Summary
Statins are 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors that exert anti-inflammatory effects. IFN-γ induction of class II MHC expression, which requires the class II transactivator (CIITA), is inhibited by statins, however, the molecular basis for suppression is undetermined. We describe that statins inhibit IFN-γ-induced class II MHC expression by suppressing CIITA gene expression, which is dependent on the HMG-CoA reductase pathway. In addition, CIITA expression is inhibited by GGTI-298 or Clostridium difficile Toxin A, specific inhibitors of Rho family protein prenylation, indicating the involvement of small GTPases. Rac1 is involved in IFN-γ inducible expression of CIITA, and statins inhibit IFN-γ-induced Rac1 activation, contributing to the inhibitory effect of statins. IFN-γ induction of the CIITA gene is regulated by the transcription factors STAT-1α, IRF-1 and USF-1. We previously reported that statins inhibit constitutive STAT-1α expression. IRF-1, a STAT-1 dependent gene, is also inhibited by statins. Therefore, statin treatment results in decreased recruitment of STAT-1α and IRF-1 to the endogenous CIITA pIV promoter. The recruitment of USF-1 to CIITA pIV is also reduced by statins, as is the recruitment of RNA Polymerase II, p300 and Brg-1. These data indicate that statins inhibit the transcriptional program of the CIITA gene.
Keywords: Statins, CIITA gene expression, Macrophages, Small G-proteins
Introduction
Class II MHC molecules play a critical role in the immune response by presenting antigens to CD4+ T cells, resulting in their activation and differentiation. Aberrant class II MHC expression is associated with diseases including type I diabetes, rheumatoid arthritis, Multiple Sclerosis and Alzheimer's Disease [1-5], although their involvement in pathogenesis is less clear. Class II MHC molecules are constitutively expressed on antigen presenting cells (APCs), whereas expression is induced by IFN-γ in cell types including endothelial cells, macrophages and microglia [2, 6, 7]. CIITA is the master regulator of class II gene activation [6]. CIITA is a non-DNA-binding co-activator, and its expression is regulated at the transcriptional level. CIITA function is further modulated by post-translational modifications such as phosphorylation and ubiquitination [8-10]. The CIITA gene is under the control of three distinct promoters, and IFN-γ induction of CIITA expression is controlled primarily by promoter IV (pIV) [6]. pIV has several important cis-acting elements including GAS, E-box and IRE. The GAS and E-box elements are bound cooperatively by activated STAT-1α and USF-1. Activated STAT-1α also induces the expression of IRF-1, which then binds IRE in pIV [6]. Histone acetylation at the CIITA pIV is increased following IFN-γ stimulation, consistent with transcriptional activity [11]. The ATPase subunit of the SWI/SNF complex, Brg-1, is needed to mediate chromatin remodeling, which is functionally required for CIITA gene transcription [12].
Statins competitively inhibit 3-hydroxy-3-methyglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme that catalyses the conversion of HMG-CoA to L-mevalonate, a key intermediate in cholesterol biosynthesis [13]. In addition to their cholesterol-lowering activity, statins have immunomodulatory and anti-inflammatory effects [14-17]. Mevalonate (MVLT) is a precursor not only of cholesterol, but also of many nonsteroidal isoprenoids, including geranylgeranyl pyrophosphate (GGPP) and farnesyl pyrophosphate (FPP). Isoprenylation is a functionally important post-translational modification of small GTP-binding proteins such as Rho, Rac and Ras, which are important for protein trafficking, signaling, cytoskeletal structure and cell motility [18, 19]. By reducing the cellular pool of isoprenoids, statins cause the accumulation of inactive forms of Rho, Rac and Ras within the cytoplasm [20]. Thus, statins have significant cholesterol-independent effects that result from inhibition of the isoprenoid pathway.
Statins inhibit functional effects of IFN-γ such as induction of class II MHC expression on APCs, thereby inhibiting T-cell activation [21-23]. Some of the effects of statins are associated with inhibition of small GTPases [16, 17, 24]. In addition, small GTPases have important roles in a variety of immune responses [25-27]. In this study, we demonstrate that statins inhibit IFN-γ inducible expression of CIITA, subsequently inhibiting class II MHC expression. IFN-γ induction of CIITA involves activation of the small GTPase Rac1, which is inhibited by statin treatment. In addition, statins affect the transcriptional program of the CIITA gene by inhibiting the recruitment of STAT-1α, IRF-1 and USF-1, and the co-activator Brg-1 to the CIITA promoter. These findings provide information on the molecular basis by which statins inhibit IFN-γ-induced class II MHC expression, thereby suggesting therapeutic targets for autoimmune and inflammatory diseases.
Results
Statins Inhibit IFN-γ-induced Class II MHC Expression by Suppressing CIITA Expression
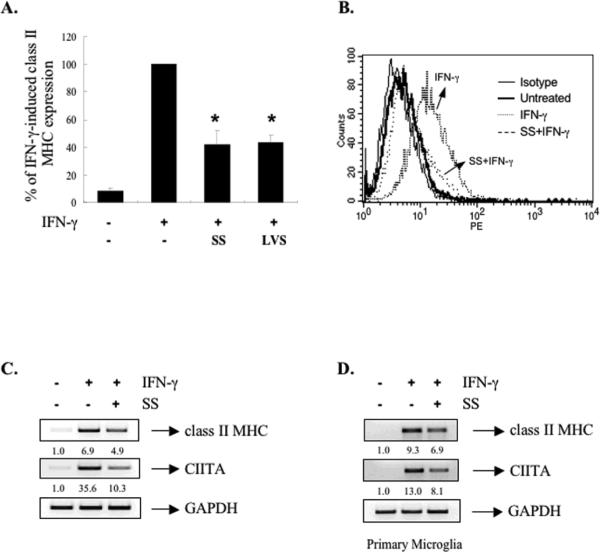
Youssef et al., [23] demonstrated that atorvastatin inhibits IFN-γ-induced class II MHC expression in macrophages and microglia by inhibiting CIITA expression. Therefore, this prompted us to explore how statins affect CIITA expression at the molecular level. We first tested the ability of simvastatin (SS) and lovastatin (LVS) to affect IFN-γ-induced class II MHC expression in bone marrow-derived macrophages (BMDM). Cells were treated with increasing concentrations of SS (0.1-10 μM) prior to IFN-γ treatment, and then class II MHC expression was examined. IFN-γ-induced class II MHC was inhibited by SS in a dose-dependent manner (data not shown), with maximal inhibition at 10 μM (~50% inhibition, Figure 1A). LVS showed a similar inhibitory effect (Figure 1A). SS and LVS alone had no effect on class II MHC expression (data not shown). Figure 1B is a representative flow cytometry result using 10 μM of SS. The inhibitory effect of SS was also observed on class II MHC mRNA expression (Figure 1C). CIITA is the master regulator of both inducible and constitutive class II MHC expression [28, 29]. SS inhibited IFN-γ-inducible CIITA mRNA expression in BMDM by ~70% (Figure 1C). Using primers for pI, pIII and pIV isoform-specific CIITA mRNA, we observed that SS inhibited IFN-γ induction of pI, pIII and pIV CIITA transcripts (data not shown). A comparable effect of SS on class II MHC and CIITA mRNA expression was observed in primary murine microglia (Figure 1D). These results indicate that statins inhibit IFN-γ induction of class II MHC expression in primary macrophages and microglia through suppression of CIITA gene expression. We also examined the effect of SS in the murine macrophage cell line RAW264.7. SS inhibited IFN-γ-induced class II MHC protein and mRNA expression, and CIITA mRNA expression (Figures 2A and 2B). LVS showed a similar inhibitory effect in RAW264.7 cells (Figure 2).
