Abstract
OBJECTIVES
We evaluated the prognostic significance of prolonged QRS duration (QRSd) relative to arrhythmic outcomes in medically- and implantable cardioverter-defibrillator (ICD)-treated patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II.
BACKGROUND
There is conflicting literature on the relationship between prolonged QRSd and arrhythmic events, including sudden cardiac death (SCD), in heart failure patients with or without ICDs.
METHODS
Using a Cox-proportional hazards model adjusting for ejection fraction (EF), heart failure class, and blood urea nitrogen, we estimated the association of prolonged QRSd ≥ 140 milliseconds with SCD in the medically-treated arm, and SCD or first ICD therapy for rapid ventricular tachycardia/fibrillation (VT/VF, cycle length ≤ 260 ms) in the ICD-treated arm.
RESULTS
In the medically-treated arm, prolonged QRSd was a significant independent predictor of SCD (HR 2.12 [95% CI 1.20–3.76], p = 0.01). However, in the ICD-treated arm, prolonged QRSd did not predict SCD or rapid VT/VF (HR 0.77 [95% CI 0.47–1.24], p = 0.28). The difference in the prognostic effect of prolonged QRSd in these two groups was significant (p<0.01). These results were not affected by varying the cycle length defining rapid VT/VF or the duration defining QRSd prolongation.
CONCLUSIONS
In patients with prior myocardial infarction and EF ≤ 30%, prolonged QRSd does not predict SCD/VT/VF in ICD-treated patients, but does predict SCD in medically-treated patients. This underscores the non-equivalence of VT/VF and SCD, and the need for caution in inferring risk of SCD when using non-randomized databases that include only patients with ICDs.
Keywords: Death, sudden, defibrillation, tachyarrhythmias, risk factors, QRS duration
INTRODUCTION
Several studies have demonstrated that prolonged QRS duration (QRSd) is associated with increased mortality in medically-treated patients with heart failure (1). However, the association between prolonged QRSd and sudden cardiac death (SCD) is less certain (2–4). While the largest study to date examining the relationship of prolonged QRSd to SCD reported a significant independent association (4), other smaller studies have had inconsistent results (2,3). Moreover, there is conflicting literature on the relationship of prolonged QRSd to arrhythmic events, including SCD, in patients treated with implantable cardiovertor-defibrillators (ICDs), with the largest study to date showing that prolonged QRSd does not predict VT/VF in patients with ICDs (5), and smaller studies reporting contradictory results (6–12).
Defining the relationship between prolonged QRSd and SCD in patients with heart failure is crucial given the increased interest in risk stratification and assessing ICD efficacy in this population (13). Potential explanations for the conflicting literature on prolonged QRSd and arrhythmic outcomes in medically- as well as ICD-treated patients have included differences in study design, definition of prolonged QRSd, and the risk profiles of the study populations. Furthermore, studies in ICD-treated populations have not included direct comparisons to comparable medically-treated populations.
To overcome these limitations and address these apparently contradictory findings, we used the randomized Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) to compare the prognostic importance of prolonged QRSd in medically- and ICD-treated patients drawn from the same population in the same study.
METHODS
Patient population
The design, protocol, and results of MADIT-II have been previously described (14,15). MADIT-II was a randomized controlled trial conducted in 76 hospital centers that enrolled 1,232 patients randomized to ICD implant plus conventional medical therapy versus conventional medical therapy alone in a 3:2 ratio. Patients 21 years or older with a left ventricular ejection fraction (LVEF) ≤ 30 % were eligible if they had a myocardial infarction greater than one month or coronary revascularization greater than 3 months before entry. There were no electrophysiologic or arrhythmic entry criteria. During an average follow-up of 20 months, total mortality was 19.8% in the conventional arm and 14.2% in the ICD arm (hazard ratio [HR] 0.69 [95% CI 0.51 to 0.93], p=0.016).
Endpoints
Medically-treated patients
SCD as determined by an independent endpoint review committee was used as the outcome for patients in the medically-treated arm. SCD was ascertained using a modified Hinkle-Thaler classification system (16). SCD was defined as a patient who died suddenly and unexpectedly within 1 hour of cardiac symptoms, during sleep, or within 24 hours after last being seen alive. For this analysis, patients with indeterminate or unknown causes of death were not included.
ICD-treated patients
In the ICD-treated patients, we endeavored to use an analogous endpoint to SCD in the medically-treated patients (i.e. patients who either experienced SCD or would have in the absence of appropriate ICD therapy). Thus, we defined the endpoint as the first occurrence of either SCD as defined above or the first appropriate therapy (shock or anti-tachycardia pacing) for rapid ventricular tachycardia/fibrillation (VT/VF). Appropriate ICD therapy for rapid ventricular tachyarrhythmias has been previously used by several investigators as a surrogate for SCD in various cardiac conditions (17–19). In the present analysis, we used a threshold of ≤ 260 milliseconds (ms) to define rapid VT/VF because it yielded a combined outcome rate in the ICD arm (of rapid VT/VF and SCD) equivalent to that of SCD in the medically-treated arm. An independent expert panel reviewed all stored ICD internal electrograms of suspected VT/VF episodes to verify their ventricular origin. The episodes were identified in terms of cycle-lengths recorded by the ICD and were also manually checked by examining the print-out electrograms.
