Abstract
Purpose of review
Pain assessment is essential for patient care in many settings, but it proves difficult when the patient is cognitively compromised or otherwise unable to produce a conventional pain report. This review describes progress in pain assessment technology that involves the coding of human facial expression.
Recent findings
It is possible to quantify facial expression by coding patterns of facial muscle contraction and relaxation. These patterns are action units, and they can gauge the intensity of pain as well as signal its occurrence. The experience of pain seems to generate a unique facial expression comprising several action units. Concerns have existed about whether demented patients produce diagnostically meaningful facial expressions of pain because they tend to generate more non-specific facial expressions and perhaps code pain intensity less well than normals. Recent work shows that facial expression reflects pain as well or better in demented patients compared to normals.
Summary
Although still nascent, coded facial expression appears to work reliably as a pain assessment tool with cognitively compromised patients. Clinical application awaits the development of technology that can automate facial coding and scoring.
Keywords: Pain assessment, dementia, facial expression
Introduction
Pain assessment proves difficult in the cognitively compromised or non-verbal older patient. Advances in research and theory show that patterns of facial expression communicate both emotional states and pain. Coding technology exists for dynamically tracking, coding and quantifying facial expression. Emerging evidence indicates that facial expression coding can both detect and gauge the intensity of pain in the cognitively compromised patient. This review integrates several lines of converging evidence, including recent findings, to reveal the promise of this approach for pain measurement in the cognitively or verbally compromised patient.
Background
The pain measurement standard put forward by the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) recognizes the right of individual patients to appropriate assessment and management of pain. Pain assessment proves challenging when the patient suffers from dementia, brain damage or some other condition that compromises his or her ability to engage in self-assessment and provide a verbal report. The consequences of poor or absent pain assessment go beyond failure to meet JCAHO standards. Lacking pain assessment, clinicians may overlook new pathologies or injuries when they occur, and they may fail to identify and alleviate the suffering of patients who experience pain that they cannot express verbally. As with any neglected painful condition, failure to recognize and address the problem ultimately leads to higher costs of care.
The cognitively compromised patient
As more of us live longer, the population of cognitively compromised patients in residential care facilities, nursing homes, home health agencies and hospitals grows. Conventional pain assessment usually involves nurse administration of an 11-point numerical rating scale anchored on both ends by word descriptors such as “No Pain” and “Worst Pain Imaginable.” Patients must introspect and provide a numerical score. As with any measure, such a pain score is valid only if it truly measures what it purports to measure, and it is reliable only if it is consistent. Many cognitively compromised patients cannot generate pain ratings at all, others can produce numbers that are invalid or unreliable, and still others find the cognitive effort distressing. Clearly, a need exists for a valid, reliable alternative pain measurement tool.
Tools for standardized observation
The most common approach to assessing pain in the cognitively compromised patient is to develop a tool standardized observation and classification of patients who potentially may have pain. In general, clinicians use a rating tool to observe the cognitively compromised patient, score the presence or absence of certain specific behavior patterns, and arrive at a pain assessment. A review by van Herk and others[1*] examined 13 such scales; still others exist in nascent form. Newly introduced or recently revised assessment tools include the Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale[2*], the Certified Nursing Assistant tool[3*], the Elderly Pain Caring Assessment 2[4*], and the Pain Assessment in Advanced Dementia Scale[5*]. Mostly these tools focus on non-coded facial expression, vocalization, motor behavior, social behavior and mood.
The Elderly Pain Caring Assessment 2[4*] is a good representative of this genre. The authors initially surveyed experienced caregivers and reviewed the literature. Ultimately they created and validated an 8 item behavioral scale to rate the intensity of pain in a nonverbal patient. Four of these pertain to patient behavior before caregiver intervention: non-coded facial expression, spontaneous posture, movements in and out of bed, and interactions with others. The remaining four items pertain to interaction during caregiver intervention: anxious reaction to intervention, reactions during the intervention, reactions when certain body parts are attended to, and complaints during intervention. Each item receives a 0 to 4 rating.