Figure 1. Simvastatin and Lovastatin Inhibit IFN-γ-induced Class II MHC Expression by Suppressing CIITA mRNA Expression.
(A) BMDM were pretreated with either SS (10 μM) or LVS (10 μ) for 24 h, and then stimulated by IFN-γ (5 ng/ml) for 15 h. Surface expression of class II MHC protein was assessed by flow cytometry. IFN-γ induction of class II MHC protein was set at 100%, and statin treatment compared to that. Mean ± S.D. of four experiments. *, p ≤ 0.05, compared with IFN-≤ treatment. (B) A representative flow cytometry result with SS (10 μM) treatment. (C) BMDM were pretreated with SS (10 μM) for 24 h prior to IFN-γ treatment for 15 h or (D) primary murine microglia were pretreated with SS (10 μM) for 12 h prior to IFN-γ treatment for 15 h. Total RNA was analyzed by RTPCR with primers specific for class II MHC, CIITA and GAPDH. Values were normalized to GAPDH mRNA. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of three independent experiments.
Figure 2. Simvastatin and Lovastatin Inhibit IFN-γ-induced Class II MHC and CIITA Expression in RAW264.7 Cells.
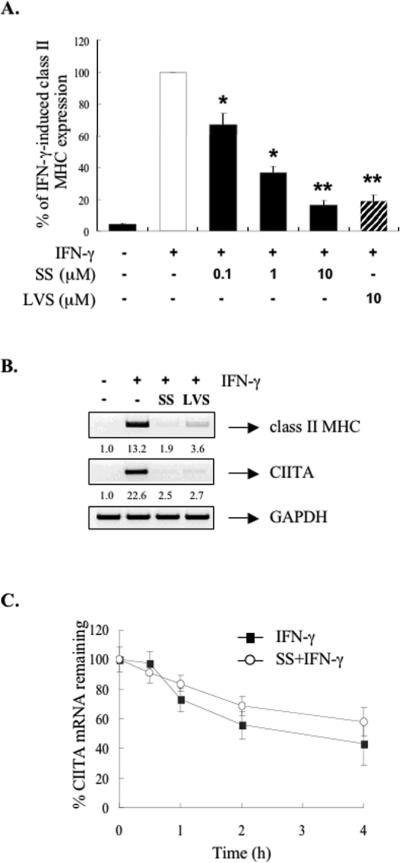
Cells were treated with different concentrations of SS (0-10 μM) or LVS (10 μM) for 12 h and then stimulated by IFN-γ (10 ng/ml) for 15 h. (A) Surface expression of class II MHC was assessed by flow cytometry. IFN-γ induction of class II MHC protein was set at 100%, and statin treatment compared to that. Mean ± S.D. of three experiments. *, p ≤ 0.05, **, p ≤ 0.001, compared with IFN-γ treatment. (B) Total RNA was analyzed by RT-PCR with primers specific for class II MHC, CIITA and GAPDH. Values were normalized to GAPDH mRNA. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of three independent experiments. (C) Cells were pretreated with either medium alone or SS (1 μM) for 12 h, and then stimulated by IFN-γ (10 ng/ml) for 15 h. Actinomycin D (Act-D) (5 μg/ml) was added at this point (time 0), and cells were harvested at the indicated times (0.5-4 h). Total RNA was analyzed by RPA for CIITA and GAPDH mRNA expression. CIITA mRNA was normalized to GAPDH mRNA in each sample, and the value for CIITA mRNA at time 0 (before the addition of Act-D) was set to 100. Mean ± S.D. of four experiments.
We next determined whether the inhibitory effect of SS on CIITA mRNA was due to destabilization of the CIITA message. RAW264.7 cells were incubated with medium, IFN-γ, or SS (1 μM) plus IFN-γ for 12 h, then actinomycin D (5 μg/ml) was added at that point (time 0). RNA was isolated at serial time points and examined for levels of CIITA mRNA. At time 0, 1 μM of SS inhibited IFN-γ-induced CIITA mRNA by ~50%. The t1/2 of IFN-γ-induced CIITA mRNA was ~ 3.5 h, while that of CIITA mRNA in the presence of SS was comparable (Figure 2C). These data indicate that SS inhibition of IFN-γ-induced CIITA mRNA levels is not due to destabilization of CIITA mRNA, suggesting an effect at the transcriptional level.