Statistical analysis
Baseline clinical characteristics of medically- and ICD-treated patients were compared using the chi-squared test for categorical variables and the student’s t-test for continuous variables.
Univariate analyses
Medically-treated arm
Freedom from SCD was assessed using the Kaplan-Meier method with patients dichotomized by QRSd ≥ 140 ms. This threshold was arbitrarily chosen a priori. Recognizing that prior studies evaluating the prognostic value of prolonged QRSd used thresholds ranging from 120 to 150 ms (2–12), we assessed the effect of this arbitrary threshold on our findings in additional sensitivity analyses (see below).
ICD-treated arm
Freedom from rapid VT/VF or SCD was assessed in a similar fashion, with patients in this arm also dichotomized by QRSd ≥ 140 ms.
Multivariate analyses
Medically-treated arm
Multivariate Cox proportional hazards regression analysis was used to assess the association of a prolonged QRSd (≥ 140 ms) with the risk for SCD, adjusting for NYHA class (≤ or > NYHA Class II), LVEF (≤ or > 25 %), and blood urea nitrogen (BUN) (≤ or > 25 mg/dL). In a prior MADIT-II secondary analysis, all three variables were identified as risk factors for SCD (20). Patients who died from non-sudden cardiac death causes were censored.
ICD-treated arm
Multivariate Cox proportional hazards analysis was used in a similar fashion to assess the association of a prolonged QRSd (≥ 140 ms) with the risk of rapid VT/VF or SCD adjusting for NYHA class, LVEF, and BUN.
Significance of effect
The adjusted hazard ratios for prolonged QRSd in each arm were compared by examining the statistical significance of the interaction term of treatment assignment-by-prolonged QRSd in a Cox proportional hazards model.
Sensitivity analyses
In sensitivity analyses, we tested the effects of using different definitions of the endpoint in the ICD arm. We varied the VT/VF threshold cycle length defining the endpoint from 240 ms to 320 ms. Sensitivity analyses were also done including and excluding SCD from the combined outcome in the ICD-treated arm. Additionally, we examined the effect of varying QRSd cut-offs at several values, from 120 ms to 150 ms.
For all analyses, a threshold two-sided p-value of 0.05 was used to determine statistical significance. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Baseline characteristics
Twenty-four patients (10 in the ICD group and 14 in the medical treatment group) were eliminated from the analysis for indeterminate or unknown cause of death. Baseline patient characteristics and medication use for the two arms are shown in Table 1. Examined clinical and electrocardiographic characteristics did not differ significantly between the two groups, except for QRSd.
Table 1.
Baseline characteristics of medically- and ICD-treated patients in the MADIT-II study*.
| Medical arm (n=476) | ICD arm (n=732) | P value | |
|---|---|---|---|
| Age in years (mean ± SD) | 64 ± 10 | 64 ± 10 | 1 |
| Male (%) | 86 | 84 | 0.4 |
| NYHA functional class (%) | 0.4 | ||
| I | 39 | 34 | |
| II | 35 | 36 | |
| III | 22 | 26 | |
| IV | 4 | 4 | |
| Treatment for hypertension (%) | 53 | 53 | 0.9 |
| Diabetes mellitus (%) | 37 | 33 | 0.2 |
| Current or former smoker (%) | 83 | 79 | 0.2 |
| Coronary bypass surgery (%) | 56 | 58 | 0.6 |
| Coronary angioplasty | 42 | 46 | 0.2 |
| Interval > 6 months between most recent MI and enrollment (%) | 87 | 88 | 0.6 |
| LVEF in percent (mean ± SD) | 23 ± 5 | 23 ± 5 | 0.9 |
| BUN > 25 mg/dL (%) | 32 | 29 | 0.2 |
| Creatinine ≥ 1.4 mg/dL (%) | 30 | 26 | 0.1 |
| Atrial fibrillation (%) | 8 | 8 | 1 |
| QRS duration in seconds (mean ± SD) | 0.120 ± 0.03 | 0.124 ± 0.04 | 0.04 |
| QRS duration ≥ 140 milliseconds (%) | 29 | 35 | 0.03 |
| Nonspecific conduction defect (%) | 26 | 23 | 0.2 |
| RBBB (%) | 7 | 9 | 0.3 |
| LBBB (%) | 17 | 18 | 0.6 |
| QTc > 480 milliseconds (%) | 33 | 33 | 0.9 |
| Medications at baseline (%) | |||
| Antiarrhythmic (class I) | 3 | 2 | 0.6 |
| ACE inhibitor or ARB | 89 | 88 | 0.7 |
| Amiodarone | 7 | 7 | 0.7 |
| Aspirin | 72 | 68 | 0.2 |
| Beta-blocker | 61 | 64 | 0.3 |
| Calcium channel blocker | 14 | 12 | 0.3 |
| Digitalis | 57 | 59 | 0.3 |
| Diuretic | 77 | 73 | 0.1 |
| Statin | 62 | 64 | 0.5 |
The MADIT-II cohort was modified by eliminating 24 patients (10 in the ICD group and 14 in the medical treatment group) for indeterminate or unknown cause of death.
