Abstract
The ventral lateral neurons (LNvs) of adult Drosophila brain express oscillating clock proteins and regulate circadian behavior. Whole cell current-clamp recordings of large LNvs in freshly dissected Drosophila whole brain preparations reveal two spontaneous activity patterns that correlate with two underlying patterns of oscillating membrane potential: tonic and burst firing of sodium-dependent action potentials. Resting membrane potential and spontaneous action potential firing are rapidly and reversibly regulated by acute changes in light intensity. The LNv electrophysiological light response is attenuated, but not abolished, in cryb mutant flies hypomorphic for the cell-autonomous light-sensing protein CRYPTOCHROME. The electrical activity of the large LNv is circadian regulated, as shown by significantly higher resting membrane potential and frequency of spontaneous action potential firing rate and burst firing pattern during circadian subjective day relative to subjective night. The circadian regulation of membrane potential, spontaneous action potential firing frequency, and pattern of Drosophila large LNvs closely resemble mammalian circadian neuron electrical characteristics, suggesting a general evolutionary conservation of both physiological and molecular oscillator mechanisms in pacemaker neurons.
INTRODUCTION
Circadian clocks in animals synchronize the timing of physiological and behavioral events to environmental cycles of day and night. Before the molecular-genetic characterization of clock genes in Drosophila, circadian rhythms of spontaneous action potential firing and membrane potential were defining features of pacemaker neurons in vertebrates and invertebrates (Colwell 2000; De Jeu and Pennartz 2002; Green and Gillette 1982; Herzog et al. 1998; Ikeda et al. 2003; Inouye and Kawamura 1979; Itri et al. 2005; Kuhlman et al. 2003; Liu et al. 1997; Michel et al. 1993; Nakamura et al. 2002; Pennartz et al. 2002; Quintero et al. 2003; Schwartz et al. 1987). Light, the primary environmental cue that entrains the circadian clock and behavior, is transduced in Drosophila pacemaker neurons cell-autonomously by the blue light—sensing protein CRYPTOCHROME (CRY) and by light-driven synaptic inputs (Emery et al. 1998; Helfrich-Förster et al. 2001; Stanewsky et al. 1998). Light sensitivity may be a general property of pacemaker neurons because most mammalian circadian neurons located in the suprachiasmatic nucleus (SCN) acutely alter their spontaneous action potential firing rate in response to changes in illumination (Meijer et al. 1986).
The molecular components of the circadian clock, including PERIOD (PER) and TIMELESS (TIM), were described first using the model organism Drosophila melanogaster (reviewed in Hall 2005; Konopka and Benzer 1971) and their detailed characterization has dominated Drosophila circadian biology for decades. Due to technical difficulty, neurophysiological characterization of Drosophila pacemaker neurons has lagged behind our molecular understanding of the circadian clock. However, this has begun to change, first by molecular genetic analysis of transgenic flies that express modified ion channels in pacemaker neurons and ion channel mutant flies (de la Paz Fernandez et al. 2007; Lear et al. 2005; Nitabach et al. 2002, 2005b, 2006). This work was followed more recently by direct patch-clamp analysis of Drosophila pacemaker neurons along with single-cell fills that permitted an unprecedented level of morphological detail of single large ventral lateral neurons (LNvs), although this study did not depict spontaneous action potentials in the large LNvs (Park and Griffith 2006).
Whether large LNvs are bona fide pacemaker neurons is controversial (Helfrich-Förster 1998; Lin et al. 2004; Shafer et al. 2002; Yang and Sehgal 2001). However, circadian molecular oscillation of tim RNA persists in large LNvs after 8 days of constant darkness (Peng et al. 2003; Stoleru et al. 2004). Although there is a clear relationship between neuronal electrical properties and phases of oscillating clocks in mammals, circadian regulation of pacemaker neuron membrane potential and spontaneous action potential firing remain unclear for Drosophila. Because of the genetic amenability of the Drosophila circadian pacemaker circuit and analysis of underlying molecular and overt behavioral rhythmicity, we set out to characterize the spontaneous electrophysiological properties of the Drosophila circadian neurons, beginning with the large LNvs because of their greater physiological accessibility. We report that large LNvs fire spontaneous action potentials, are acutely light regulated, and their action potential firing rate and pattern are circadian regulated after weeks of constant darkness.
METHODS
Fly strains
All experiments unless specified were done on the line yw; pdfGal4; UAS-ΔdORK-NC1-GFP/+. This line enables visualization of LNvs due to the membrane-delimited expression of a green fluorescent protein (GFP)—tagged nonconducting Drosophila open rectifier potassium channel (ΔdORK-NC1) using the UAS-Gal4 driver system as described in Nitabach et al. (2002). Since ΔdORK-NC1 is nonconducting and does not act as a dominant-negative subunit, it does not modify the electrical activity of pdf-positive cells (Nitabach et al. 2002). To study the electrophysiological properties of the large LNvs in the whole brain we used the general methods described previously (Gu and O’Dowd 2006). Cold temperature acts as a rapidly reversible anesthesia for flies. With the exception of constant darkness (DD) experiments, all initial current-clamp recording experiments were performed on flies maintained under 12:12-h light/dark (LD) cycles. Flies were killed during a wide 7-h “daytime” window: Zeitgeber Time (ZT) 1 to ZT8 (where ZT0 denotes time at which lights turn on under LD cycles). Whole brains were dissected from flies immobilized by placing the fly vial on ice. The dissected whole brain included the photoreceptors, which allowed us to test for light responsiveness of the large LNvs. Brains were dissected under light intensity of about 4–7 klux in a solution containing 20 units/ml papain with 1 mM L-cysteine to partially activate the enzyme, dissolved in standard external solution. Dissected whole brains were then placed into an RC-26 perfusion chamber (Warner Instruments, Hamden, CT) and secured using a nylon-fiber holder. The chamber temperature (24 ± 0.5°C) was controlled and continuously monitored using a CL-100 system (Warner Instruments). All recordings were made at a recording chamber light level of 0.1–1 klux unless indicated otherwise. Light level was determined using an LI-250A light meter (LI-COR, Lincoln, NE) using the photometric sensor. An Olympus BX51WI upright fluorescent microscope platform with a mechanical XY stage was used for most patch-clamp recordings (New Jersey/New York Scientific, Middlebush, NJ). Glass patch pipettes (10-MΩ resistance) were fabricated using a Narishige PP-83 two-step gravity puller. Patch pipettes were directed to GFP-labeled large LNv neurons using a Sutter MP 285 micromanipulator. Signals were measured using an Axopatch 200B patch-clamp amplifier (Molecular Devices/Axon Instruments, Sunnyvale, CA) with pClamp8 Clampex software and a Digidata 1322A 16-bit data acquisition board (Molecular Devices/Axon Instruments). All electrical signals were treated through a standard 5-kHz low-pass Bessel filter. Cell-attached patch configuration was established by a gentle negative pressure on the pipette holder; subsequently stronger negative pressure pulses were applied to break through the membrane to obtain whole cell configuration. Once formed, the whole cell recording configuration was stable for ≤30 min as determined by membrane potential stability. Standard external bath solution consisted of (in mM) 101 NaCl, 1 CaCl2, 4 MgCl2, 3 KCl, 5 glucose, 1.25 NaH2PO4, and 20.7 NaHCO3, with osmolality 250 mOsm and a pH of 7.2, aerated by a gas mixture of 95% O2-5% CO2; internal solution consisted of (in mM) 102 K-gluconate, 0.085 CaCl2, 1.7 MgCl2, 17 NaCl, 0.94 EGTA, 8.5 HEPES, with an osmolality of 235 mOsm and a pH of 7.2 (Gu and O’Dowd 2006). The liquid junction potential for this preparation was measured and found to be -5 mV and subtracted from all measurements of membrane potential. Analysis of electrophysiological data was performed using pClamp8 ClampFit (Molecular Devices/Axon Instruments) and Matlab software (The MathWorks, Natick, MA). The whole cell capacitance and input resistance were estimated by measuring the area under the capacitative current and the steady-state current generated by a 6-mV depolarizing voltage step from a holding potential of -80 mV immediately after breaking into the cell.