The Inhibitory Effect of Simvastatin is Dependent on the HMG-CoA Reductase Pathway
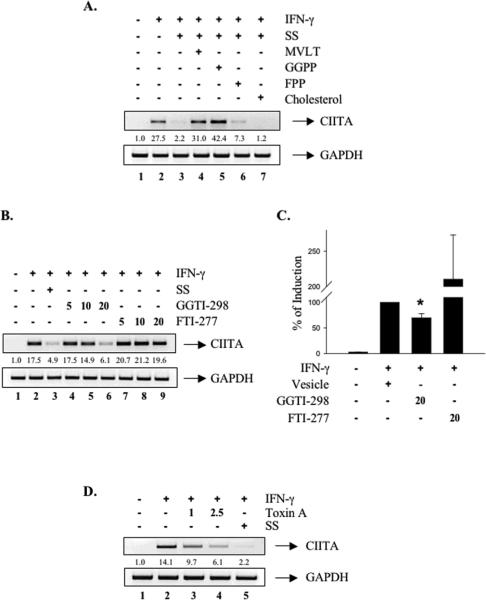
To elucidate the mechanism(s) by which statins inhibit CIITA expression, we determined whether the inhibitory effect of SS was via the HMG-CoA reductase pathways. RAW264.7 cells were incubated with several metabolic intermediates (MVLT, GGPP, FPP or cholesterol) in the presence of SS prior to IFN-γ treatment. The addition of MVLT reversed SS inhibition of IFN-γ-induced CIITA mRNA expression (Figure 3A, lanes 3 and 4), demonstrating that its effect was the result of inhibition of HMG-CoA reductase. GGPP and FPP are required for the post-translational modification of GTP-binding proteins of the Rho and Ras family, respectively. GGPP reversed the inhibitory effect of SS, while FPP had a very modest effect on reversal (Figure 3A, compare lane 3 with lanes 5 and 6). In addition, cholesterol had no effect on the inhibitory influence of SS (Figure 3A, compare lanes 3 and 7). To further examine if inhibition of GTP-binding protein activation was responsible for the inhibitory effect of SS, cells were treated with GGTI-298 and FTI-277, which are specific inhibitors for Ras superfamily protein prenylation [24, 30]. Treatment with GGTI-298 (5-20 μM) inhibited IFN-γ-induced CIITA mRNA expression in a dose-dependent manner (Figure 3B, lanes 4-6), mimicking the effect of SS (lane 3). In contrast, FTI-277 was without effect (Figure 3B, lanes 7-9). We also investigated whether IFN-γ-induced CIITA pIV activity was affected by GGTI-298 and FTI-277 treatment. RAW264.7 cells were transiently transfected with a CIITA pIV construct, and then treated with GGTI-298 (20 μM) or FTI-277 (20 μM) for 12 h. Cells were stimulated with IFN-γ and luciferase activity assessed. GGTI-298 treatment significantly reduced IFN-γ-induced CIITA promoter activity, while FTI-277 had no inhibitory effect (Figure 3C). There was a two-fold increase in CIITA promoter activity in the presence of FTI-277, but this was not significant due to the large standard error (Figure 3C). These results indicate that the effect of SS is mediated by inhibition of HMG-CoA reductase, subsequently inhibiting isoprenylation of the Rho family of G-proteins by decreasing the pools of GGPP.
Figure 3. The Inhibitory Effect of Simvastatin is Dependent on the HMG-CoA Reductase Pathway.
RAW264.7 cells were pretreated with SS (10 μM), SS plus metaboli cintermediates (500 μM MVLT, 5 μM GGPP, 5 μM FPP, or 500 μM Cholesterol) (A), FTI-277 (5-20 μM) or GGTI-298 (5-20 μM) (B) or Toxin A (1-2.5 nM) (D) for 12 h, and then were stimulated by IFN-γ (10 ng/ml) for 12 h. (A, B, D) Total RNA was analyzed by RT-PCR with primers specific for CIITA and GAPDH. Values were normalized to GAPDH mRNA. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of three independent experiments. (C) Cells were transiently transfected with the CIITA pIV construct. Cells were pretreated with FTI-277 (20 μM) or GGTI-298 (20 μM)for 12 h, and then stimulated by IFN-γ (10 ng/ml) for 12 h. Luciferase activity was determined from the cell lysates, as described in Materials and Methods. The results are shown as fold induction (mean ± S.D.) of three independent experiments in which all samples were assayed in duplicate, *, p ≤ 0.05, compared with IFN-γ treatment.
The Rho family of G-proteins, including Rho, Rac, and Cdc42, are isoprenylated primarily by GGPP [16]. As the inhibition by GGTI-298 mimicked the inhibitory effect of SS on CIITA expression, this suggested that SS inhibition was a consequence of inhibition of Rho protein function. We investigated this by using a specific inhibitor of this class of GTPases, C. difficile Toxin A, which specifically inhibits Rho family proteins [31]. Cells were treated with increasing concentrations (1-2.5 nM) of Toxin A prior to IFN-γ treatment. Toxin A treatment reduced IFN-γ-induced CIITA mRNA levels (Figure 3D, lanes 3 and 4), comparable to the extent of SS-mediated inhibition (lane 5). These results suggest that the inhibitory effect of SS is in part derived from its ability to suppress Rho family GTPase function by limiting GGPP levels and subsequently inhibiting isoprenylation.
IFN-γ-induced Rac1 Activation is Involved in CIITA Expression
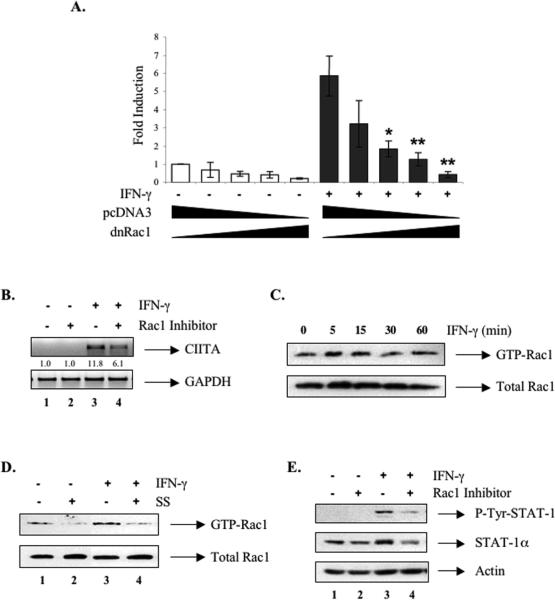
To examine whether IFN-γ induction of CIITA involves the activity of the Rho family of GTPases, we tested the influence of mutant forms of RhoA and Rac1 on CIITA pIV activity. RAW264.7 cells were co-transfected with a CIITA pIV construct and mutant forms of RhoA or Rac1. Overexpression of both dominant-negative and constitutively active RhoA constructs did not influence IFN-γ-induced CIITA pIV activity (data not shown). In contrast, IFN-γ-induced CIITA pIV activity was diminished with increasing concentrations of dominant-negative Rac1 (Figure 4A), while constitutively active Rac1 had no effect (data not shown). We further examined the involvement of Rac1 by using the Rac1 inhibitor NSC23766, which is a cell-permeable Rac1-specific inhibitor [32]. Treatment with NSC23766 (100 μM) for 24 h resulted in suppression of IFN-γ-induced CIITA mRNA expression (Figure 4B, lanes 3 and 4). IFN-γ stimulates the activation of Rac1 in rat astrocytes [26]. Therefore, we examined whether IFN-γ induced Rac1 activation in macrophages. Cells were stimulated with IFN-γ, and GST-PBD-bound active Rac1 detected by immunoblotting. Active Rac1 levels were enhanced by IFN-γ treatment, peaked at 15-30 min, and then decreased (Figure 4C). Collectively, these data indicate that Rac1 activation is involved in IFN-γ-induced CIITA gene expression. We next investigated the effect of SS on Rac1 activation by IFN-γ. Cells were pretreated with SS (10 μM) for 12 h, and then stimulated by IFN-γ for 15 min. SS treatment inhibited IFN-γ-induced Rac1 activation (Figure 4D, lane 4) as well as the basal level of active Rac1 (lane 2). These results suggest that the inhibitory effect of SS on CIITA expression is in part due to inhibition of Rac1 activation. We previously reported that SS inhibits STAT-1α activation by suppressing expression of total STAT-Aα levels in RAW264.7 cells [33]. To determine if Rac1 is involved in STAT-Aα expression, cells were treated with NSC23766, and STAT-A expression examined. We observed that the Rac1 inhibitor attenuated IFN-γ-induced STAT-A activation by suppressing STAT-A expression (Figure 4E, lanes 3 and 4). STAT-A mRNA expression was also inhibited by the Rac1 inhibitor (data not shown), suggesting that Rac1 activity may be involved in STAT-A expression. These results connect Rac1 as being involved in CIITA expression through STAT-A.