MADIT-II = Multicenter Automatic Defibrillator Trial II; ICD = implantable cardioverter-defibrillator; SD = standard deviation; NYHA = New York Heart Association; BUN = blood urea nitrogen; MI = myocardial infarction; LVEF = left ventricular ejection fraction; RBBB = right bundle branch block; LBBB = left bundle branch block; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker.
Outcomes
In the medically-treated arm, the SCD rate was 10.3% (49/476). In the ICD-treated arm, the combined outcome of SCD or first appropriate ICD therapy for rapid VT/VF (cycle length ≤ 260 ms) was 10.5% (77/732).
Univariate analysis
Kaplan-Meier curves dichotomized by QRSd depicting time to SCD in medically-treated patients and SCD or first appropriate ICD therapy for rapid VT/VF in ICD-treated patients are shown in Figure 1 and Figure 2, respectively. In the medically-treated arm, the 138 patients (29%) with QRSd ≥ 140 ms exhibited over a two-fold chance of SCD (HR 2.26 [95% CI 1.29–3.98], log-rank p=0.005). However, in the ICD-treated arm, the 255 patients (35%) with QRSd ≥ 140 ms showed no difference in time to SCD or first appropriate ICD therapy for rapid VT/VF compared to those with QRSd < 140 ms (HR 0.91 [95% CI 0.56–1.46], log-rank p=0.68).
Figure 1. Time to sudden cardiac death in medically-treated arm by QRSd < or ≥ 140 milliseconds (ms).
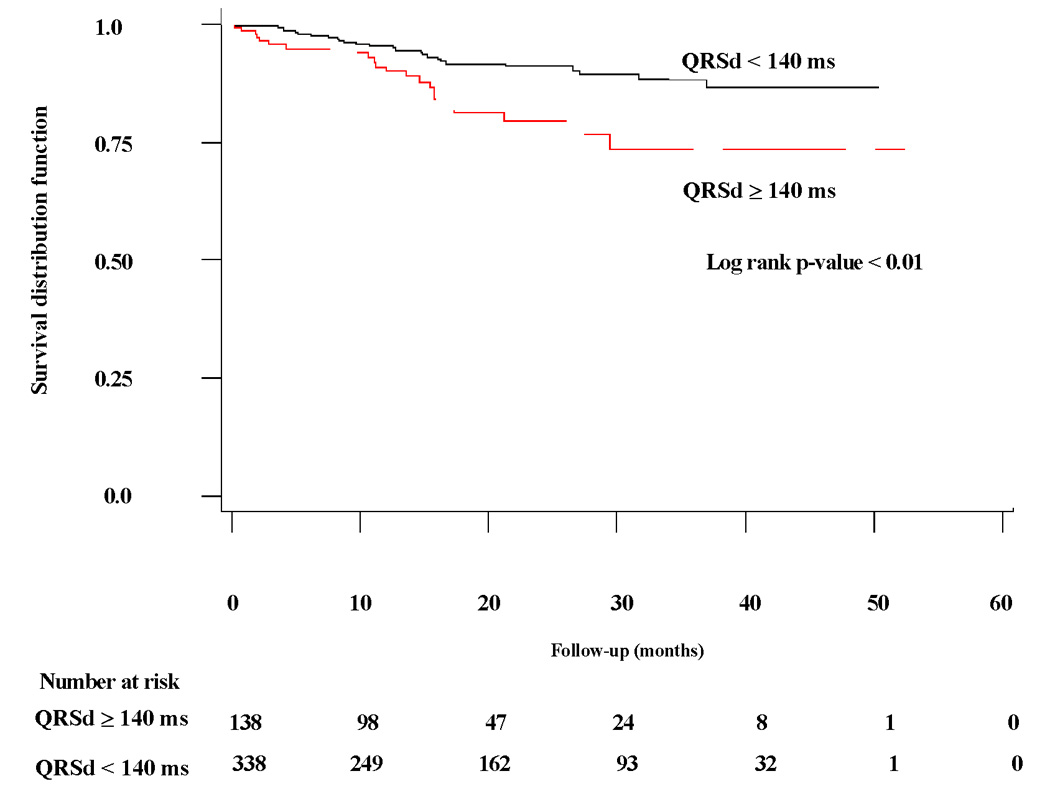
Kaplan-Meier curve of freedom from sudden cardiac death in medically-treated arm in patients with QRSd ≥ 140 milliseconds (ms) compared to patients with QRSd < 140 ms.