To determine whether large LNv electrical activity is circadian regulated, the locomotor activity of large numbers of flies was monitored in constant darkness for 15 days before being used for electrophysiological analysis. Individual flies were removed from the incubator under low-intensity far red light (to prevent phase shifts of other flies; Suri et al. 1998) at circadian sampling times of CT1, CT6, CT11, and CT18. Although, in principle, flies can entrain to far red light, this requires repeated and longer-exposure and greater-intensity far red light than that used in our experiments (Helfrich-Förster et al. 2002; Klarsfeld et al. 2004). Remaining flies in behavioral monitors showed no evidence of phase shifting following low-intensity far red light exposure. Traces were low-pass filtered using the standard three-point boxcar method and leak current corrected. Statistical analysis of data was performed with Statistica software (StatSoft, Tulsa, OK). Action potential firing rate was first determined by hand count of spikes over the duration of record, and then divided by the time of duration of the record. Spectral analyses of action potential firing and oscillations in membrane resting potential were analyzed by Fourier analysis and Lomb—Scargle time-series analysis using Clocklab analysis software (Actimetrics, Wilmette, IL) with traces that were low-pass filtered by resampling data at 5-ms intervals. The resultant time series was analyzed using Fourier and Lomb—Scargle analyses with a significance level of ≤0.01 used for all hypothesis and time-series tests. The mathematical algorithm used in Lomb—Scargle analysis detects periodic oscillation patterns in a time series. This method effectively detects regular oscillations in resting potential that underlie regular firing of action potentials <3.3 Hz (as determined by the filtering cutoff, which in turn was based on the mean and variance of earlier hand counts of the tonic firing action potentials). The Clocklab software was modified to adopt the timescale and sampling bins of our experiments. Significant patterns obtained from the Lomb—Scargle analysis were also detected in the Fourier analysis and were close to periodicities estimated by hand counts. Lomb—Scargle analysis, which has been used extensively to detect circadian patterns in time-series data, reliably detects frequencies that make statistically significant contribution. For comparison of the action potential firing patterns statistical significance for difference was determined using ANOVA.
RESULTS
Large LNvs fire spontaneous tonic and burst pattern action potentials
To determine the electrophysiological properties of the circadian pacemaker neurons of adult Drosophila melanogaster, we recorded from neurons in acutely dissected adult Drosophila whole brain preparations with intact photoreceptors. Initial current-clamp recording experiments were performed on flies maintained under 12:12-h light/dark (LD) cycles. Flies were killed during a wide 7-h “daytime” window: ZT1 to ZT8 (ZT0 denotes time at which lights turn on in the LD cycles). The large LNvs are close to the brain surface and are easily accessible for electrophysiological measurements. Therefore all whole cell recordings in this study are from the large LNv, the identity in some cases verified by post hoc analysis using neurobiocytin cell fills using the method described previously (Wilson et al. 2004; data not shown).
Large LNvs recorded in whole cell current-clamp mode fire spontaneous action potentials in the absence of injected holding current. Two distinct patterns of spontaneous action potential firing activity are seen during the day in large LNvs: tonic firing (9/13 cells recorded) and burst firing (4/13) (Fig. 1, A-D). Although most neurons exhibit one or the other firing pattern over the duration of the recording, short-duration spontaneous alternations between tonic and burst firing can be observed in single LNv neurons (data not shown). For the 9 large LNv neurons that exhibit predominantly tonic firing, 3 of these neurons show episodes of burst firing of ≤10-s duration. Similarly for the 4 large LNv neurons that exhibit predominantly burst firing, one of these cells exhibited several short stretches of tonic firing that lasted ≤10 s. This shows that the firing mode is state dependent rather than neuron subtype specific.
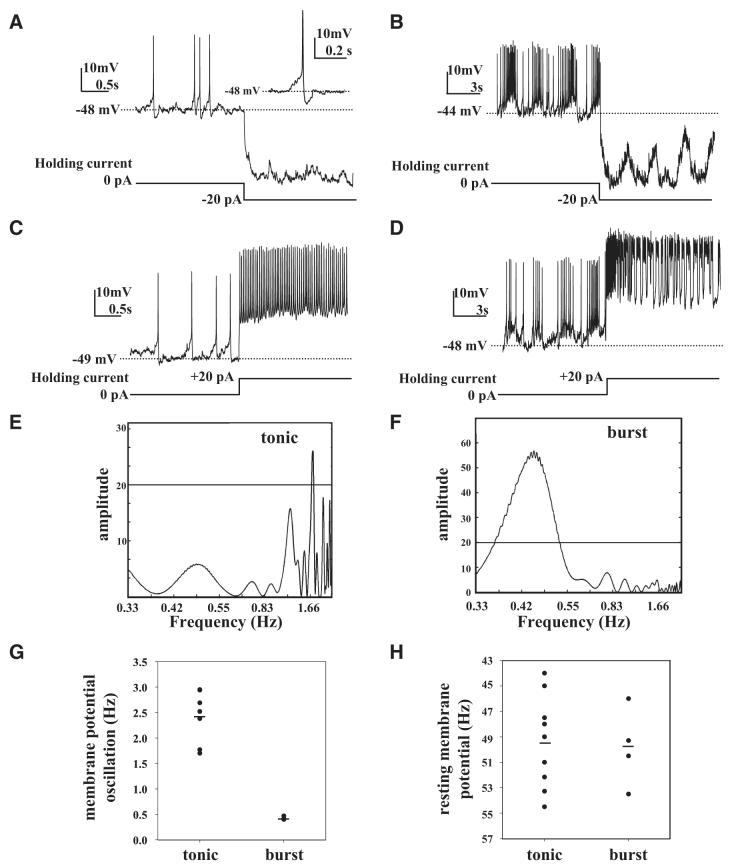
Fig. 1.
Spontaneous tonic and burst firing action potentials of large ventral lateral (LNv) pacemaker neurons. A: representative trace of spontaneous action potentials measured by whole cell recording from a large LNv of freshly dissected brain from an adult fly showing tonic firing pattern. Spontaneous changes in membrane potential were measured in current-clamp configuration with 0-pA holding current. Hyperpolarizing current injection (-20 pA) step from 0-pA holding current blocks spontaneous firing. At 0 holding current spontaneous action potentials (APs) show gradual depolarization approaching spike threshold, then a depolarizing spike followed by repolarization and afterhyperpolarization (inset: detail of single tonic AP). Tonic firing large LNv resting membrane potential is -49 ± 1 mV, n = 9. B: representative traces of large LNv spontaneous exhibiting burst pattern of AP firing measured in current-clamp configuration with 0-pA holding current followed by hyperpolarization (-20 pA) that blocks AP firing but not membrane potential oscillations. C: depolarizing current injection (+20 pA) step from 0 pA holding current increases tonic firing AP firing rate. D: depolarizing (+20 pA) current injection increases firing rate during burst firing. Mean resting membrane potential during burst firing is -50 ± 2 mV (mean ± SE, n = 4). E and F: representative periodograms obtained by Lomb—Scargle time-series analysis during tonic firing showing a single statistically significant period with frequency of 1.76 Hz (frequency range for tonic firing is 1.7 to 3.0 Hz, n = 9) and burst firing that shows a significant spectral peak around 0.45 Hz (frequency range for burst firing is 0.4 to 0.47 Hz, n = 4). During burst firing large LNvs exhibit low-frequency (0.49 ± 0.07 Hz, n = 4), high-amplitude (7 ± 1 mV, n = 4) slow oscillations in membrane potential that underlie the burst firing. G: scatterplot of frequency of oscillation in membrane potential as measured by Lomb—Scargle analysis during tonic and burst firing modes showing a higher range during tonic firing and no frequency overlap between tonic and burst firing modes. H: mean resting membrane potential during burst firing is -50 ± 2 mV (mean ± SE, n = 4), which does not differ significantly from tonic firing mode, as shown by scatterplot comparison.