Figure 4. IFN-γ-induced Rac1 Activation is Involved in CIITA Expression.
(A) RAW264.7 cells were transiently co-transfected with the CIITA pIV construct and increasing amounts of pRK5-Rac1N17 (dnRac1) (0, 0.1, 0.25, 0.5, 1 μg) or pcDNA3 for 24 h, and then treated with IFN-γ (10 ng/ml) for 12 h. Luciferase activity was determined as described in Materials and Methods. The results are shown as fold induction (mean ± S.D.) of three independent experiments in which all samples were assayed in duplicate. *, p ≤ 0.05, **, p ≤ 0.001, compared with IFN-γ treatment. (B) RAW264.7 cells were pretreated with the Rac1 inhibitor (100 μM) for 24 h, and then stimulated with IFN-γ for 12 h. Total RNA was analyzed by RT-PCR with primers specific for CIITA and GAPDH. Values were normalized to GAPDH mRNA. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of three independent experiments. Cells were treated with IFN-γ (10 ng/ml) for the indicated times (C) or pretreated with SS (10 μM) for 12 h and then stimulated with IFN-γ for 15 min (D). The amount of Rac1 bound to the GST-PBD fusion protein was analyzed by immunoblotting as described in Materials and Methods. Representative of three experiments. (E) RAW264.7 cells were pretreated with the Rac1 inhibitor (100 μM) for 24 h, and then incubated in the absence or presence of IFN-γ for 30 min. Total cell lysates were analyzed by immunoblotting with phospho-STAT-α, stripped, and reprobed with total STAT-α and actin antibodies. Representative of three experiments.
Simvastatin Inhibits STAT-Aα and IRF-1 Expression in BMDM
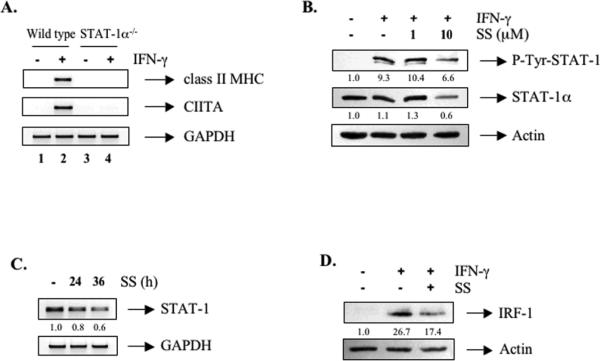
IFN-γ-inducible expression of CIITA and subsequent class II MHC expression requires STAT-Aα [28, 34]. We confirmed this in microglia from STAT-Aα-deficient mice (Figure 5A, compare lanes 2 and 4). To determine if SS inhibits STAT-A in primary macrophages, BMDM were pretreated with SS (1-10 μM) for 24 h prior to IFN-γ treatment, and protein lysates subjected to immunoblotting. SS inhibited activation of IFN-γ-induced STAT-Aα in BMDM through decreasing levels of the STAT-Aα protein (Figure 5B), confirming our previous findings. SS also suppressed STAT-Aα mRNA expression in BMDM (Figure 5C). IFN-γ-induced IRF-1 gene expression is dependent on STAT-Aα [6]. As shown in Figure 5D, IRF-1 protein expression was also inhibited by SS in BMDM, which results from SS-mediated inhibition of STAT-Aα.
Figure 5. Simvastatin Inhibits STAT-Aα and IRF-1 Expression in BMDM.
(A) Primary murine microglia isolated from wild type or STAT-A-deficient 129 mice were incubated with medium or IFN-γ (10 ng/ml) for 12 h. Total RNA was analyzed by RT-PCR with primers specific for class II MHC, CIITA and GAPDH. Representative of three experiments. (B) BMDM were pretreated with SS (1-10 μM) for 24 h, and then stimulated by IFN-γ for 30 min. Total cell lysates were analyzed by immunoblotting with phospho-STAT-Aα, stripped, and reprobed with total STAT-Aα and actin antibodies. Representative of three experiments. (C) BMDM were treated with SS (10 αM) for 0-36 h. Total RNA was analyzed by RT-PCR with primers specific for STAT-A and GAPDH. Values were normalized to the respective GAPDH levels. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of four independent experiments. (D) BMDM were pretreated with SS (10 αM) for 24 h, and then stimulated by IFN-γ for 3 h. Total cell lysates were analyzed by immunoblotting with IRF-1, stripped, and reprobed with actin antibodies. Representative of three experiments.
Simvastatin Does Not Inhibit USF-1 Expression
Binding of STAT-Aα to the GAS element in CIITA pIV is stabilized by USF-1. Moreover, binding of USF-1 to the E-box element is stabilized by STAT-Aα [28]. We examined the effect of SS on USF-1 expression. RAW264.7 cells were treated with increasing concentrations of SS for 16 h, and analyzed by immunoblotting or RT-PCR. SS did not inhibit USF-1 protein or mRNA expression (Figures 6A and 6B).
Figure 6. Simvastatin Does Not Suppress USF-1 Expression.
RAW264.7 cells were incubated with increasing concentrations of SS (0-10 μM) for 16 h (A, B). Nuclear extracts were prepared, and analyzed by immunoblotting with USF-1, HDAC1, or Sp1 antibodies (A), while total RNA was analyzed by RT-PCR with primers specific for USF-1 and GAPDH (B). Representative of three independent experiments.