Figure 2. Time to rapid VT/VF/SCD in ICD-treated arm by QRSd < or ≥ 140 milliseconds.
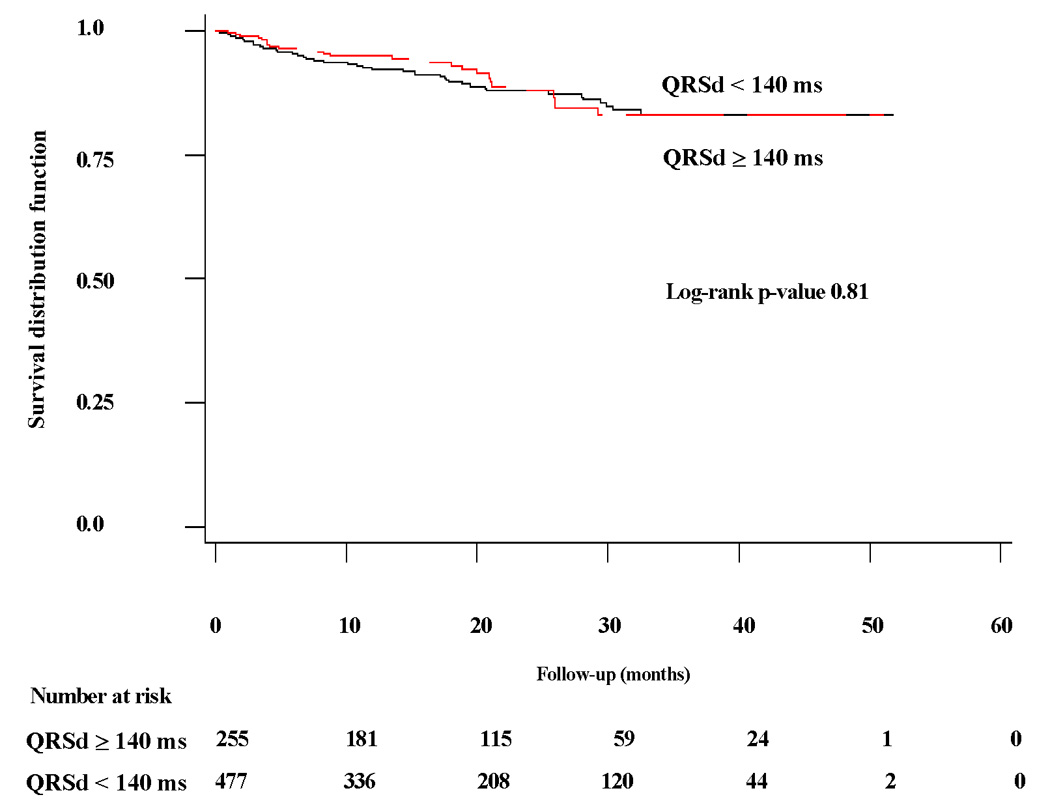
Kaplan-Meier curve of freedom from rapid VT/VF/SCD in ICD-treated arm in patients with QRSd ≥ 140 milliseconds (ms) compared to patients with QRSd < 140 ms.
VT/VF = ventricular tachycardia/ventricular fibrillation; SCD = sudden cardiac death.
Multivariate analysis
As shown in Table 2, multivariate Cox proportional hazards modeling adjusting for NYHA class, LVEF, and BUN did not appreciably change the estimated hazard ratios for prolonged QRSd in either the medically- or the ICD-treated arm. Prolonged QRS duration was independently associated with a statistically significant increased risk of the outcome in the medical arm, but not the ICD arm. Examination of the treatment-by-QRSd interaction term demonstrated that the difference between the hazard ratios of the two arms was statistically significant (p<0.01). Furthermore, multivariate analysis adjusting for additional covariates (age, gender, prior coronary artery bypass surgery, and baseline use of amiodarone, β-blockers, digoxin, and diuretics) did not appreciably alter the estimated hazard ratios in either arm, nor did it change the significance of the interaction term (medically-treated arm, HR 1.97 [95% CI 1.07–3.63]; ICD-treated arm, HR 0.71 [95% CI 0.43–1.16]; interaction term p-value=0.01).
Table 2.