In the tonic firing class, individual large LNv action potentials reach firing threshold at the crest of membrane depolarization, typically initiating between -40 and -50 mV with a rapidly depolarized spike (Fig. 1, A and C). Membrane repolarization is followed by monophasic afterhyperpolarization (AHP). Current injection of a 20-pA hyperpolarizing current step from zero holding current blocks the spontaneous tonic action potentials (Fig. 1A), whereas injection of a 20-pA depolarizing current step from zero holding current increases tonic firing frequency (Fig. 1C). For tonic firing pattern mode, the mean resting membrane potential is -49 ± 1 mV (mean ± SE, n = 9) and the mean firing frequency is 1.57 ± 0.24 Hz (mean ± SE, n = 9). AHP (Fig. 1, A and C, see inset in Fig. 1A for more detail) is readily observed during tonic firing mode with amplitude of 5 ± 1 mV (mean ± SE, n = 9). In contrast AHP was not reliably measurable during burst firing.
The spikes of bursting action potentials occur on the crests of large-amplitude slow oscillations of large LNv membrane potential (Fig. 1, B and D). Injection of a 20-pA hyperpolarizing current step from zero holding current abolishes the spontaneous burst action potentials but underlying large-amplitude slow oscillations of membrane potential robustly persist in the presence of hyperpolarizing current injections (Fig. 1B). Injection of a 20-pA depolarizing current in a step from zero holding current increases burst firing frequency (Fig. 1D). The frequency of slow oscillation in burst firing mode is 0.49 ± 0.07 Hz (mean ± SE, n = 4) and the mean amplitude of the large LNv slow oscillation during burst firing is 7 ± 1 mV (mean ± SE, n = 4). The mean resting membrane potential of large LNvs, while in burst firing mode, is -50 ± 2 mV (mean ± SE, n = 4). Mean firing frequency during burst firing is significantly higher compared with tonic firing mode at 3.24 ± 0.89 Hz (t-test, P < 0.01). The mean whole cell capacitance and input resistance estimated from four cells is 12 ± 2 pF and 305 ± 30 MΩ (means ± SE, n = 4), respectively.
Large LNv membrane potential slow oscillation was isolated and quantified in low-pass filtered current-clamp records during both tonic and burst firing modes using Lomb—Scargle analysis, a mathematical algorithm used to detect periodic patterns across different time series (the filtering step was done to exclude high-frequency values from the action potentials themselves). This analysis effectively detects regular oscillations in resting potential that underlie regular firing of action potentials (<3.3 Hz, as determined by the filtering cutoff, which in turn was based on the mean and variance of earlier hand counts of large LNv firing action potentials). Representative Lomb—Scargle time-series analysis records are shown for an individual large LNv exhibiting tonic firing with a significant peak of resting potential oscillation at 1.76 Hz (Fig. 1E) and an individual large LNv in burst firing mode that exhibits a single significant peak at 0.43 Hz (Fig. 1F). During tonic firing mode, neurons exhibit a predominant regular oscillating frequency in membrane potential in the range of 1.7 to 3.0 Hz (Fig. 1G, n = 9). These values closely match “hand-counted” estimates of frequency of small-amplitude oscillation for membrane potential during tonic firing mode. The values for actual tonic spike firing frequency for each recorded cell are lower than those for the frequency of membrane potential oscillation, reflecting that numerous failures to fire occur because not all peaks of oscillating membrane potential result in action potential spikes. Lomb—Scargle time-series analysis indicates that during burst firing mode, significant large-amplitude oscillations in membrane potential in the range of 0.4 to 0.47 Hz (n = 4) are seen. These values are in close agreement with hand-counted estimates of the large-amplitude oscillation frequency of membrane potential during burst firing. The summary of large LNv membrane potential oscillation shows that there is no overlap in oscillation frequency between burst firing and tonic firing modes (Fig. 1G). In contrast to their differences in firing rate and oscillation frequency, the mean membrane potential during tonic and burst firing modes does not significantly differ (Fig. 1H).
Large LNv spontaneous tonic and burst pattern action potentials are abolished by treatment with pharmacological blockers of voltage-gated sodium and calcium channels
The observed threshold of activation of spontaneous action potential firing for large LNv neurons is close to the reported value for the Drosophila voltage-gated sodium channel para (O’Dowd and Aldrich 1988; Wicher et al. 2001), suggesting that the depolarizing phase of the large LNv action potential is mediated by the opening of voltage-dependent sodium channels. Bath application of 100 nM tetrodotoxin (TTX, a voltage-gated sodium channel blocker) rapidly abolishes spontaneous action potential firing in large LNvs (Fig. 2, A-C and Supplemental Fig. S1, A1-3).1 In contrast to the profound suppression of spontaneous action potential firing, 100 nM TTX treatment has no effect on mean resting potential (Fig. 2, A-C and Supplemental Fig. S1A1-3). TTX suppression of spontaneous action potential firing is relatively resistant to washout, but two of six cells tested showed discernible washout (Fig. 2C). The poor washout of TTX is consistent with the high affinity of this blocker (Gitschier et al. 1980). TTX treatment also dampens low-frequency membrane oscillations (Fig. 2B and Supplemental Fig. S1A2). These data demonstrate that the spontaneous action potentials require flux of sodium through voltage-gated channels and that sodium channel activity also contributes to generation of slow-wave oscillations (Fig. 2, A and B, Supplemental Fig. S1A1-3).
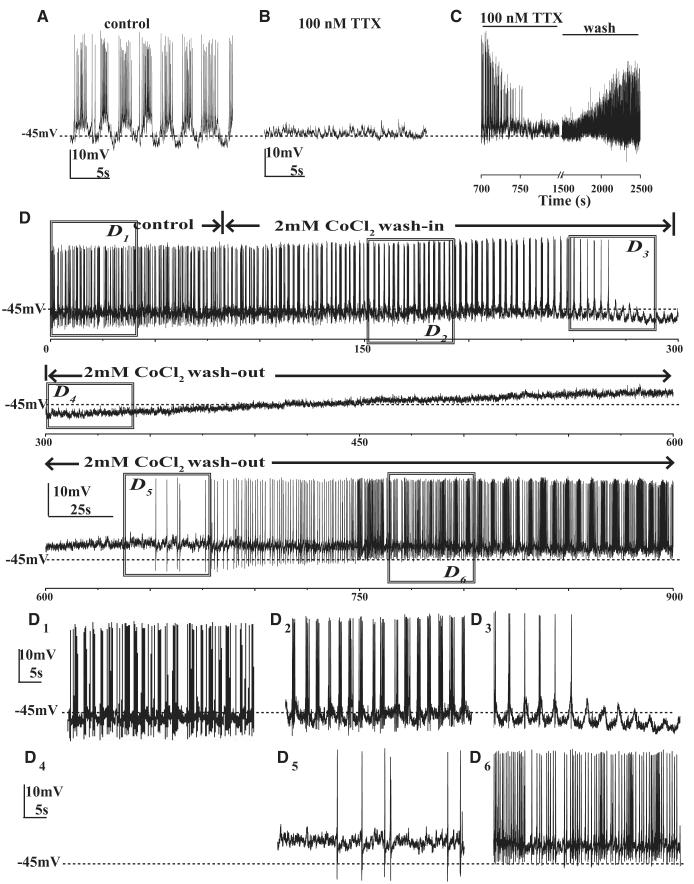
Fig. 2.