Simvastatin Affects the Transcriptional Program of the Endogenous CIITA pIV
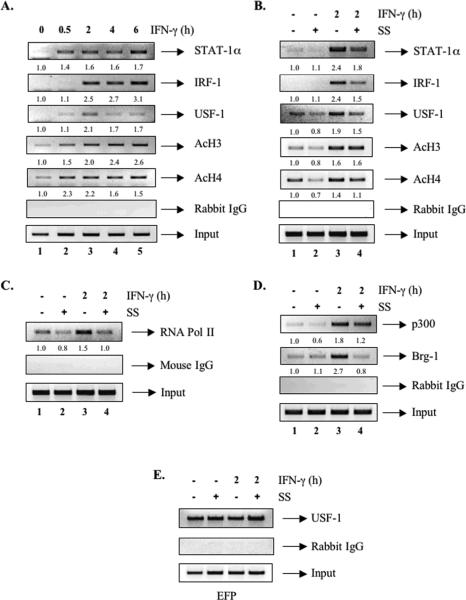
To investigate further whether the inhibitory effect of SS is mediated at the level of CIITA transcription, we examined the influence of SS at the endogenous CIITA pIV. ChIP assays revealed that STAT-Aα recruitment occurred within 30 min of IFN-γ treatment, and persisted for 6 h (Figure 7A). IRF-1 binding was detected at 2 h (lane 3), and was maintained through 6 h. USF-1 was recruited to the CIITA promoter at 2 h (Figure 7A). Acetylation of histones H3/H4 was also induced in a time-dependent manner by IFN-γ (Figure 7A). Histone acetylation may be mediated by the co-activators CBP/p300 [35]. We observed recruitment of p300 to CIITA pIV after 2 h of IFN-γ treatment (Figure 7D, lane 3), but negligible levels of CBP were detected (data not shown). IFN-γ induces Brg-1 recruitment to the CIITA promoter, and chromatin remodeling induced by Brg-1 is required for CIITA gene transcription [12, 35]. We observed IFN-γ induced recruitment of Brg-1 to the CIITA pIV (Figure 7D, lane 3).
Figure 7. Simvastatin Affects the Transcriptional Program of the Endogenous CIITA pIV Promoter.
RAW264.7 cells were either stimulated with IFN-γ for up to 6 h (A) or pretreated with SS (10 μM) for 12 h, and then stimulated with IFN-γ for 2 h (B-E). Cells were cross-linked with formaldehyde, and the soluble chromatin was subjected to immunoprecipitation with antibodies against STAT-Aα, IRF-1, USF-1, AcH3, AcH4, RNA Pol II, p300, Brg-1, rabbit IgG or mouse IgG, followed by PCR for either the CIITA pIV (AD) or the EFP promoter (E). Input chromatin was subjected to PCR to control for variations in immunoprecipitation starting material. Polyclonal rabbit IgG or monoclonal mouse IgG was used as a negative immunoprecipitation control for nonspecific binding. The basal level of the untreated sample was set as 1.0, and fold induction was compared with that. Representative of three independent experiments.
We next examined whether SS inhibits the recruitment of STAT-Aα, IRF-1 and/or USF-1 to CIITA pIV. The 2 h time point of IFN-γ treatment was chosen since all three transcription factors are present at that time (Figure 7A, lane 3). Recruitment of STAT-Aα, IRF-1 and USF-1 was decreased by SS treatment (Figure 7B, compare lanes 3 and 4). H3 acetylation was unaffected by SS treatment, while H4 acetylation was reduced to a modest level (Figure 7B). The IFN-γ-induced recruitment of both p300 and Brg-1 was also decreased by SS, particularly that of Brg-1 (Figure 7D, lanes 3 and 4). Because binding of STAT-A and USF-1 increases recruitment of RNA Pol II to the CIITA promoter after IFN-γ treatment [35], we examined the effect of SS on Pol II recruitment. Pol II recruitment to pIV was observed after a 2 h treatment with IFN-γ, and was inhibited by SS (Figure 7C). STAT-Aα and USF-1 bind to the GAS/E box motifs in a cooperative manner [28]. As shown in Figure 6, USF-1 expression was not inhibited by SS, while recruitment to pIV was inhibited by SS (Figure 7B). To examine whether SS affects the DNA binding ability of USF-1, we investigated the effect of SS on USF-1 recruitment to the estrogen-responsive finger protein (EFP) promoter, which is regulated by USF-1 [36]. USF-1 was recruited to the EFP promoter under basal conditions, and this recruitment was not affected by treatment with SS, IFN-γ or both (Figure 7E). These results suggest that SS destabilizes the binding of USF-1 to the CIITA pIV by reducing recruitment of STAT-Aα, which results from inhibiting its expression. Taken together, these data indicate that SS inhibits CIITA gene expression at the transcriptional level.
Discussion
The ability of statins to inhibit CIITA gene transcription has been demonstrated by several groups [21, 23]. In this study, we demonstrate that statins inhibit IFN-γ-induced CIITA gene expression by inhibiting transcriptional events at CIITA pIV (Figure 8). SS inhibits the expression of STAT-Aα and IRF-1 proteins, resulting in less recruitment to the CIITA pIV. Although USF-1 expression is not affected by SS, recruitment of USF-1 to CIITA pIV is diminished by SS. In addition, we reveal for the first time that Rac1 GTPase is involved in both STAT-Aα expression and IFN-γ-induced CIITA expression, and that SS inhibits Rac1 activation, subsequently inhibiting CIITA expression. Inhibition of class II MHC expression by statins may help to explain their beneficial effects in the treatment of autoimmunity.
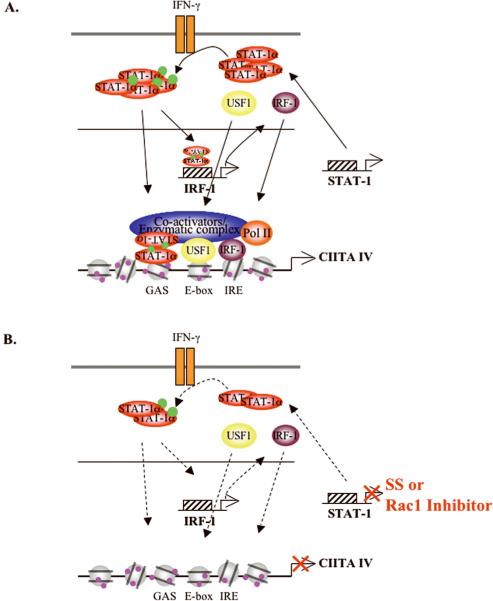
Figure 8. Proposed Model of Statin-mediated Inhibition of CIITA IV Gene Expression.
(A) CIITA IV promoter elements include GAS, E-box and IRE. IFN-γ-induced CIITA gene expression involves recruitment of STAT-Aα, IRF-1 and USF-1 to the CIITA pIV, as well as acetylation of histones H3 and H4 (shown as purple circles). In addition, co-activators such as p300 and Brg-1 and RNA Pol II are recruited to the CIITA pIV, initiating CIITA gene transcription. (B) SS or the Rac1 inhibitor suppress constitutive STAT-Aα expression. This results in inhibition of STAT-Aα activation, subsequently leading to a reduction of IFN-γ-induced IRF-1 gene expression. This inhibition is associated with reduced recruitment of STAT-Aα and IRF-1 to the CIITA pIV. In addition, recruitment of USF-1 is decreased, whereas its expression is not affected. The recruitment of p300, Brg-1 and RNA Pol II is decreased by SS treatment, thereby inhibiting CIITA gene transcription. Green circles indicate phosphorylation.