The various pain observation scales available and emerging differ in numbers of items, types of categories, and in their documented reliability and validity. Not surprisingly, some scales by design score specific types of pain rather than pain in general. One of the challenges in this approach to pain assessment is the fundamental issue of validity. Do the response patterns observed reflect pain per se or a general pattern of distress in someone who happens to also have pain? It will be some time before one or more of these scales emerges as the new gold standard for observational pain assessment in the cognitively compromised patient.
Facial expression of pain
In an editorial, Prkatchin[6*] addressed pain assessment in the cognitively compromised patient and heralded the coding of facial expression as “coming of age.” A well-developed literature exists on coding facial expression to quantify pain in infants[7*, 8*] and laboratory studies of adults using the facial action coding system (FACS) demonstrate the distinct and prototypical configuration of pain as well as basic emotion expressions in the face[9*]. Although the expression of pain largely involves the corrugator, orbicularis oculi, and levator muscles, clinical application in adults proves complex. Socialization tends to override spontaneous facial expression, and cognitive processes, including interpretation of the immediate situation, can generate complex emotions that may concomitantly drive facial expression. Nonetheless, consensus holds that the FACS technology can identify a reliable basic pain expression.
The FACS originated in the work of Ekman and Friesen who sought to code the basic emotions of anger, disgust, fear, joy (happiness), sadness, and surprise. In 2002, it underwent a major refinement and revision[10]. The basic unit of human facial expression is a pattern of muscle contraction and relaxation termed the action unit (AU). Using FACS one can manually code nearly any anatomically possible facial expression, decomposing it into the specific AUs and their temporal segments that produced the expression. FACS employs 32 AUs and a number of broader motor patterns, termed Action Descriptors, such as head movement and jaw thrust. The labeling of expressions currently requires trained experts. However, work is underway to develop computer-based scoring that can automatically identify FACS codes[11, 12*].
Rationale
Darwin first identified mammalian facial expression as communication during his voyage on the Beagle. Current evolutionary theory recognizes the adaptive value of emotion and its communication as behavioral adaptation[13]. Facial expressions are coordinated, stereotyped behavioral phenotypes that integrate with vocalization, gestures and postures to make higher order social interaction possible. In the human infant, a facial expression conveying pain increases survival by eliciting protection and nurturance. In the child and adult, facial expression of pain elicits social support and assistance.
Physiological basis of facial expression: Autonomic mechanisms
Porges[14] proposed polyvagal theory as a biological basis for social behavior, asserting that evolution of neural regulation within the autonomic nervous system has passed through three stages, each representing a behavioral strategy. In the first stage, the primitive unmyelinated visceral vagus that supports digestion could respond to threat only by depressing metabolic activity and generating immobilization behaviors. In the second stage, the sympathetic nervous system made it possible to increase metabolic output and inhibit the visceral vagus, thus enabling fight/flight behaviors. The third stage, which is uniquely mammalian, involves a myelinated vagus that can quickly regulate cardiac output to facilitate rapid engagement and disengagement with the environment. The mammalian vagus is neuroanatomically linked to the cranial nerves that regulate social engagement via facial expression and vocalization and also cranial nerves V, VII originating in medullary nucleus ambiguous. Corticofugal processes involving in the frontal lobes normally modulate medullary activity. With evolution the interplay of the autonomic nervous system with the hypothalmo-pituitary-adrenocortical axis and the immune system and cortex change to maximize response to stressors. Nociception is a stressor, and the ANS operates in concert with the endocrine and immune systems to generate stress[15*]. Therefore, within polyvagal framework, facial expression of pain reflects dynamic autonomic response patterns triggered by noxious signaling.
Physiological basis of facial expression: Central mechanisms
At the cortical level, recognition of particular faces depends heavily on the fusiform face area of the fusiform gyrus located on the ventral surface of the temporal lobe, although this area is not specific to faces. The bilateral superior temporal sulcus processes facial expression in others, and such expression is rich in information, particularly emotion. Communication via facial expression is possible because of “mirroring systems” brain circuits.