Voltage-gated sodium and calcium channel blockers abolish the spontaneous AP firing of large LNvs. A: firing of a representative large LNv measured in whole cell current clamp at 0-pA holding membrane current in control perfusion solution. B: recording from the same cell in the presence of 100 nM tetrodotoxin (TTX). The application of 100 nM TTX completely abolishes large LNv spontaneous APs (n = 6). C: example trace showing recovery of spontaneous firing after washout of TTX (2/6 cells). D: representative long-duration whole cell current-clamp recording of one large LNv at 0-pA holding current in control solution during washin and washout of 2 mM CoCl2 shows state modulation of a large LNv showing burst firing becoming tonic and then silent with 2 mM CoCl2 washin followed by washout recovery to tonic and then burst firing pattern (x-axis shows time in seconds, n = 6). D1–6: detail of traces sampled from the above trace for 20 s. D1: burst firing before CoCl2 washin, (D2) initial broadening of slow-wave oscillation and crests of firing in response to CoCl2 washin, (D3) during transition from tonic to silent, (D4) silent phase, (D5) transition from silent to tonic showing recovery of large LNv spontaneous firing after CoCl2 washout, and (D6) transition from tonic to burst firing showing complete washout.
To determine whether voltage-gated calcium channels also contribute to spontaneous (and evoked) action potentials and or low-frequency membrane potential oscillations, the effect of bath-applied 2 mM CoCl2 on large LNv activity was tested. We subsequently measured whether the effects of 2 mM CoCl2 on large LNv activity are reversible by washout with fresh bath solution. Five of six cells examined in whole cell recordings were initially burst firing cells and one of the six cells was tonic firing before application of CoCl2. All burst firing cells initially show broader crests of firing on slower-frequency membrane potential oscillations along with more depolarized membrane potential in response to CoCl2 washin (n = 5; Fig. 2D; compare D1 and D3). All five cells then transitioned from slow burst firing to tonic firing (Fig. 2, D1 and D3). For two of the five initial burst-firing cells, this tonic firing pattern persisted throughout CoCl2 treatment. In the three other initial burst-firing cells, CoCl2 caused the cells to transition from tonic firing to silent (no firing) state (Fig. 2, D, D3, and D4). On washout of CoCl2, in the three cells that were silenced by CoCl2, spontaneous tonic firing appeared first followed by recovery of bursting pattern (Fig. 2, D and D5), whereas the two cells that remained at tonic firing during CoCl2 washin transitioned back to burst firing during the washout (Fig. 2, D and D6). Similarly, the single large LNv that was initially tonic firing became silent in response to CoCl2 washin followed by a recovery back to tonic firing following CoCl2 washout. These results indicate that the firing pattern in large LNv is state modulated, and that state transitions between firing patterns in response to CoCl2 washin and washout behave in the sequence: from burst firing to tonic firing to silent with CoCl2 washin and recovery from silent to tonic firing to burst firing with CoCl2 washout (in some cases, cells “stall” at intermediate transition states, but they do not appear to bypass any intermediate transition states).
CoCl2 application also dampens high-amplitude, slow-frequency oscillations in membrane potential that is especially apparent in burst firing cells (Fig. 2, D and D4). Three of six cells showed significant depolarization of membrane potential on CoCl2 application, whereas two others did not show consistent significant change during CoCl2 application and one cell was slightly hyperpolarized. The ability of some neurons to continue firing in the presence of 2 mM CoCl2 and the ability of those that were silenced by CoCl2 application to fire action potentials when injected with depolarizing current (Supplemental Fig. S2B) demonstrate that action potentials in large LNvs do not require activation of voltage-gated calcium channels. However, activity of these channels does modulate the firing pattern and firing rate of the neurons.
Rapid light-induced increase in large LNv resting membrane potential and spontaneous action potential firing rate is attenuated in cryb mutants
Light is the predominant environmental sensory cue for entrainment of the circadian clock. The Drosophila pacemaker neural circuit receives light input via synaptic inputs from ocular and extraocular photoreceptors as well as the cell-autonomous photopigment CRY (Emery et al. 1998; Helfrich-Förster et al. 2001; Stanewsky et al. 1998). Because our recording preparation had intact photoreceptors, we tested the effect of light exposure on the electrophysiological properties of the large LNv in control versus cryb mutant Drosophila whole brains dissected at midday (ZT3—ZT6.5) from flies maintained in 12:12-h LD cycles. To test comparable genetic backgrounds, the control and cryb flies both express the membrane delimited GFP marker on the second chromosome, control (yw; pdfGal4/UAS-dORK-NC1-GFP/+); cry-mutant (yw; pdfGal4/UAS-dORK-NC1-GFP; crybss). The other flies used in this study express the membrane-delimited GFP marker on the third chromosome. There are no measurable electro-physiological or behavioral differences between control flies expressing the GFP marker on the second versus third chromosome.
Whole cell current-clamp recordings were made while alternating between darkness (light intensity = 0 klux, dark upper bar; Fig. 3A) and lights-on (light intensity = 7–10 klux, white upper bar; Fig. 3A). Light exposure rapidly increases the spontaneous action potential firing rate of large LNvs (Fig. 3, A-D). Cessation of light causes an equally rapid reversible decrease in the spontaneous action potential firing rate of large LNvs (Fig. 3A) as shown in a representative 4-min-long recording of spontaneous action potentials from a representative large LNv in response to alternating lights-off and lights-on. The light-induced increase and reversible decrease in large LNv spontaneous action potential firing rate occurred consistently up to 10 cycles of alternating lights-on and lights-off (≤20 min of recording). The previous “history” of acute changes in light exposure had no measurable effect on firing frequency as shown by the consistent increase in firing rate in response to lights-on and the consistent return to baseline firing on lights-off (Fig. 3A). Light-induced changes in large LNv firing parameters occurred rapidly (typically within 1–2 s after switching lights on or off), although delays ≤10 s for acute changes in firing rate following lights-on or lights-off were observed in one recording (out of seven). We observed robust 1.5-fold increases in firing rate when light levels were switched between darkness and 7–10 klux when measured during the day (Fig. 3, A-D). Although no measurable changes in neuronal firing rate occurred when light levels were switched between darkness and 0.1 klux when tested between early morning to late day, we cannot exclude the possibility that 0.1 klux may alter membrane electrical properties when measured during the night. Lights-on also evoked a rapid and significant depolarization of large LNv membrane potential (Fig. 3, A and E and F). Analysis comparing the light-induced change in large LNv firing rate and resting potential showed that these two parameters are significantly correlated (product-moment correlation test, P < 0.05; correlation coefficient = 0.78). Large LNv electrophysiological parameters recorded are: firing frequency for lights-off is 1.38 ± 0.32 Hz and for lights-on is 2.98 ± 0.59 Hz (Fig. 3C; mean ± SE, n = 7, significantly different at P < 0.01, paired t-test); resting membrane potential for lights-off is -51 ± 1 mV and for lights-on is -49 ± 1 mV (Fig. 3E, mean ± SE, n = 7, lights-off and -on populations are significantly different at P < 0.001, paired t-test); there is no change in AHP amplitude, lights-off (5 ± 1 mV), and lights-on (5 ± 1 mV, mean ± SE, n = 7). Although all wild-type large LNvs recorded increased their firing rate and depolarized resting membrane potential in response to lights-on, some neurons showed larger-magnitude response than that of others (Fig. 3, C and E). Recordings of the wild-type LNv neurons tested for light responsiveness were made between ZT3 and ZT6.5. To determine whether light responsiveness varies with time of day within this interval of time we estimated correlation coefficient between time of measurement and firing frequency and resting membrane potential. The magnitude of the large LNv light response for both firing frequency and resting membrane potential varied with time of day. Recordings earlier in the day showed larger responses than those later in the day (control firing rate change in response to light vs. time of recording, correlation coefficient = 0.68; control resting membrane potential change in response to light vs. time of recording, correlation coefficient = 0.71). The preceding measurements included cells that showed tonic and burst firing and, in some cases, cells alternated between the two types of firing patterns within the duration of the dark phase or light exposure. Since temperature can also modulate circadian clock entrainment, we continuously monitored temperature in the recording chamber bath solution throughout all changes in light intensity to determine whether any observed electrophysiological changes were due to temperature artifacts; no change in the recording chamber temperature was detected (data not shown).