Adjusted hazard ratios for risk of SCD in medically-treated arm and risk of rapid VT/VF or SCD in ICD-treated arm for patients with QRS duration of ≥ 140 milliseconds (ms) versus those with QRS duration of < 140 ms.
| Medical arm | ICD arm | P value (interaction term)* | |
|---|---|---|---|
| HR QRSd ≥ 140 ms | 2.12 | 0.77 | <0.01 |
| 95 % CI | 1.20–3.76 | 0.47–1.24 | |
| P value (main effect of QRSd) | 0.01 | 0.28 |
The p-value of the interaction term of treatment assignment-by-prolonged QRS duration signifies the statistical difference between the two hazard ratios. A p-value threshold of 0.05 was used for this purpose. Rapid VT/VF was defined as any tachyarrhythmia with cycle length of ≤ 260 milliseconds.
ICD = implantable cardioverter-defibrillator; ms = milliseconds; VT/VF = ventricular tachycardia/ventricular fibrillation; SCD = sudden cardiac death; HR = hazard ratio; CI = confidence interval; ms = milliseconds.
Sensitivity analyses
To demonstrate the consistency of our findings over a range of VT/VF cycle length cut-offs, we performed sensitivity analyses varying the definition of the rapid VT/VF outcome in the ICD-treated arm. Details of the composition of various combined rapid VT/VF/SCD outcomes in the ICD-treated arm by varying VT/VF cycle length cut-offs are shown in Table 3. As longer cycle lengths (i.e. slower VT) were included in the outcome, the hazard ratio for VT/VF increased only marginally (Table 4). The overall ability of prolonged QRSd to predict rapid VT/VF events remained poor despite varying cycle length cut-offs defining appropriate therapy for rapid VT/VF. Excluding SCD in the endpoint definition did not appreciably alter these findings (Table 4) nor did examining SCD alone as the endpoint in the ICD-treated arm (not shown). Of the patients that died of SCD in the ICD-treated arm, seventeen (3.6%) had a QRSd < 140 ms and eleven (4.3%) a QRSd ≥ 140 ms. Furthermore, the findings were consistent across different thresholds defining prolonged QRSd (Figure 3). The overall prognostic ability of prolonged QRSd to predict rapid VT/VF/SCD in the ICD-treated arm did not improve with more prolonged QRSd (up to 150 ms) even as these more prolonged thresholds conferred more risk for SCD in the medically-treated arm (Figure 3).
Table 3.
Details of the composition of the combined rapid VT/VF/SCD endpoint in the ICD-treated arm by varying VT/VF cycle length cut-off.*
| VT/VF cycle length cut-off in ms. (Equivalent heart rate in bpm) | Number of patients with appropriate ICD therapy for given VT/VF cycle length cut-off | Number of patients with SCD‡ | Number of patients with either an appropriate ICD therapy for given VT/VF or SCD | Outcome rate (%)† |
|---|---|---|---|---|
| ≤ 240 (≥250) | 40 | 22 | 62 | 8.5 |
| ≤ 260 (≥230) | 57 | 20 | 77 | 10.5 |
| ≤ 280 (≥214) | 78 | 19 | 97 | 13.3 |
| ≤ 300 (≥200) | 99 | 18 | 117 | 16.0 |
| ≤ 320 (≥187) | 120 | 18 | 138 | 18.9 |
The combined VT/VF/SCD endpoint was defined as the first occurrence of either an appropriate ICD-therapy for rapid VT/VF, or SCD.
For any given VT/VF cycle length cuff-off, the combined VT/VF/SCD outcome was calculated by adding the number of patients with an appropriate therapy for VT/VF to the number of patients who died of SCD in the ICD-treated arm. Each outcome rate was calculated by dividing this sum with the number of patients in the ICD-treated arm (i.e. total/732). For a given VT/VF cycle length cut-off, each outcome rate can be compared to the outcome rate of SCD of 10.3% in the medically-treated arm.
Patients originally classified as SCD were reclassified to ICD-therapy for VT/VF because they experienced and survived appropriate ICD-therapies for VT/VF well before their SCD. As the cycle length definition for VT/VF changed, those patients were reclassified depending on the cycle length of their VT/VF event.
ICD = implantable cardioverter-defibrillator; ms = milliseconds; bpm = beats per minute; VT/VF = ventricular tachycardia/ventricular fibrillation; SCD = sudden cardiac death.
Table 4.
