Abstract
Autism encompasses a wide spectrum of disorders arising during brain development. Recent studies reported that sequence polymorphisms in neuroligin-3 (NLGN3) and neuroligin-4 (NLGN4) genes have been linked to autism spectrum disorders indicating neuroligin genes as candidate targets in brain disorders. We have characterized a single mutation found in two affected brothers that substituted Arg451 to Cys in NL3. Our data show that the exposed Cys causes retention of the protein in the endoplasmic reticulum (ER) when expressed in HEK-293 cells. To examine whether the introduction of a Cys in the C-terminal region of other α/β-hydrolase fold proteins could promote the same cellular phenotype, we made homologous mutations in acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) and found a similar processing deficiency and intracellular retention (De Jaco et al., J Biol Chem. 2006, 281:9667-76). NL3, AChE and BChE mutant proteins are recognized as misfolded in the ER, and degraded via the proteasome pathway. A 2D electrophoresis coupled with mass spectrometry based approach was used to analyze proteins co-immunoprecipitating with NL3 and show differential expression of factors interacting with wild type and mutant NL3. We identified several proteins belonging to distinct ER resident chaperones families, including calnexin, responsible for playing a role in the folding steps of the AChE and NLs.
Keywords: α/β-Hydrolase fold proteins, Protein folding, ER chaperone proteins
1. Introduction
NLs belong to the α/β-hydrolase fold superfamily of proteins and are structurally related to cholinesterases. NLs associate within the synaptic cleft with protein members of the neurexin (Nxs) family to facilitate maturation of the synapses during development. Rearrangements of chromosomal regions coding for NLGN and NRXN genes have been found in autistic patients suggesting that genetic alterations are likely to be important for explaining the synaptic defects associated with autism spectrum disorders. A single point mutation of a conserved Arg451 in NL3 was found substituted with a Cys in two autistic affected brothers [1]. The R451C mutation causes impaired intracellular protein trafficking in rat NL1 and NL3, demonstrating that most of the mutant protein is retained within the ER [2]. NL3, BChE and AChE share a common fold and over 30% of amino acid identity in their extracellular domains. Arg451 in NL3 is mapped to the C-terminus of the cholinesterase homologous region. The homologous Arg is conserved in both AChE and BChE and cysteine substitution for the conserved arginine in BChE was found as a rare mutation in a patient population with post-succinylcholine apnea [3].
2. Experimental methods
Flag-tagged constructs NL3 WT or NL3 R451C were transiently transfected into HEK-293 cells. Forty eight hours post-transfection, cells were lysed and centrifuged to obtain the detergent-soluble fraction. NL proteins were immunoprecipitated with Flag antibodies (Sigma) and Protein A/G beads (Pierce) for 12 h at 4 °C. The beads were washed and resuspended in 2D electrophoresis buffer (2 M thiourea, 5 M urea, 0.25% CHAPS, 0.25% Tween-20, 0.25% SB-3, 10% isopropanol, and 12.5% water saturated butanol) containing 15 mg DTT and 0.5% ampholytes (pI ranges 3-10; BioRad), and passively absorbed onto immobilized pH gradient strips (pI range 3-10 NL; BioRad) for 4 h at 20 °C followed by active rehydration (50 V at 20 °C) for 8 h. Isoelectric focusing was performed for 35,000 V h on a Protean IEF System (BioRad), followed by traditional SDS-PAGE using the Criterion gel system (BioRad). Proteins were visualized by silver staining. Other methods have been described in previous publications [2,4].
3. Results and discussion
The Arg to Cys mutation was inserted in the c-DNAs coding for NL1 and NL3, two different oligomeric forms of AChE (soluble and GPI-linked), and the soluble tetrameric form of BChE. Cellular phenotypes of the mutant compared to the wild type proteins were analyzed. Sensitivity to endoglycosidase H and immunofluorescence co-localization studies with the ER resident protein, calnexin, revealed that all the mutant proteins are retained in the ER [4] indicating similar protein processing for the mutant proteins belonging to the NL and to the cholinesterase families. To assess whether the mutant proteins are globally or locally misfolded, the functionality of R395C AChE enzyme was studied. Catalytic properties were determined by pS curves and by organophosphate titration for purified soluble AChE wild type and the Arg to Cys mutant protein. AChE R395C is still active but the mutated Cys affects the properties of the enzyme having a greater Km and smaller kcat, resulting in slower catalysis with diminished affinity for the substrate [4]. The changes in the catalytic parameters following substitution of the cysteine could be explained by a local protein misfolding or by the association of the mutant protein with ER resident chaperones that recognize the protein as misfolded.
