10.1159/000021976. [DOI] [PubMed] [Google Scholar]
- 50.Sterpetti AV, Cucina A, Lepidi S, Randone B, Stipa F, Aromatario C, Travi D, D’Angelo LS, Cavallaro A, Stipa S. Progression and regression of myointimal hyperplasia in experimental vein grafts depends on platelet-derived growth factor and basic fibroblastic growth factor production. J Vasc Surg. 1996;23:568–575. doi: 10.1016/s0741-5214(96)80034-6. [DOI] [PubMed] [Google Scholar]
- 51.Hoch JR, Stark VK, Turnipseed WD. The temporal relationship between the development of vein graft intimal hyperplasia and growth factor gene expression. J Vasc Surg. 1995;22:51–58. doi: 10.1016/s0741-5214(95)70088-9. [DOI] [PubMed] [Google Scholar]
- 52.George SJ, Williams A, Newby AC. An essential role for platelet-derived growth factor in neointima formation in human saphenous vein in vitro. Atherosclerosis. 1996;120:227–240. doi: 10.1016/0021-9150(95)05717-x. [DOI] [PubMed] [Google Scholar]
- 53.Englesbe MJ, Hawkins S, Hsieh pch, Davies MG, Daum G, Kenagy RD, Clowes AW. Concomitant Blockade of PDGF Receptors –a and –b Induces Intimal Atrophy in Baboon PTFE Grafts. J Vasc Surg. 2004;39:440–446. doi: 10.1016/j.jvs.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 56.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med. 2006;145:152–153. doi: 10.7326/0003-4819-145-2-200607180-00020. [DOI] [PubMed] [Google Scholar]