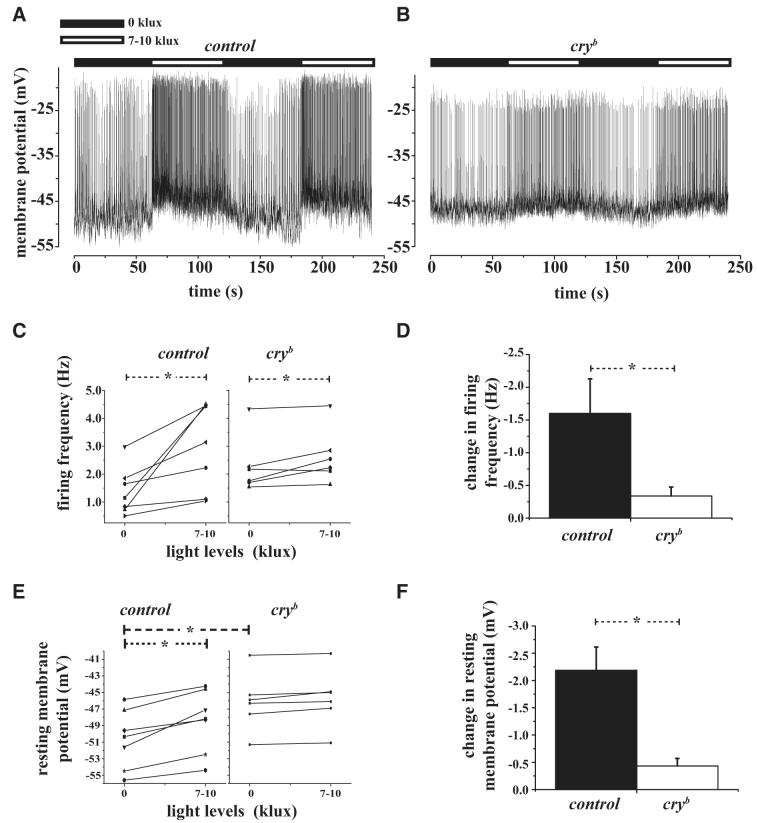
Fig. 3.
Spontaneous AP firing of large LNv increase rapidly in response to light in a CRYPTOCHROME (CRY)-dependent manner. A: representative large LNv whole cell current-clamp recording from control large LNv showing depolarized resting membrane potential and increased spontaneous AP firing rate within seconds in response to light increase from 0 to 7–10 klux (light levels indicated by the horizontal bars above each trace: black bar, lights-off; white bar, lights-on) where light levels were at 0 lux (off) during the 1st and 3rd min and increased to 7–10 klux (on) during the 2nd and 4th min of the recording. Control large LNv cells show depolarized resting membrane potential and increased spontaneous AP firing rate in response to light and hyperpolarized resting membrane potential and decreased spontaneous AP firing in response to the cessation of light. B: representative trace from cryb mutant flies recorded under similar condition as control. C: mean spontaneous AP firing frequency increases significantly from 0 to 7–10 klux light levels as determined by paired t-test for large LNv control (P = 0.01) and cryb mutant flies (P = 0.02). D: the light response is not detectable in cryb mutant large LNv cells because increase in spontaneous AP firing frequency is significantly higher for controls compared with cryb mutant flies (t-test, P = 0.03). E: large LNv resting membrane potential becomes significantly more depolarized in response to lights-on for controls (paired t-test P = 0.001) but not in cryb mutant flies. The baseline resting membrane potential in cryb mutant large LNv at 0 klux is significantly greater than controls. F: the relative change in resting membrane potential is significantly greater in controls compared with cryb mutant flies (t-test, P = 0.002).
Drosophila entrain to light by both cell-autonomous activation of the CRY blue light photopigment and by light-driven synaptic inputs from ocular and extraocular photoreceptors (Helfrich-Förster et al. 2001). To determine whether CRY protein mediates the ability of large LNvs to respond to light, whole cell current-clamp recordings during ZT3—ZT7 were compared between control and cryb mutant flies that were maintained in 12:12-h LD cycles. Although control flies showed robust increase in large LNv firing rate and resting membrane potential in response to light, such light responses were significantly blunted (but not completely abolished) in recordings prepared from cryb mutant flies (Fig. 3, B-F). Furthermore, the response kinetics of action potential firing rate to both lights-on and lights-off was weaker in cryb mutant flies (Fig. 3B). In summary, the electrophysiological parameters of cryb mutants are: firing frequency is 2.29 ± 0.42 Hz for lights-off and 2.63 ± 0.39 Hz for lights-on (Fig. 3, C and D, means ± SE, n = 6, significantly different, P < 0.05, paired t-test); for resting membrane potential is -46 ± 1 mV for both lights-off and lights-on (Fig. 3, E and F, means ± SE, n = 6, lights-off and -on conditions are not significantly different, P < 0.05, paired t-test); and AHP amplitude is 4 ± 1 mV for lights-off and 3 ± 1 mV for lights-on (means ± SE, n = 6, not significantly different, paired t-test). Curiously, for light response recordings made between ZT4 and ZT7 in the large LNv of cryb flies, larger-amplitude light responses in firing rate tended to occur later in the day (cryb firing rate change in response to light vs. time of recording, correlation coefficient = -0.62).
Long-term circadian regulation of electrophysiological properties in large LNvs
Circadian variation of membrane electrophysiological properties is a hallmark of pacemaker neurons (Green and Gillette 1982; Inouye and Kawamura 1979; Michel et al. 1993; Schwartz et al. 1987). To determine whether spontaneous action potential firing and resting potential are circadian regulated in the large LNv, patch-clamp recordings were made at four circadian time points spaced about 6 h apart from whole brains dissected from adult flies that had been maintained for 15 days in constant darkness. Adult Drosophila males were placed in glass tubes for behavioral monitoring and entrained in 12:12-h LD for 10 days in an incubator maintained at a constant temperature of 25°C; lights were then switched off and the flies were maintained in constant darkness and temperature for the next 15 days. Each time individual flies crossed an infrared beam across the middle of the glass tube, a record was collected. Such counts were binned for 15-min intervals and a behavioral time series was generated for each fly. Representative behavioral actograms show typical behavioral results for this protocol, as indicated by lights-on (white bar) and lights-off (black bar) shown above the activity plot for both LD and constant darkness (Fig. 4A, double plotted to permit better visualization of the circadian behavioral rhythm). The flies used for these experiments “free run” in constant darkness with a circadian behavioral period of ≥24 h (Fig. 4A). Thus after 15 days, the phase of circadian time cannot be accurately estimated based solely on the previous LD entrainment schedule. To overcome this, we monitored the circadian locomotor behavior of each individual fly to determine its precise circadian phase in real time, for which the offset of behavioral activity reflects circadian time at 12 h (CT12). Individual flies were removed from the incubator under low-intensity far red light [to prevent light-induced phase shifts of other flies (Suri et al. 1998)] at circadian sampling times of CT1 (beginning of subjective day), CT6 (mid-subjective day), CT11 (just before end of subjective day), and CT18 (mid-subjective evening) for immediate rapid brain dissection and patch-clamp recording under standard conditions (Fig. 4A, as indicated by the black arrows on the bottom of each representative behavioral actogram).