Sensitivity analysis by varying VT/VF cycle length cut-off used to define the rapid VT/VF outcome and by including or excluding SCD towards the combined outcome in the ICD-treated arm.
| Including Sudden Cardiac Death | Excluding Sudden Cardiac Death | |||||||
|---|---|---|---|---|---|---|---|---|
| VT/VF | Outcome | HR | P value | P value | Outcome | HR | P value | P value |
| cycle length cut-off (ms) | rate (%) | QRSd ≥ 140 ms [95% CI] | (QRSd effect) | (interaction) | rate (%) | QRSd ≥ 140 ms [95% CI] | (QRSd effect) | (interaction) |
| ≤ 240 | 8.5 | 0.64 [0.37–1.12] | 0.12 | <0.01 | 5.5 | 0.41 [0.19–0.90] | 0.03 | <0.001 |
| ≤ 260 | 10.5 | 0.77 [0.47–1.24] | 0.28 | <0.01 | 7.8 | 0.64 [0.39–1.16] | 0.14 | <0.01 |
| ≤ 280 | 13.3 | 1.04 [0.69–1.57] | 0.86 | 0.05 | 10.7 | 1.02 [0.64–1.63] | 0.93 | 0.04 |
| ≤ 300 | 16.0 | 1.09 [0.75–1.60] | 0.64 | 0.06 | 13.5 | 1.12 [0.74–1.68] | 0.60 | 0.06 |
| ≤ 320 | 18.9 | 1.05 [0.74–1.49] | 0.78 | 0.03 | 16.4 | 1.06 [0.73–1.55] | 0.75 | 0.03 |
In the medically-treated arm, the outcome rate of SCD was 10.3% and the hazard ratio for SCD in patients with QRSd ≥ 140 milliseconds was 2.12 (95% CI 1.20–3.76). The p-value of the interaction term of treatment assignment-by-prolonged QRSd signifies the statistical significance of the difference between the hazard ratios presented here compared with the hazard ratio for SCD in patients with prolonged QRSd in the medically-treated arm. A p value threshold of 0.05 was used for statistical significance.
VT/VF = ventricular tachycardia/ventricular fibrillation; SCD = sudden cardiac death; ICD = implantable cardioverter-defibrillator; ms = milliseconds; HR = hazard ratio; CI = confidence interval; QRSd = QRS duration.
Figure 3. Sensitivity analysis of hazard ratios in ICD-arm by varying cycle length cut-off used to define the combined rapid VT/VF/SCD outcome, and in both arms by varying the definition of prolonged QRSd.
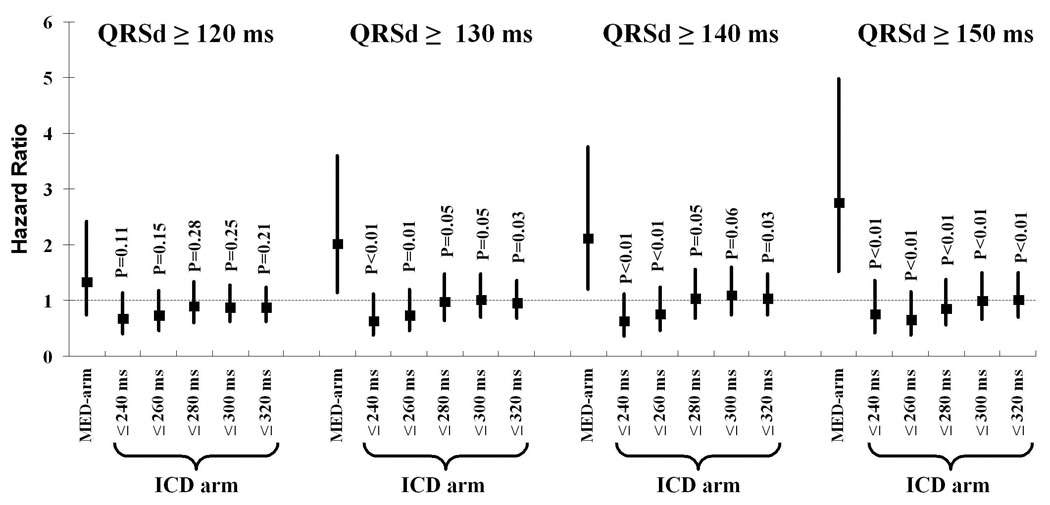
The p-values of QRSd as a main effect for each VT/VF cycle length definition are all not statistically significant and not displayed. The p-values displayed above are of the interaction term of treatment assignment-by-prolonged QRS duration for each VT/VF cycle length definition. Each signifies the statistical difference between its corresponding hazard ratio compared to the hazard ratio of SCD in the medically-treated arm for patients within each dichotomized QRSd category. A p-value threshold of 0.05 was used for this purpose.
VT/VF = ventricular tachycardia/fibrillation; SCD = sudden cardiac death; s = second; MED-arm = medically treated arm; ICD arm = implantable cardioverter defibrillator-treated arm.
DISCUSSION
To our knowledge, this is the first analysis to compare the association of prolonged QRSd to arrhythmic outcomes in medically-treated patients versus a comparable ICD-treated population. It is also the largest study to date to examine the relationship between prolonged QRSd and appropriate ICD therapy in patients with ICDs. Using data from the MADIT-II study, we observed that prolonged QRSd is highly predictive for SCD in medically-treated patients, but not at all predictive of VT/VF/SCD in a comparable ICD-treated population. The difference in the prognostic importance of prolonged QRSd between the two groups is statistically significant and robust to sensitivity analysis. Results were similar regardless of the duration defining QRSd prolongation, the cycle length cut-off used to define rapid VT/VF, or whether or not SCD was counted along with the VT/VF events in the ICD-treated arm.