The inhibitory effect of statins on expression of IFN-γ-induced class II MHC has been shown in numerous cell types [21, 23, 37, 38], however, the molecular basis for suppression is varied. Statins inhibit IFN-γ-induced class II MHC expression at the transcriptional level in microglia and macrophages, which is mediated through inhibition of CIITA expression [21, 23]. However, Buttice et al., demonstrated that the inhibitory effect of SS occurred in a CIITA-independent mechanism in human aortic smooth muscle cells by inhibiting RFX5 expression, which assists in recruiting CIITA to the class II promoter [37]. Kuipers et al., showed that SS does not significantly inhibit the transcription/translation pathway of class II MHC in several human cell types. Instead, SS impairs class II MHC transport to the cell surface by disruption of cholesterol-containing microdomains, which transport and concentrate class II MHC molecules to the cell surface [38]. We have previously shown that ectopic expression of Suppressor Of Cytokine Signaling (SOCS)-1 inhibits IFN-γ-induced activation of CIITA pIV in macrophages by suppression of STAT-Aα activation [34]. SOCS-3 protein induction by LVS contributes to inhibition of STAT-Aα activation in RAW264.7 cells [39]. However, in our hands, SS or LVS did not induce expression of SOCS-1/3 in macrophages (data not shown), indicating that the inhibitory effect of the statins in our study was not due to SOCS expression. Rather, our data indicate that the inhibitory effect of SS on class II MHC expression in macrophages/microglia results from inhibiting CIITA transcription.
IFN-γ-induced CIITA expression is regulated at the transcriptional level by histone modifications and the transcription factors STAT-Aα, IRF-1 and USF-1. SS-mediated suppression of STAT-Aα expression results in inhibition of STAT-Aα activation [33], which contributes to inhibition of the STAT-Aα dependent gene, IRF-1. This ultimately results in the reduced recruitment of STAT-Aα and IRF-1 to the endogenous CIITA pIV. Binding of STAT-Aα stabilizes the binding of USF-1 to CIITA pIV [28]. Although SS did not inhibit USF-1 expression, the DNA binding ability of USF-1 was diminished by SS treatment. Collectively, we propose that SS-mediated inhibition of STAT-Aα contributes to destabilization of USF-1 binding, subsequently leading to less recruitment/retention of USF-1 to CIITA pIV. ATP-dependent chromatin remodeling by Brg-1 is required for CIITA transcription [12, 35]. Our ChIP data demonstrated that Brg-1 is present at CIITA pIV in untreated cells at a low level, and substantially increased upon IFN-γ treatment (Figure 7D, lanes 1 and 3). It has been shown that Brg-1 is present at the promoter before STAT-A and IRF-1-binding [35]. Therefore, Brg-1 seems to be required not only for chromatin changes but also for the initial recruitment of STAT-A. We demonstrated that SS inhibits IFN-γ-induced recruitment of Brg-1 to CIITA pIV (Figure 7D, lane 4), whereas expression of Brg-1 is not suppressed by SS treatment (data not shown). The mechanism by which SS inhibits Brg-1 recruitment is unclear at this time.
The inhibitory effect of SS on IFN-γ-induced CIITA expression was reversed by the addition of MVLT or GGPP (Figure 3A). In addition, the inhibitory effect of SS was mimicked by GGTI-298 and Toxin A (Figures 3B-D), suggesting the inhibitory effect of SS is the result of decreased geranylgeranylation of Rho family GTPases. Therefore, geranylgeranylation of Rho family GTPases may be involved in IFN-γ-induced CIITA expression. Small GTPases play an important role in a variety of immune responses [25, 40]. Rac2 activates Th1-specific signaling and IFN-γ gene expression, thus controlling Th1 cell activation [40], and Rac contributes to the assembly of a multiprotein transduction complex associated with the TCR [25]. We observed that overexpression of the dominant-negative form of Rac1, but not RhoA, inhibited IFN-γ activation of CIITA pIV (Figure 4A and data not shown). Furthermore, a Rac1 inhibitor suppressed IFN-γ-induced CIITA mRNA expression (Figure 4B). In accordance with this result, IFN-γ-induced activation of STAT-A was inhibited by the Rac1 inhibitor, which was mediated through suppression of STAT-A protein and mRNA expression (Figure 4E and data not shown), contributing to inhibition of CIITA expression. Therefore, Rac1 may be involved in STAT-A as well as CIITA expression. We further determined that IFN-γ induces Rac1 activation (Figure 4C), and SS inhibits this activation as well as the basal level of activated Rac1 (Figure 4D). Taken together, these data support the idea that Rac1 contributes to IFN-γ induction of CIITA expression in macrophages, and is one possible target for SS-mediated inhibition of CIITA expression. Regarding STAT-A and Rho family GTPases, Park et al., [26] showed that Rac1 contributes to maximal IFN-γ activation of STAT-A in astrocytes. Rac1 associated with the IFN-γRα and augmented the interaction of IFN-γRα with STAT-A in response to IFN-γ. Besides affecting STAT-A expression, Rac1 may contribute to IFN-γ-induced CIITA expression by affecting the transcriptional activity of transcription factors, enhancing receptor signaling through IFN-γ or affecting other factors. Future experiments are planned to address these possibilities. Because Rho family GTPases are involved in a variety of immune responses as well as cellular functions, statin-mediated inhibition of Rho family GTPases' function provides a better understanding of the molecular mechanisms and diverse actions of statins. Other signaling pathways such as activation of PKCδ, PKCα and PKCβII have been implicated in CIITA gene expression [41, 42]. It will be of interest to determine if statins influence these signaling pathways.
Atorvastatin treatment of patients with rheumatoid arthritis showed a modest beneficial effect [43], and atorvastatin is currently being tested in ongoing clinical trials for patients with relapsing-remitting MS. In addition, oral administration of SS (Zocor) reduced the number of new gadolinium-enhancing lesions in relapsing-remitting MS patients by 44% in a small 6-month open-label trial [44]. Our previous [33] and present findings suggest that the ability of statins to mediate beneficial effects may be a result of blunting class II MHC and CD40 expression on macrophages, thereby lessening antigen presentation and subsequent T cell activation. Moreover, our present findings provide a better understanding of the molecular basis underlying the clinical benefits of statin therapy in autoimmune diseases.