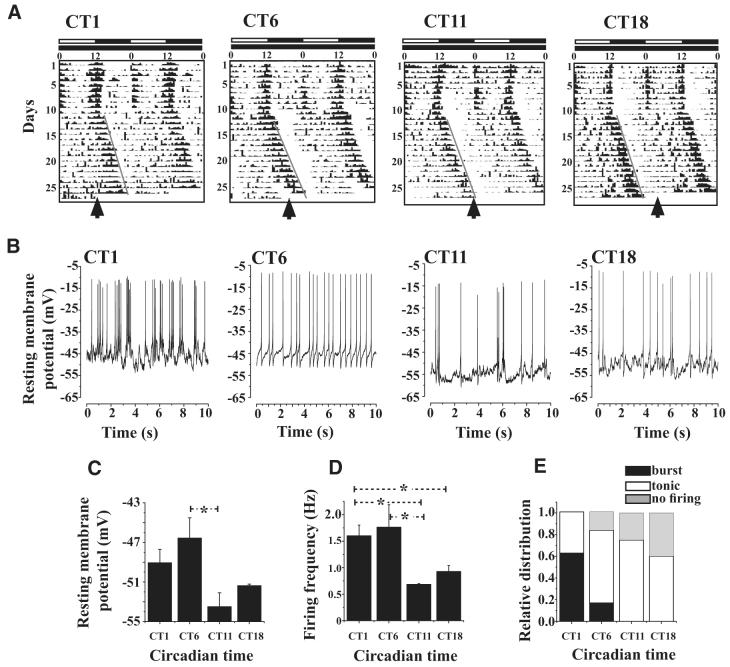
Fig. 4.
Electrophysiological properties of large LNv cells exhibit circadian oscillation. A: representative locomotor activity/rest records of individual flies maintained under 12 h/12 h light/dark (LD) cycle for 10 days following which they were transferred to constant darkness (DD). During LD, lights remained on from 0 to 12 h as indicated by the white horizontal bar and remained off for the next 12 h as indicated by the black horizontal bar. The activity/rest recordis double plotted to enable better visualization of the circadian rhythm. Locomotor activity synchronizes to the imposed LD cycle as indicated by the increased levels of activity around lights-on and -off and relative quiescence during night and midday. After release into DD, the activity/rest rhythms “free-runs” with a period slightly longer than 24 h, reflecting the endogenous period of circadian clock of each individual fly. By convention the offset of activity is referred to as circadian time CT12 and was used as a phase marker in our experiments. Flies were sampled at 4 distinct phases, CT1, 6, 11, and 18, based on the predicted offset of activity after 15 days of DD as indicated by an arrow. B: representative current-clamp traces showing that large LNv spontaneous AP firing frequency and resting membrane potential values are higher during subjective day compared with night phases (sample sizes for CT1, 6, 11, and 18 were 8, 6, 4, and 5 brains, respectively). All recordings were made under identical low-light conditions of 0.1 klux. C: mean resting membrane potential is highest at CT6 and lowest at CT11 (one-way ANOVA, P = 0.0246; * indicates significant differences, Tukey’s HSD, CT6 vs. CT11, P = 0.02). D: large LNv firing frequency is significantly highest at CT6 and least at CT11 (one-way ANOVA, P = 0.045; * indicates significant differences, Fisher’s least-significant difference test; P < 0.05; CT6 vs. CT11, P = 0.025; CT6 vs. CT18, P = 0.04; CT1 vs. CT11, P = 0.04). E: burst firing pattern predominates at CT1. Patterns of firing were classified as tonic or bursting based on the frequencies obtained using Lomb—Scargle periodogram analysis and verified by visual examination of the traces. No burst firing is observed at CT18. The greatest number of instances of cells that do not fire spontaneous APs occur at CT18 (although nonfiring large LNv mean resting potentials recorded at this time point are within the normal range relative to tonic firing mode).
Whole cell current-clamp recordings of large LNvs revealed a striking pattern of circadian regulation of spontaneous action potential firing. Large LNv spontaneous action potential firing rate was significantly higher during the circadian subjective day than that during the circadian subjective evening (Fig. 4, B and D, ANOVA, P = 0.045; Fisher’s least-significant difference test: CT6 vs. CT11, P = 0.025; CT6 vs. CT18, P = 0.04; CT1 vs. CT11, P = 0.04). Furthermore, the pattern of spontaneous action potential firing was circadian regulated, as shown by a high proportion of burst firing early in the subjective day (CT1), which then progressively decreased by mid-subjective day (CT6), and was absent by the end of the subjective day through the mid-subjective evening (Fig. 4, B and E). Consistent with these results, large LNv mean resting potential was significantly higher during the early and mid-subjective day, then decreasing to more hyperpolarized potentials during the end of subjective day through the subjective evening (Fig. 4, B and C, ANOVA, P = 0.0246; Tukey’s test, CT6 vs. CT11, P = 0.02).
DISCUSSION
The circadian pacemaker circuit in Drosophila controls circadian locomotor behavior and other circadian regulated physiological processes in the fly (Hall 2005). Until recently, the structural and functional features of this circuit have been defined almost entirely by molecular genetic studies—and physiological features of the pacemaker neurons such as spontaneous action potential firing could only be inferred. Our earlier results showing that electrical silencing of the LNv stops the free-running circadian clock predicts that such electrical activity is a critical component of the free-running clock (Nitabach et al. 2002, 2005a,b). These results predict that electrical activity fluctuates in a circadian manner in the LNv. Patch-clamp recordings of pacemaker neurons in the Drosophila isolated whole brain preparation verify this prediction and allow mechanistic access (e.g., future studies that identify native currents and their functional contributions) to circadian-regulated intrinsic spontaneous neuronal activity. The Drosophila isolated whole brain preparation is similar in many ways to the widely used mammalian brain slice preparation for electrophysiological recording, but with a number of additional notable advantages over traditional mammalian brain slice preparations. The Drosophila whole brain preparation with intact photoreceptors retains a much larger extent of intact neural circuitry than does a typical mammalian brain slice. Sensory processing can be observed, as demonstrated earlier for light-driven increase in activity in the large LNv (Fig. 3). These results suggest that this general method can be applied to study other visually driven neurons and circuits in the fly brain. In addition to excellent physiological and pharmacological accessibility afforded by the Drosophila whole brain preparation (Gu and O’Dowd 2006), the preparation also facilitates the powerful combination of electrophysiology and genetics, as shown by the analysis of the large LNv light response in control versus mutant cryb flies (Fig. 3). Previous genetic studies indicate that light modulates long-term entrainment in pacemaker neurons both by synaptic inputs downstream from ocular and extraocular photoreceptors and by CRY (Helfrich-Förster et al. 2001). Acute light exposure evokes a rapid (within 1–10 s) increase in resting membrane depolarization and action potential firing rate in the large LNv neurons (Fig. 3), whereas degradation of TIM can occur within 1–10 min of light exposure (Hunter-Ensor et al. 1996; Yang et al. 1998). In mammals, light causes a rapid transient increase in spike-firing rate in SCN neurons (Meijer et al. 1986, 1998). Exposure to light in light-sensitive SCN neurons evokes immediate-early gene induction (including mPer1 and mPer2) peaking between 30 and 60 min after light exposure (Kornhauser et al. 1996; Shearman et al. 1997). This is followed by longer-lasting changes in SCN neuronal electrical activity that appear between 3 and 5 h after light exposure (Kuhlman and McMahon 2006; Kuhlman et al. 2003). Although there is a brief 5-min exposure to light for the preparation of the Drosophila whole brains dissected after 15 days in constant darkness, the activity of the large LNv exhibits long-term circadian regulation. Furthermore, the light responsiveness of the large LNv is stable over time and shows no signs of chronic changes in response to light for the timescale of the light-on/lights-off experiments (Fig. 3). We note that brief exposure to low temperature, light, dissection of the brain from the body or exposure to recording bath solution prior to the electrophysiological measurements may all cause instantaneous phase shifts in circadian pacemakers. Therefore the observed waveform and the timing of the peak in the rhythms of firing frequency and resting membrane potential may not be identical to the physiological state of the unperturbed circadian pacemaker. The pattern of light response in the LNv suggests that genetic manipulations that alter the balance between firing rate or membrane potential in these neurons between day and night could contribute to a shift in the relative level of locomotor behavior between day and night. We predict that genetic manipulations that increase depolarization at night will correspondingly lead to greater locomotor activity at night.