Contribution to existing literature
While there is consistent evidence that QRSd is an independent predictor of total mortality, studies examining the relationship of prolonged QRSd to arrhythmic mortality in non-ICD-treated patients have yielded less consistent results (1–3). However, the largest of these prior studies, a subanalysis of the Multicenter Unsustained Tachycardia Trial (MUSTT) trial, found a significant and independent relationship between prolonged QRSd and SCD in medically-treated patients (4), consistent with our present observation in MADIT-II.
There has also been conflicting data on the ability of QRSd to predict ventricular tachyarrhythmic events requiring ICD therapy in ICD-treated populations (5–12). The largest prior study, a sub-analysis of 431 ICD-treated patients in the PainFREE II study, found no association between prolonged QRSd and rapid VT/VF events (5). Our study confirms their findings, albeit in a sicker, more uniform, and larger (n=732) patient population, and is able to make direct comparisons with a near-identical control population. While other investigators have reported that prolonged QRSd did predict VT/VF events in patients with ICDs (7,9–12), these studies were smaller and had less consistent follow-up rendering them more vulnerable to ascertainment bias.
Reconciling this conflicting literature is limited by the fact that these studies were done in different populations with varying degrees of heart failure and risk of arrhythmic outcomes. This is relevant given the recognition that the prognostic value of prolonged QRSd may be closely related to the extent of underlying heart disease (21). In addition, previous studies relied on a single cut-off for defining prolonged QRSd or VT/VF. The present study overcomes these limitations by demonstrating consistency across various definitions for prolonged QRSd and rapid VT/VF, and importantly, by comparing the prognostic significance of prolonged QRSd relative to arrhythmic outcomes in two near-identical medically- and ICD-treated populations.
Potential explanations/implications
Based on prior comparisons of randomized trials testing ICDs in various populations, it is well recognized that VT/VF is much more common than SCD, indicating that many ventricular arrhythmias terminate spontaneously (22). Thus, one possible explanation for our findings is that prolonged QRSd may not be associated with a greatly increased risk of VT/VF but may be associated with a higher fatality rate when VT/VF does occur (in the absence of ICD treatment). If tachyarrhythmias that occur in patients with prolonged QRSd were more complex, more likely to degenerate, and less likely to spontaneously terminate, then a higher rate of SCD in these patients would be expected (in the absence of ICD therapy) even if their rate of VT/VF were similar to patients with normal QRSd. Furthermore, the observation that prolonged QRSd is prognostically related to SCD but not to VT/VF implies that prolonged QRSd may not be mechanistically related to VT/VF, but may be related to other etiologies of SCD (e.g. electromechanical dissociation or asystole). Regardless of the explanation, the results of our study underscore the non-equivalence of VT/VF events and SCD. The results also underscore the need for caution in inferring risk of SCD, and the likelihood of benefiting from ICDs, using non-randomized databases that include only patients with ICDs, since the presence or absence of higher risk for VT/VF does not automatically imply the presence or absence of a higher risk of treatable SCD.
Study limitations
This is a post-hoc analysis of a clinical trial, and therefore we acknowledge that the results may be due to chance. However, the effect is very strong and robust to multiple sensitivity analyses. Second, we recognize that classification of mode of death in MADIT-II was unblinded and could have resulted in a classification bias in favor of a higher rate of SCD in the medically-treated arm. This observation, however, does not jeopardize the major conclusions of our study, as any misclassification errors should be randomly distributed among all QRS widths. Therefore, while misclassification errors might have affected the rates in the medical versus the ICD arm, they should not affect the hazard ratio associated with prolonged QRSd within each of the treatment arms (i.e. a higher rate of SCD does not imply a higher hazard ratio for prolonged QRSd). Likewise, errors in the storage and interrogation of ICD therapy for patients in the ICD-treated arm would not affect the risk of outcome associated with prolonged QRSd since these missed episodes would also be distributed randomly among all QRS widths.
Conclusions
This analysis of the MADIT II trial indicates that prolonged QRSd is associated with an approximately two-fold increase in SCD in medically-treated patients, but not with any increase in ICD-treated VT/VF events or SCD in patients with an implanted defibrillator. If confirmed in other randomized databases, the finding suggests that ICD-treated VT/VF events may be a poor surrogate marker for SCD in patients with ischemic cardiomyopathy. Future work should examine possible mechanisms by which prolongation of QRSd might increase the risk of SCD without increasing the risk of VT/VF.