Materials and methods
Recombinant Proteins and Reagents
Recombinant murine IFN-γ and M-CSF were purchased from R&D Systems (Minneapolis, MN), and PE-conjugated rat anti-mouse class II MHC antibody was from SouthernBiotech (Birmingham, AL). Activated SS and LVS, Clostridium difficile Toxin A, FTI-277, GGTI-298 and the Rac1 inhibitor (NSC23766) were from Calbiochem (San Diego, CA). Phospho-STAT-A (Tyr701) was from Cell Signaling Technology (Beverly, MA). STAT-A, AcH3, and AcH4 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY), and all other antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). L-mevalonate, geranylgeranyl pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), and cholesterol were obtained from Sigma-Aldrich (St. Louis, MO). MMLV RT (Moloney Murine Leukemia Virus Reverse Transcriptase) was from Promega (San Luis Obispo, CA).
Cells
BMDM were generated as previously described [45]. Briefly, bone marrow was flushed from the femurs of 6- to 12-week-old C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME). Red blood cells were lysed, and cells were seeded in 60 mm dishes at a concentration of 1 × 106 cells. After a 2 h incubation, suspended cells were removed, and attached cells were cultured for 6 days in the presence of 20 ng/ml of M-CSF. The purity of BMDM was measured by flow cytometry for CD11b staining, and > 98% of the cells were positive. Primary murine microglia from C57BL/6J mice, and WT and STAT-Aα-deficient mice on the 129S6/SvEv background (Taconic, Germantown, NY) were prepared as described previously [46]. The care and maintenance of the mice and the research was performed according to the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. The murine macrophage cell line RAW264.7 was maintained in DMEM supplemented with 10% FBS as previously described [46].
Quantitative Analysis of Class II MHC Protein Expression by Flow Cytometry
Cells were plated at 5 × 105 cells/well and allowed to grow for 12-16 h in media supplemented with 10% FBS. The cells were stimulated with IFN-γ (5 or 10 ng/ml) for 15 h in 1% FBS containing media, and then stained for class II MHC protein expression as previously described [47]. The cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA). Negative controls were stained with an isotype matched control antibody. Ten thousand cells were analyzed for each sample.
RNA Isolation, RT-PCR, and Ribonuclease Protection Assay
Total cellular RNA was isolated, and class II MHC, CIITA, STAT-A, USF-1 and GAPDH mRNA expression were analyzed by RT-PCR, as previously reported [33]. The primers used are shown in Table I. For assessment of CIITA mRNA stability, RPA was performed as described previously [48]. Quantification of the protected RNA fragments was performed by scanning with the PhosphorImager (Molecular Dynamics, Sunnyvale, CA). GAPDH mRNA was used as a housekeeping gene because its levels are not affected by cytokine or statin treatment.
Immunoblotting
Twenty-30 μg of whole cell lysates or nuclear extracts were used for immunoblotting as previously reported [33]. Densitometry was used to quantify the immunoblots, and all results were normalized by the respective actin values. The basal level of the untreated sample was set as 1.0, and fold induction (or inhibition) upon treatment with IFN-γ and/or SS was compared to that.
Plasmids
The pGEX construct encoding the GTPase-binding domain of PAK1, PBD, dominant-negative mutants of Rac1 (Rac1N17) and RhoA (RhoAN19), and constitutively active mutants of Rac1 (Rac1L61) and RhoA (RhoAL63) were a gift from Dr. W.-C. Xiong (Medical College of Georgia, GA, USA) [27, 49].
GST-PAK-PBD-Binding Assays
Rac1 activation was determined as previously reported [50]. Briefly, the pGEX construct encoding the GTPase-binding domain of PAK1 was expressed in E. coli as a GST fusion protein (GST-PBD). After treatment with IFN-γ, cells were lysed and incubated with 10 μg of GST-PBD. To detect GTP-bound Rac1, proteins were separated by SDS-PAGE, and immunoblot analysis was performed using an antibody against Rac1.
Transient Transfection and Luciferase Assays
A luciferase reporter plasmid driven by 1703 bp of the human CIITA pIV was used as previously reported [51]. Transient transfection was performed by Lipofectamine Plus (Invitrogen Life Technologies) as described previously [46]. Transfected cells were stimulated with IFN-γ and the luciferase activity of each sample was normalized to the total protein concentration of each well. Luciferase activity from the untreated sample was arbitrarily set at 1.0 for calculation of fold induction.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described previously [52]. Nuclei from formaldehyde cross-linked cells were resuspended in Tris-EDTA buffer and sonicated. The soluble chromatin was adjusted into RIPA buffer and precleared. Immunoprecipitation was performed with 4 μg of appropriate antibodies, and the immune complexes were absorbed with protein A beads blocked with salmon sperm DNA. Immunoprecipitated DNA was amplified by primer pairs corresponding to the mouse CIITA pIV or Estrogen-responsive Finger Protein (EFP) promoter, and subjected to PCR (Table I). The PCR products were resolved in 1.5% agarose gels in 1X TAE electrophoresis buffer, and stained with ethidium bromide. Densitometry was used to quantify the PCR results, and all results were normalized by the respective input values.
Statistical Analysis
Data are presented as mean ± S.E.M., and the Student's t-test was used to determine statistical differences. P values of ≤ 0.05 were considered to be statistically significant. All experiments were repeated at least three times.
Acknowledgements
This work was supported in part by NIH grants NS45290, NS50665 and NS57563 to E.N.B., and a Pilot Research Grant from the National Multiple Sclerosis Society (PP1129) to E.N.B.. We acknowledge the support of the University of Alabama at Birmingham Flow Cytometry Core Facility (AM20614).
Abbreviations
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- CIITA
class II transactivator
- SS
simvastatin
- LVS
lovastatin
- MVLT
mevalonate
- GGPP
geranylgeranyl pyrophosphate
- FPP
farnesyl pyrophosphate
- BMDM
bone marrow-derived macrophages
- Pol II
RNA Polymerase II
Footnotes
Conflict of interest The authors declare no financial or commercial conflicts of interest.