To study the degradation pathway of wild type and R to C mutant proteins, HEK-293 transiently transfected cells were treated with the proteasome inhibitor lactacystin. Expression levels of soluble wild type and mutant NL3, BChE and AChE proteins are increased after treatment with the drug [4]. Cells transfected with the c-DNA encoding for NL3 soluble showed an increase in protein expression both in the cell lysates [4] and in the media (Fig. 1). The increase is greater in cell extracts and media expressing the mutant proteins suggesting that the incompletely processed mutant protein, retained in the ER, is preferentially shuttled to the proteasome for degradation.
Fig. 1.
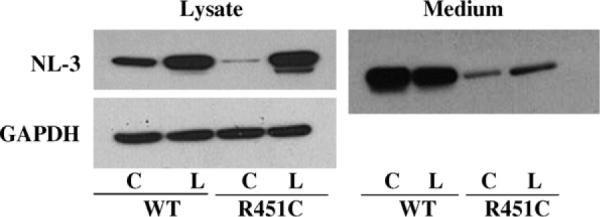
Western blot analysis of the expression levels of neuroligin in cells transfected with c-DNA for neuroligin-3 wild type and R451C mutant protein. C = control, L = lactacystin. (Lysate data are modified from Ref. [4].)
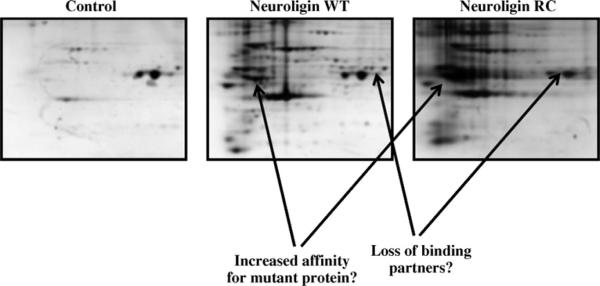
To understand the molecular mechanism responsible for the ER-retention of the mutant proteins, the study of the proteins interacting with the NLs has been approached by 2D-electrophoresis technique (Fig. 2). Preliminary results obtained by 2D-electrophoresis analysis of purified full length NL3 proteins show that wild type and mutant proteins interact with a unique subset of ER molecular chaperones. These data suggest that differences in protein-protein interactions may be critical for the sub-cellular localization and function of the wild type and mutant NLs. The identity of the partner proteins after immunoprecipitation of the flag-tagged NLs is revealed by mass spectrometry analysis of single spots obtained after the 2D-gel electrophoresis and by western blot analysis using commercial antibodies for specific ER chaperones. We found calnexin and calreticulin to be candidate chaper-one proteins involved in the processing of the NLs, together with members of the Hsp70 family of proteins. The processing of NL3 proteins over the time and the association of these chaperone proteins at each folding step could explain the ER retention mechanism of the R451C mutant in the ER in HEK-293 cells.
Fig. 2.
2D SDS-PAGE gels of immunoprecipitated neuroligin-3 wild type and R451C mutant. Differences in associated proteins between neuroligin WT and neuroligin RC are shown by the arrows.
Our findings show that a conserved arginine in a region near the C-terminus of proteins of the α/β-hydrolase fold family plays a role in processing of the nascent peptide and further trafficking towards the cell membrane. Mutation to cysteine perturbs biosynthetic processing of different synaptic proteins belonging to the α/β-hydrolase fold family of proteins. Identification of such proteins NL, AChE and BChE and their aberrant structures and functions are important for understanding molecular mechanisms mediating synaptogenesis in the central nervous system and for finding linkages between naturally occurring mutations and disease states.
Acknowledgements
This work is supported by NIH GM18360 and P42-ES10337 to PT and Cure Autism Now pilot research award to DC.
References
- [1].Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, et al. Paris Autism Research International Sibpair Study. Nat. Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, et al. J. Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yen T, Nightingale BN, Burns JC, Sullivan DR, Stewart PM. Clin. Chem. 2003;49:1297–1308. doi: 10.1373/49.8.1297. [DOI] [PubMed] [Google Scholar]
- [4].De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radić Z, Lockridge O, et al. J. Biol. Chem. 2006;281:9667–9676. doi: 10.1074/jbc.M510262200. [DOI] [PubMed] [Google Scholar]