A growing body of brain research on empathy is revealing the link between empathic communication and facial expression. An overlap exists between brain areas activated when a person undergoes painful stimulation and when he/she observes another undergoing such painful stimulation. Functional magnetic resonance imaging (fMRI) studies indicate bilateral activation in the anterior cingulate cortex and the anterior insula during both self-experienced pain and observation of pain in others. Saarela et al.,[16*] showed that, when subjects observed pain from the faces of chronic pain patients, activations in bilateral anterior insula (AI), left anterior cingulate cortex, and left inferior parietal lobe in the observers' brains correlated with their estimates of the intensity of observed pain. These and other studies in this area show that the “intersubjective representation of pain” is an aspect of human empathy and, as such, a natural basis for pain measurement.
Assessing pain in the cognitively compromised patient
Although there are many kinds of cognitively compromised patients, the elderly patient with dementia is the most common. Kunz and others[17**] studied the facial expression of pain in dementia patients experiencing mechanically induced pain, contrasting their observations with similar expressions in normals of similar age experiencing the same painful stimulation. Subjects underwent repeated trials with a pressure algometer applied to right and left forearms at two intensities. To score event-related facial expressions, they videotaped faces under varying experimental conditions and analyzed the recordings using the FACS.
Recognizing the potential limitations of the FACS approach with this patient population, Kunz and colleagues[17**] evaluated three hypotheses: 1) demented patients experiencing noxious stimulation exhibit more intense and frequent facial expressions than normals; 2) this is due to nonspecific increases in facial responses in the demented vs normal patient; and 3) demented patients encode the intensity of noxious stimuli less well than normals.
Analysis of the data provided support for the first hypothesis. Kunz and others[17**] observed that the demented patients displayed higher frequencies and higher intensities of facial responses; that is, enhanced AUs during painful stimulation. Figure 1 provides a graphical depiction of their effect sizes for seven AUs. It reveals more AUs for the demented patients and also greater effect sizes for AUs common to the two groups. The graph suggests that facial expression is less socially inhibited in demented patients than normals. This resembles the familiar observation of seemingly exaggerated facial expression in otherwise normal persons who are blind from birth and therefore have never experienced visual social feedback in response to their facial expressions.
Figure 1. Facial action unit effect sizes for demented and normal patients experiencing pain.
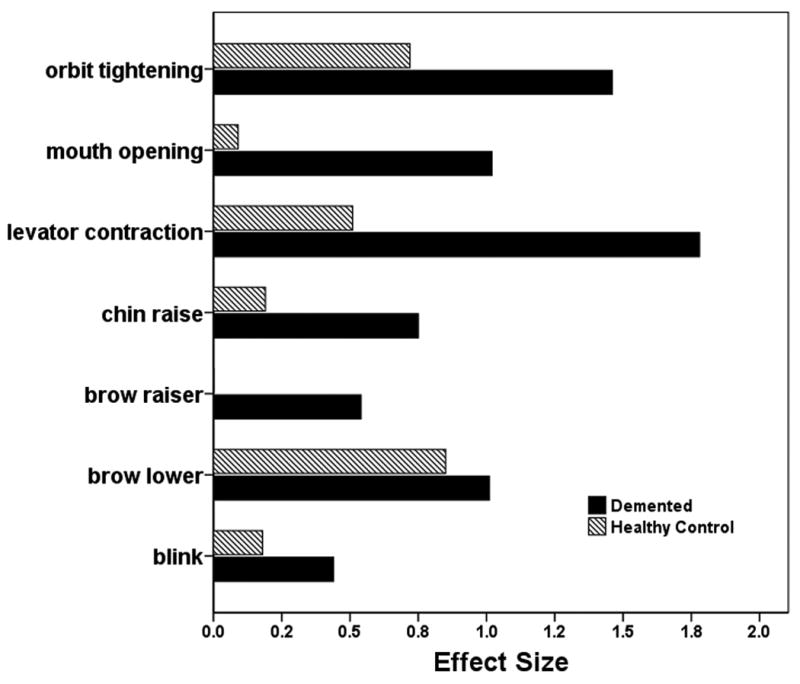
The data plotted derive from the work of Kunz and others[17**]. Effect sizes come from repeated painful versus non-painful testing with a pressure algometer; that is, they gauge the impact of pain on the facial action units. The ordinate lists the relevant action units. A small effect size corresponds to .2, a medium effect size to .5 and a large effect size to .8. [this figure is original]
The second hypothesis failed to gain support because the pain-relevant AUs were more frequent and intense in the demented patients than in the normals. Thus, it was enhanced specific rather than nonspecific facial expression that distinguished the demented patients from the normals. Finally, the third hypothesis failed because demented patients did as well or better than normals in the stimulus-response relationship, indicating that nociception and pain reflect just as validly in the facial expressions of dementia patients as in the facial expressions of normals.