The rapid acute light response of the large LNv is attenuated in cryb mutant flies (Fig. 3) and preliminary data suggest the immediate loss of the light response following acute treatment with the nicotinic acetylcholine receptor antagonist tubocurare (data not shown). These results raise the possibility that the large LNv light response is mediated dually by CRY and light-driven synaptic inputs. We attempted to address this question experimentally by patch-clamp recordings of the large LNv in glass60j mutant flies—this mutation eliminates all ocular and extraocular photoreceptors. However, for our initial efforts, we could not reliably patch the large LNv in the glass60j mutant flies (data not shown). Although we cannot rule out the contribution of light-driven synaptic inputs to the large LNv light response, CRY appears to make a large contribution based on the observation that the large LNv light response is severely attenuated in cryb flies (Fig. 3). The large LNvs appear to receive a direct projection from the Hofbauer—Buchner extraretinal photoreceptor eyelet (Helfrich-Förster et al. 2002). Although it is not difficult to imagine direct or multiorder light-driven synaptic inputs from extraocular and ocular photoreceptors evoking rapid changes in membrane potential and action potential firing rate in the large LNv, the mechanism of the observed functional contribution of CRY to the rapid light response of these pacemaker neurons is not obvious and could potentially occur through a number of very different mechanisms. CRY is expressed in the LNv and could mediate direct effects on one or more membrane channels or receptors through physical coupling of light-induced conformational changes of the CRY protein. Alternatively, because CRY has been shown to act as a transcriptional repressor in photoreceptors (Collins et al. 2006), decreased CRY activity could alter the expression of membrane proteins that underlie the rapid light response. Supporting this possibility, a previous report showed that the circadian rhythm in olfactory response is attenuated in cryb flies (Krishnan et al. 2001).
Large LNvs show robust circadian regulation of membrane potential and spontaneous action potential firing rate and pattern (Fig. 4). The general features of circadian-regulated spontaneous action potential firing in large LNvs are remarkably similar to those observed in mammalian SCN neurons. For both Drosophila and mammalian SCN pacemaker neurons during the circadian subjective day relative to the circadian subjective night, resting potential is depolarized, membrane potential oscillation amplitude is high, spike frequency is high, and action potential AHP is low (reviewed in Kuhlman and McMahon 2006; see Fig. 4). Based on the observed functional conservation of spontaneous action potential firing properties, the circadian-regulated ionic mechanisms occurring in pacemaker neurons are potentially conserved between Drosophila and mammals.
The magnitude of resting membrane potential difference between day and night is similar for Drosophila and mammalian pacemaker neurons (respectively, ∼7 and 10 mV; Fig. 4 and de Jeu et al. 1998). In contrast, a previous study reported that large LNvs exhibit hyperpolarized membrane potential in subjective day after 1 day of constant darkness (Park and Griffith 2006), whereas we report relative membrane potential depolarization in circadian subjective day after 15 days of constant darkness (Fig. 4). The reasons for these difference are not clear, although it is worth noting that the membrane potential values are -5 mV (or more) hyperpolarized in Park and Griffith (2006) relative to the membrane potential values reported herein, and that spontaneous action potential firing was not shown in the Park and Griffith (2006) study. Studies in mammalian pacemaker neurons tend to show similar electro-physiological parameters for day/night and subjective day/subjective night by comparison of recordings made under LD and DD conditions (Kuhlman and McMahon 2004, 2006). Along these lines, Park and Griffith (2006) reported that large LNvs are relatively depolarized in the day based on recordings made from flies kept in standard LD cycles—these results are in agreement with the results reported herein and with data from recordings from mammalian and other invertebrate circadian neurons. Neither we nor investigators studying the pacemaker neurons of other animals have yet determined the molecular identity of the potassium channels underlying the circadian-regulated basal resting conductance. Two-pore open-rectifier (dORK) potassium channels are possible candidates (reviewed in Kuhlman and McMahon 2006). Notably, constitutive transgenic expression of mutant high-conductance dORK potassium channels in Drosophila LNv neurons abolishes circadian locomotor behavioral rhythm and disrupts molecular PER and TIM cycling after several days in constant darkness (Nitabach et al. 2002, 2005b). Furthermore, expression of dORK channels in the LNv increases the amount of injected current required for evoked action potential firing (Park and Griffith 2006). Similarly, we found that dORK expression in the LNv lowers mean resting potential by about 15 mV below baseline resting potential values recorded in the day and abolishes spontaneous action potential firing (data not shown). Other potassium channels are candidates for modulation of circadian neuronal membrane potential and firing rate (Cloues and Sather 2003; Itri et al. 2005; Kuhlman and McMahon 2004; Michel et al. 1993; Panda et al. 2002).
Our results that pacemaker neuronal resting membrane potential is circadian regulated after 15 days of constant darkness—thus entirely clock driven—are in agreement with those of Kuhlman and McMahon (2004) who showed that basal potassium conductance circadian rhythm is robust in SCN neuronal recordings prepared from animals that had been housed for long periods of time in constant darkness. Thus clock-dependent regulation of resting membrane potential is conserved between Drosophila and mammals. In addition to setting membrane potential, potassium conductance via voltage-gated potassium channels contributes to action potential repolarization and modulates spike-firing frequency in circa-dian pacemaker neurons in mammals (Itri et al. 2005). Rapidly activating voltage-gated potassium channels are particularly important for maintaining high action potential firing frequencies (Itri et al. 2005). Although we do not yet know the molecular identity of the voltage-gated potassium channels underlying large LNv neuron potassium currents, previous work in our laboratory rules out contribution of the Shaker potassium channel by two independent lines of evidence: 1) there is no immune-positive signal in the LNv neurons using Shaker-specific antisera (Rogero and Tejedor 1995) and 2) expression of a dominant-negative Shaker subunit (Mosca et al. 2005) in the LNv neurons has no effect on circadian locomotor behavior or electrophysiological parameters (data not shown). The activation and inactivation kinetics of isolated potassium currents recorded in voltage-clamp mode from LNv are consistent with a mixture of Shab, Shal, and Shaw currents (data not shown).
High-amplitude oscillations in membrane potential are found predominantly in the day in the large LNv (Figs. 1 and 4). Mammalian SCN pacemaker neurons exhibit similar circadian-regulated oscillations in membrane potential mediated by L-type voltage-gated calcium channels (Pennartz et al. 2002). Calcium-mediated oscillations in membrane potential constitute one of the determinants of reaching spike threshold, although persistent sodium currents appear to underlie the primary ionic conductance responsible for spike initiation in mammalian SCN pacemaker neurons (Jackson et al. 2004; Kuhlman and McMahon 2006). Furthermore, we find that TTX treatment also blocks large-amplitude, slow-frequency oscillations in membrane potential (Fig. 3); thus it is unlikely that calcium is the sole charge carrier mediating membrane oscillations that underlie burst firing. In addition to its relatively minor contribution as a charge carrier, calcium has been proposed as a critical signal transducer in pacemaker cells coupling membrane events with the circadian clock (Colwell 2000; Hamasaka and Nassel 2006; Ikeda et al. 2003; reviewed by Imaizumi et al. 2007; Lundkvist et al. 2005; Nitabach et al. 2002). Circadian regulation of large LNvs shows transitions between predominantly high firing rates and burst firing pattern during the circadian day giving way to lower firing rates and tonic or silent neurons during the circadian night. Our results show that cobalt block (and washout of cobalt block) of voltage-gated calcium channels also leads to similar changes in large LNv firing rate and pattern. This suggests that modulation of calcium flux is a potential mechanism that may underlie circadian regulation of large LNv electrical activity.