ACKNOWLEDGEMENTS
The authors acknowledge the MADIT-II investigators for use of the MADIT-II database.
FUNDING SOURCES
Dr. Ritesh Dhar was funded by a T32 training grant awarded by the Agency for Healthcare Research and Quality. Dr. Alsheikh-Ali is a recipient of a faculty development award from Pfizer/Tufts-New England Medical Center. MADIT-II was supported by a research grant from Guidant Corp., St. Paul, Minnesota, to the University of Rochester School of Medicine and Dentistry.
Abbreviations and Acronyms
- QRSd
QRS duration
- SCD
sudden cardiac death
- ICD
implantable cardiovertor-defibrillator
- MADIT II
the Multicenter Automatic Defibrillator Implantation Trial II
- VT/VF
ventricular tachycardia/fibrillation
- LVEF
left ventricular ejection fraction
- ms
milliseconds
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Drs. Moss, Zareba, and Greenberg have received research grants from Boston Scientific; Dr. Zareba has also received a research grant from Medtronic. Dr. Moss has received honoraria for lectures from Boston Scientific. Dr. Daubert has ownership interest in Medtronic and Boston Scientific. Dr. Estes has spoken for Medtronic, Boston Scientific, and St. Jude Medical. Drs. Dhar, Alsheikh-Ali, Case, and Kent have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 2.Iuliano S, Fisher SG, Karasik PE, et al. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 3.Kearney MT, Zaman A, Eckberg DL, et al. Cardiac size, autonomic function, and 5-year follow-up of chronic heart failure patients with severe prolongation of ventricular activation. J Card Fail. 2003;9:93–99. doi: 10.1054/jcaf.2003.15. [DOI] [PubMed] [Google Scholar]
- 4.Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–769. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Sweeney MO, Wathen MS, et al. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310–316. doi: 10.1016/j.jacc.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Bode-Schnurbus L, Bocker D, Block M, et al. QRS duration: a simple marker for predicting cardiac mortality in ICD patients with heart failure. Heart. 2003;89:1157–1162. doi: 10.1136/heart.89.10.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guttigoli AB, Wilner BF, Stein KM, et al. Usefulness of prolonged QRS duration to identify high-risk ischemic cardiomyopathy patients with syncope and inducible ventricular tachycardia. Am J Cardiol. 2005;95:391–394. doi: 10.1016/j.amjcard.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Ashwath ML, Sogade FO. Ejection fraction and QRS width as predictors of event rates in patients with implantable cardioverter defibrillators. South Med J. 2005;98:513–517. doi: 10.1097/01.SMJ.0000149390.50866.74. [DOI] [PubMed] [Google Scholar]
- 9.Arya A, Haghjoo M, Dehghani MR, et al. Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am J Cardiol. 2006;97:389–392. doi: 10.1016/j.amjcard.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Klein G, Lissel C, Fuchs AC, et al. Predictors of VT/VF-occurrence in ICD patients: results from the PROFIT-Study. Europace. 2006;8:618–624. doi: 10.1093/europace/eul082. [DOI] [PubMed] [Google Scholar]
- 11.Stecker EC, Zargarian M, Dogra V, et al. Native QRS duration predicts the occurrence of arrhythmic events in ICD recipients. Europace. 2006;8:859–862. doi: 10.1093/europace/eul090. [DOI] [PubMed] [Google Scholar]
- 12.Hreybe H, Saba S. A clinical risk score to predict the time to first appropriate device therapy in recipients of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2007;30:385–389. doi: 10.1111/j.1540-8159.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 13.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: Lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1158–1160. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Cannom DS, Daubert JP. Multicenter Automatic Defibrillator Implantation Trial II (MADIT II): design and clinical protocol. Ann Noninvasive Electrocardiol. 1999;4:83–91. doi: 10.1111/j.1542-474X.2005.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg H, Case RB, Moss AJ, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Cardiol. 2004;43:1459–1465. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Bocker D, Bansch D, Heinecke A, et al. Potential benefit from implantable cardioverter-defibrillator therapy in patients with and without heart failure. Circulation. 998;98:1636–1643. doi: 10.1161/01.cir.98.16.1636. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, Shen WK, Link MS, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 19.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 20.Zareba W, Piotrowicz K, McNitt S, et al. Implantable cardioverter-defibrillator efficacy in patients with heart failure and left ventricular dysfunction (from the MADIT II population) Am J Cardiol. 2005;95:1487–1491. doi: 10.1016/j.amjcard.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Olshansky B. Wide QRS, narrow QRS: what's the difference? J Am Coll Cardiol. 2005;46:317–319. doi: 10.1016/j.jacc.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Germano JJ, Reynolds M, Essebag V, et al. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–1261. doi: 10.1016/j.amjcard.2005.11.048. [DOI] [PubMed] [Google Scholar]