The findings of Kunz and others[17**] suggest that facial expression is less socially inhibited in demented patients and therefore an even better indicator of pain than it is in normal people, who tend to conform facial expression to the social context. This finding is consistent with polyvagal theory in demonstrating that noxious signaling drives autonomic arousal and this in turn generates specific, socially significant facial expressions. It is also consistent with the observations of Cole and others [18**] who showed using fMRI that pain-related brain activation is greater in demented vs. age-matched healthy control patients. Hsu and others[19**] used the FACS to gauge pain in dental patients undergoing gingival injection of local anesthetic. They compared cognitively impaired with normal older adults. Changes in facial expression proved to be the most useful measure overall in identifying pain in both cognitively intact and cognitively impaired older patients, and it was more sensitive in the cognitively impaired patients.
Limitations and pitfalls in assessing pain via facial expression
The history of facial coding extends over three decades. Although these results and related findings with normal subjects are encouraging, a few concerns remain before the FACS approach to pain assessment is ready for clinical application. Fortunately, none of them is insurmountable.
First, visual observation of muscle activity is a low sensitivity methodology. Skin obscures muscle, and many small or rapid changes in contraction or relaxation almost certainly escape visual detection. Moreover, the technique is likely to work better in the lean, thin-skinned individual than in the obese subject. If these concerns are valid, then the FACS is under-sampling the information available in human facial expression during pain. Systematic studies based on electromyography could evaluate the sensitivity and precision of measurement in FACS methodology, but there has been little work along these lines to date.
Much of the work on facial expression and pain presumes that a single, primal face of pain exists[20*]. This may well be the case, but it is difficult to prove. In the study by Kunz and others[17**] normals and demented patients had the same prototypical pain expression, but the demented patients revealed much more of the expression. Measurement of pain with verbal tools proves difficult across different types of pain because pain quality and temporal features can vary. This problem may or may not complicate pain assessment by facial expression. For example, visceral pains are subjectively very different in quality from pain originating in the skin and tend to involve much more parasympathetic nervous system activation. Both of these differ from neuropathic pain. Whether facial expression codes can differentiate pains arising from different origins is an open question.
Finally, the greatest limitation of the FACS approach in its current form is that it is clinically infeasible. It requires videotaping and coding by a trained observer. This approach is academically valuable but out of the question for everyday nursing practice or medical evaluation. Clearly, the next step towards application must involve computer technology that can observe and recognize human AUs, identifying and scoring emotional states and pain dynamically.
Related Applications
Although this review has focused on the older, cognitively compromised patient, there are other potential applications for pain assessment through coded facial expression in special populations. As already noted above, this approach has proven valuable the study of infants and preverbal children. Potentially, it can also provide a way to assess pain in the non-English speaking patient. Several possibilities merit consideration.
First, facial expression of pain may prove valuable as an outcome measure for acute pain. Although some applications will prove too difficult with human coders, computer technology for capturing and assessing facial expression should eventually become available. When it does, the FACS approach should provide a noninvasive and automated way to evaluate acute pain states. Applications could include postoperative pain in hospitals and ambulatory clinics, as well as acute pain in the emergency department including sickle cell crisis, dentistry, and numerous settings where potentially painful procedures take place. Facial action coding could in principle identify how soon after administration an intervention begins to act, the maximum impact of the intervention, and its duration.
Second, facial expression of pain may prove valuable as an indicator of noxious signaling in the patient undergoing partial sedation. It is difficult for such patients to sustain attention and generate reliable pain reports. They are temporarily and intentionally cognitively compromised. If the FACS approach works with such patients, then this approach could help improve the quality of care by helping clinicians minimize the stress and discomfort that patients experience.