Given that the circadian clock continues to cycle in electrically silenced LNv neurons in flies kept in LD cycles (Nitabach et al. 2002, 2005b), elevated LNv calcium levels is a likely component of the LNv physiologic light response. Further evidence for the importance of calcium signaling in pacemaker neurons is shown by the expression of both large- and small-conductance calcium-activated potassium channels in mammalian SCN pacemaker neurons (Cloues and Sather 2003). Expression of large-conductance calcium-activated potassium (BK) channel mRNA and functional currents is circadian regulated in mammals (Panda et al. 2002) with high levels of BK current peaking at night (Meredith et al. 2006; Pitts et al. 2006). Pharmacological or genetic disruption of mammalian BK channels attenuates the circadian rhythm in spike frequency. The most likely mechanism for BK channel contribution to regulation of action potential firing rate is modulation of action potential AHP. Consistent with these results in SCN, we observe lower-frequency action potential spike rates and, due to the paucity of burst firing, more robust AHP in large LNvs during subjective circadian night (Fig. 4). Recent genetic evidence in Drosophila implicates the fly mutant BK channel slowpoke for modulating output of the circadian pacemaker circuit (de la Paz Fernandez et al. 2007). Even though direct expression of slowpoke in LNv neurons could not be determined unambiguously, immunocytochemistry suggests that slowpoke may be expressed in pigment—dispersing factor (PDF)—positive neurites (de la Paz Fernandez et al. 2007).
Circadian-regulated neuronal electrical activity was traditionally used to define circadian function in pacemaker neurons until the characterization of oscillating molecular components of the circadian clock (Green and Gillette 1982; Inouye and Kawamura 1979; Michel et al. 1993; Schwartz et al. 1987). Although large LNvs do not appear to have sustained long-term molecular PER protein oscillation in constant darkness (Lin et al. 2004), these neurons do show robust sustained circadian rhythm for multiple parameters of electrical activity, including the firing frequency and tonic versus burst firing pattern of action potentials and the fluctuating pattern and absolute level of membrane potential (Fig. 4). The robust persistence of circadian-regulated oscillation of electrical activity in large LNv could be a consequence of cell—cell communication with other pacemaker neurons with more robust self-sustained oscillators (e.g., small LNvs). Alternatively, patch-clamp measurement of electrical activity could be a more sensitive assay for oscillator function than present immunocytochemical methods for measuring oscillation of clock protein cycling. In support of this, Peng et al. (2003) and Stoleru et al. (2004) reported that oscillations of tim gene RNA expression persists in large LNvs after prolonged darkness, suggesting that the circadian molecular machinery continues to operate in these neurons, although immunocytochemical data measuring clock protein oscillations suggest otherwise (Lin et al. 2004).
Anatomical studies suggest that large LNvs are important for coupling neurons of the pacemaker circuit between the two brain hemispheres. The large LNvs exhibit extensive cross-hemispheric projections (Helfrich-Förster and Homberg 1993; Park and Griffith 2006). The Drosophila mutant small optic lobe/sine oculis lacks normal cross-hemispheric connections between large LNvs (as well as disrupted connectivity between the LN and DN pacemaker groups) and these mutant flies show desynchronized locomotor behavioral rhythms (reviewed in Helfrich 1986; Helfrich-Förster 2002). The large LNvs express the neuropeptide PDF (Renn et al. 1999). Pigment-dispersing hormone (a peptide related to PDF) injection in the cockroach brain resets the phase of circadian locomotor behavioral rhythm and is proposed to couple the bilateral sides of the pacemaker circuit (Petri and Stengl 1997). For future studies, we will examine the electrophysiological response of the LNv to local application of PDF.
In summary, we measure tonic and burst spontaneous action potential firing patterns in Drosophila large LNv pacemaker neurons. Spontaneous action potential firing in the large LNv is circadian- and light regulated. The electrical properties of Drosophila circadian pacemaker neurons are remarkably similar to those observed in mammalian circadian pacemaker neurons and suggest a functional evolutionary conservation of circadian pacemaker neurophysiology between flies and mammals. Future neurophysiology studies will identify the Drosophila pacemaker neurons ion channels whose activities are functional components of the circadian clock.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Rubovszky for technical assistance performing the patch-clamp recordings, M. Kaneko and two anonymous reviewers for constructive comments on previous versions of this manuscript, J. Hall for providing the pdfGAL4 and cryb lines, and the Bloomington Stock Center for other fly lines.
GRANTS This work was supported by National Science Foundation Grants IBN-0323466 and IBN-0092753 and National Institutes of Health Grants NS-046750 and DA-016352 to T. C. Holmes and NS-27501 to D. K. O’Dowd.
Footnotes
The online version of this article contains supplemental data.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- de Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- de la Paz Fernandez M, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF. Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci USA. 2007;104:5650–5655. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Gitschier J, Strichartz GR, Hall LM. Saxitoxin binding to sodium channels in head extracts from wild-type and tetrodotoxin-sensitive strains of Drosophila melanogaster. Biochem Biophys Acta. 1980;595:291–303. doi: 10.1016/0005-2736(80)90091-7. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Gu H, O’Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. Systems approaches to biological rhythms in Drosophila. Methods Enzymol. 2005;393:61–185. doi: 10.1016/S0076-6879(05)93004-8. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Nassel DR. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J Comp Neurol. 2006;494:314–330. doi: 10.1002/cne.20807. [DOI] [PubMed] [Google Scholar]
- Helfrich C. Role of the optic lobes in the regulation of the locomotor activity rhythm of Drosophila melanogaster: behavioral analysis of neural mutants. J Neurogenet. 1986;3:321–343. doi: 10.3109/01677068609106857. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol A Sens Neural Behav Physiol. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology. 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photo-receptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Schroeder JI, Kay SA. In SYNC: the ins and outs of circadian oscillations in calcium. Sci STKE. 2007;390:pe32. doi: 10.1126/stke.3902007pe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. AG protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ding JM, Faiman LE, Gillette MU. Coupling of muscarinic cholinergic receptors and cGMP in nocturnal regulation of the suprachiasmatic circadian clock. J Neurosci. 1997;17:659–666. doi: 10.1523/JNEUROSCI.17-02-00659.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993;259:239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
- Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci USA. 2005;102:3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Holmes TC, Blau J. Membranes, ions, and clocks: testing the Njus-Sulzman-Hastings model of the circadian oscillator. Methods Enzymol. 2005a;393:682–693. doi: 10.1016/S0076-6879(05)93036-X. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2005b;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd DK, Aldrich RW. Voltage-clamp analysis of sodium channels in wild-type and mutant Drosophila neurons. J Neurosci. 1988;8:3633–3643. doi: 10.1523/JNEUROSCI.08-10-03633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol. 2006;95:3955–3960. doi: 10.1152/jn.00117.2006. [DOI] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:e13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Petri B, Stengl M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci. 1997;17:4087–4093. doi: 10.1523/JNEUROSCI.17-11-04087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rogero O, Tejedor FJ. Immunochemical characterization and developmental expression of Shaker potassium channels from the nervous system of Drosophila. J Biol Chem. 1995;270:25746–25751. doi: 10.1074/jbc.270.43.25746. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci. 2002;22:5946–5954. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Suri V, Qian Z, Hall JC, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21:225–234. doi: 10.1016/s0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Wicher D, Walther C, Wicher C. Non-synaptic ion channels in insects—basic properties of currents and their modulation in neurons and skeletal muscles. Prog Neurobiol. 2001;64:431–525. doi: 10.1016/s0301-0082(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Yang Z, Emerson M, Su HS, Sehgal A. Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron. 1998;21:215–223. doi: 10.1016/s0896-6273(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.