Conclusions
Progress in the domain of facial coding and pain assessment is steady and promising. Facial expression appears to reflect integrated mesencepahlic response to noxious signaling, with modulation under normal conditions from cortex. Because of cortical mediation in the normal subject, this type of measurement may not be free from bias; future research will need to determine whether subjects can intentionally exaggerate or constrain pain intensity. This avenue appears valuable for pain assessment in special populations where verbal report is difficult or unreliable. Ultimately, it may provide an objective method for measuring pain. At present, however, pain assessment requires skilled human coding, and this limits clinical application. Development of automated coding technology will greatly advance both research and application.
Acknowledgments
Support for this work came from NIH award NR009542 to the author.
Bibliography
- *1.van Herk R, van Dijk M, Baar FP, Tibboel D, de Wit R. Observation scales for pain assessment in older adults with cognitive impairments or communication difficulties. Nursing Research. 2007;56(1):34–43. doi: 10.1097/00006199-200701000-00005. [DOI] [PubMed] [Google Scholar]; Comparison of 13 pain observations scales designed for use with cognitively impaired patients revealed substantial herterogeniety and as yet insufficient information on validity and reliability.
- *2.Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Snow AL, Ljunggren AE. Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. Journal of Pain and Symptom Management. 2007;34(1):67–80. doi: 10.1016/j.jpainsymman.2006.10.016. [DOI] [PubMed] [Google Scholar]; A newly developed scale with seven rating items (observing at rest, mobilization of the hands, arms, legs, turn over in bed, sitting on bedside, and teeth/mouth care) demonstrated that such ratings could disclose more pain in cognitively impaired patients than standard pain rating methods.
- *3.Cervo FA, Raggi RP, Bright-Long LE, Wright WK, Rows G, Torres AE, et al. Use of the certified nursing assistant pain assessment tool (CPAT) in nursing home residents with dementia. American Journal of Alzheimer's Disease and other Dementias. 2007;22(2):112–9. doi: 10.1177/1533317506298907. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nursing pain assessment tool developed for use with dementia patients did not result in any change in patient behavior, medication use or function. This underscores the limitations of the rating approach.
- *4.Morello R, Jean A, Alix M, Sellin-Peres D, Fermanian J. A scale to measure pain in non-verbally communicating older patients: the EPCA-2 Study of its psychometric properties. Pain. 2007;133(13):87–98. doi: 10.1016/j.pain.2007.03.007. [DOI] [PubMed] [Google Scholar]; This paper demonstrates a thorough approach to validation of a pain rating scale designed for use with cognitively impaired patients.
- *5.Schuler MS, Becker S, Kaspar R, Nikolaus T, Kruse A, Basler HD. Psychometric properties of the German “Pain Assessment in Advanced Dementia Scale” (PAINAD-G) in nursing home residents. Journal of the American Medical Directors Association. 2007;8(6):388–95. doi: 10.1016/j.jamda.2007.03.002. [DOI] [PubMed] [Google Scholar]; A pain rating scale developed for dementia patients involves observation of breathing, vocalization, facial expression, body language, and consolability. Psychometrically, these reduced to one dimension. Although reliability was good, it did nto correlate well with pain ratings. This raises the question of what the gold standard should be for pain assessment in this population.
- *6.Prkachin KM. The coming of age of pain expression. Pain. 2007;133(13):3–4. doi: 10.1016/j.pain.2007.10.015. [DOI] [PubMed] [Google Scholar]; The author discusses the findings of Kunz and others against the historical background facial expression in psychology. He points out that this methodology can sometimes identify a phenomenon that conventional pain assessment misses.
- *7.Serpa AB, Guinsburg R, Balda Rde C, dos Santos AM, Areco KC, Peres CA. Multidimensional pain assessment of preterm newborns at the 1st, 3rd and 7th days of life. Sao Paulo Medical Journal = Revista Paulista de Medicina. 2007;125(1):29–33. doi: 10.1590/S1516-31802007000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]; A neonatal version of the FACS proved useful in assessing pain in preterm infants. This supports the hypothesis that a primal pain expression exists in humans.
- *8.Lehr VT, Zeskind PS, Ofenstein JP, Cepeda E, Warrier I, Aranda JV. Neonatal facial coding system scores and spectral characteristics of infant crying during newborn circumcision. The Clinical Journal of Pain. 2007;23(5):417–24. doi: 10.1097/AJP.0b013e31805476f2. [DOI] [PubMed] [Google Scholar]; Using a neonatal version of the FACS, the authors found a strong relationship between the pain score and the pitch of infant crying. This is consistent with polyvagal theory.
- *9.Simon D, Craig KD, Gosselin F, Belin P, Rainville P. Recognition and discrimination of prototypical dynamic expressions of pain and emotions. Pain. 2008;135(12):55–64. doi: 10.1016/j.pain.2007.05.008. [DOI] [PubMed] [Google Scholar]; This report demonstrated that the dynamic facial expression of the pain experience differs from that of basic emotional states. This lends support to the hypothesis that a pain-specific facial expression exists.
- 10.Hager JC, Paul E, Friesen Wallace V. Facial action coding system. 2002 [Google Scholar]
- 11.Dailey MN, Cottrell GW, Padgett C, Adolphs R. EMPATH: a neural network that categorizes facial expressions. Journal of Cognitive Neuroscience. 2002;14(8):1158–73. doi: 10.1162/089892902760807177. [DOI] [PubMed] [Google Scholar]
- *12.Susskind JM, Littlewort G, Bartlett MS, Movellan J, Anderson AK. Human and computer recognition of facial expressions of emotion. Neuropsychologia. 2007;45(1):152–62. doi: 10.1016/j.neuropsychologia.2006.05.001. [DOI] [PubMed] [Google Scholar]; This study compared human and support vector machine facial recognition performance. The automated evaluation performed well across different emotions. This suggests that automated detection and scoring of human facial pain expression will ultimately be possbile.
- 13.Schmidt KL, Cohn JF. Human facial expressions as adaptations: Evolutionary questions in facial expression research. American Journal of Physical Anthropology. 2001 33:3–24. doi: 10.1002/ajpa.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- *15.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9(2):122–45. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dynamic physiological arousal associated with pain involves coordinated allostatic changes in the nervous, endocrine and immune systems. The physiology underlying the facial expression of pain probably reflects all of these processes.
- *16.Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cereb Cortex. 2007;17(1):230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]; Empathy for others in pain has objective correlates in brain activity. When an observer watches the faces of chronic pain patients experiencing pain, both the presence of pain and also the intensity of the observed pain is encoded in the observer's brain, closely resembling the brain activity pattern that occurs during the observer's own pain experience.
- **17.Kunz M, Scharmann S, Hemmeter U, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain. 2007;133(13):221–8. doi: 10.1016/j.pain.2007.09.007. [DOI] [PubMed] [Google Scholar]; This study showed that coding the facial expression of pain in dementia patients provides as good or better pain assessment as similar coding in normal patients.
- **18.Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer's disease. Brain. 2006;129(Pt 11):2957–65. doi: 10.1093/brain/awl228. [DOI] [PubMed] [Google Scholar]; Brain activity during pain is preserved and enhanced in the demented patient versus normal older person.
- **19.Hsu KT, Shuman SK, Hamamoto DT, Hodges JS, Feldt KS. The application of facial expressions to the assessment of orofacial pain in cognitively impaired older adults. Journal of the American Dental Association (1939) 2007;138(7):963–9. doi: 10.14219/jada.archive.2007.0293. quiz 1021-2. [DOI] [PubMed] [Google Scholar]; Using a dental pain model, the authors demonstrated that FACS assessment proved to be the most useful measure overall in identifying pain in both cognitively intact and cognitively impaired older patients. This is consistent with the observations of Kunz and others.
- *20.Schiavenato M, Byers J, Scovanner P, Windyga P, Shah M. Is there a Primal Face of Pain? A methodology answer. Conf Proc IEEE Eng Med Biol Soc. 2007:3559–62. doi: 10.1109/IEMBS.2007.4353100. [DOI] [PubMed] [Google Scholar]; This article endorses the concept of a primal pain expression and articulates an approach to computerized quantification in newborns. It suggests that similar methods would apply for pain assessment in demented elderly patients.