References
- 1.Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr. Pharm. Des. 2004;10:129–143. doi: 10.2174/1381612043453414. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe GM, Nguyen VT, Benveniste EN. Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: Implications in neurological diseases. J. Neurovirol. 2002;8:496–512. doi: 10.1080/13550280290100941. [DOI] [PubMed] [Google Scholar]
- 3.Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW, Iacomini J. Prevention of type 1 diabetes by gene therapy. J. Clin. Invest. 2004;114:969–978. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 5.Dyment DA, Sadovnick AD, Ebers GC. Genetics of multiple sclerosis. Hum. Mol. Genet. 1997;6:1693–1698. doi: 10.1093/hmg/6.10.1693. [DOI] [PubMed] [Google Scholar]
- 6.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 7.Cullell-Young M, Barrachina M, Lopez-Lopez C, Gonalons E, Lloberas J, Soler C, Celada A. From transcription to cell surface expression, the induction of MHC class II I-A alpha by interferon-gamma in macrophages is regulated at different levels. Immunogenetics. 2001;53:136–144. doi: 10.1007/s002510100312. [DOI] [PubMed] [Google Scholar]
- 8.Tosi G, Jabrane-Ferrat N, Peterlin BM. Phosphorylation of CIITA directs its oligomerization, accumulation and increased activity on MHCII promoters. EMBO J. 2002;21:5467–5476. doi: 10.1093/emboj/cdf557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greer SF, Harton JA, Linhoff MW, Janczak CA, Ting JP, Cressman DE. Serine residues 286, 288, and 293 within the CIITA: a mechanism for down-regulating CIITA activity through phosphorylation. J. Immunol. 2004;173:376–383. doi: 10.4049/jimmunol.173.1.376. [DOI] [PubMed] [Google Scholar]
- 10.Greer SF, Zika E, Conti B, Zhu XS, Ting JP. Enhancement of CIITA transcriptional function by ubiquitin. Nat. Immunol. 2003;4:1074–1082. doi: 10.1038/ni985. [DOI] [PubMed] [Google Scholar]
- 11.Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- 12.Pattenden SG, Klose R, Karaskov E, Bremner R. Interferon-gamma-induced chromatin remodeling at the CIITA locus is BRG1 dependent. EMBO J. 2002;21:1978–1986. doi: 10.1093/emboj/21.8.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 14.Weber MS, Youssef S, Dunn SE, Prod'homme T, Neuhaus O, Stuve O, Greenwood J, et al. Statins in the treatment of central nervous system autoimmune disease. J. Neuroimmunol. 2006;178:140–148. doi: 10.1016/j.jneuroim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordle A, Landreth G. 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J. Neurosci. 2005;25:299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 19.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 20.Menge T, Hartung HP, Stuve O. Statins - a cure-all for the brain? Nat. Rev. Neurosci. 2005;6:325–331. doi: 10.1038/nrn1652. [DOI] [PubMed] [Google Scholar]
- 21.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 22.Neuhaus O, Strasser-Fuchs S, Fazekas F, Kieseier BC, Niederwieser G, Hartung HP, Archelos JJ. Statins as immunomodulators: comparison with interferon-beta 1b in MS. Neurology. 2002;59:990–997. doi: 10.1212/wnl.59.7.990. [DOI] [PubMed] [Google Scholar]
- 23.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 24.Dunn SE, Youssef S, Goldstein MJ, Prod'homme T, Weber MS, Zamvil SS, Steinman L. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J. Exp. Med. 2006;203:401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrieumerlou C, Randriamampita C, Bismuth G, Trautmann A. Rac is involved in early TCR signaling. J. Immunol. 2000;165:3182–3189. doi: 10.4049/jimmunol.165.6.3182. [DOI] [PubMed] [Google Scholar]
- 26.Park EJ, Ji KA, Jeon SB, Choi WH, Han IO, You HJ, Kim JH, et al. Rac1 contributes to maximal activation of STAT1 and STAT3 in IFN-gammastimulated rat astrocytes. J. Immunol. 2004;173:5697–5703. doi: 10.4049/jimmunol.173.9.5697. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier S, Duhamel F, Coulombe P, Popoff MR, Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G proteincoupled receptors. Mol. Cell Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlethaler-Mottet A, Di Berardino W, Otten LA, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 29.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 30.Finder JD, Litz JL, Blaskovich MA, McGuire TF, Qian Y, Hamilton AD, Davies P, Sebti SM. Inhibition of protein geranylgeranylation causes a superinduction of nitric-oxide synthase-2 by interleukin-1beta in vascular smooth muscle cells. J. Biol. Chem. 1997;272:13484–13488. doi: 10.1074/jbc.272.21.13484. [DOI] [PubMed] [Google Scholar]
- 31.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Qin H, Benveniste EN. Simvastatin inhibits IFN-gamma-induced CD40 gene expression by suppressing STAT-Aalpha. J. Leukoc. Biol. 2007;82:436–447. doi: 10.1189/jlb.1206739. [DOI] [PubMed] [Google Scholar]
- 34.O'Keefe GM, Nguyen VT, Ping Tang LL, Benveniste EN. IFN-gamma regulation of class II transactivator promoter IV in macrophages and microglia: involvement of the suppressors of cytokine signaling-1 protein. J. Immunol. 2001;166:2260–2269. doi: 10.4049/jimmunol.166.4.2260. [DOI] [PubMed] [Google Scholar]
- 35.Ni Z, Karaskov E, Yu T, Callaghan SM, Der S, Park DS, Xu Z, et al. Apical role for BRG1 in cytokine-induced promoter assembly. Proc. Natl. Acad. Sci. U S A. 2005;102:14611–14616. doi: 10.1073/pnas.0503070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda K, Inoue S, Orimo A, Tsutsumi K, Muramatsu M. Promoter analysis of mouse estrogen-responsive finger protein (efp) gene: mouse efp promoter contains an E-box that is also conserved in human. Gene. 1998;216:155–162. doi: 10.1016/s0378-1119(98)00307-2. [DOI] [PubMed] [Google Scholar]
- 37.Buttice G, Miller J, Wang L, Smith BD. Interferon-gamma induces major histocompatibility class II transactivator (CIITA), which mediates collagen repression and major histocompatibility class II activation by human aortic smooth muscle cells. Circ. Res. 2006;98:472–479. doi: 10.1161/01.RES.0000204725.46332.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum. Immunol. 2005;66:653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Huang KC, Chen CW, Chen JC, Lin WW. Statins induce suppressor of cytokine signaling-3 in macrophages. FEBS Lett. 2003;555:385–389. doi: 10.1016/s0014-5793(03)01297-3. [DOI] [PubMed] [Google Scholar]
- 40.Li B, Yu H, Zheng W, Voll R, Na S, Roberts AW, Williams DA, et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–2222. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 41.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J. Biol. Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 42.Kwon MJ, Yao Y, Walter MJ, Holtzman MJ, Chang CH. Role of PKCdelta in IFN-gamma-inducible CIITA gene expression. Mol. Immunol. 2007;44:2841–2849. doi: 10.1016/j.molimm.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 44.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 45.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular Mechanism of Lipopolysaccharide-Induced SOCS-3 Gene Expression in Macrophages and Microglia. J. Immunol. 2007;179:5966–5976. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen VT, Benveniste EN. Involvement of STAT-A and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J. Biol. Chem. 2000;275:23674–23684. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen VT, Walker WS, Benveniste EN. Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur. J. Immunol. 1998;28:2537–2548. doi: 10.1002/(SICI)1521-4141(199808)28:08<2537::AID-IMMU2537>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.O'Keefe GM, Nguyen VT, Benveniste EN. Class II transactivator and class II MHC gene expression in microglia: modulation by the cytokines TGF-beta, IL-4, IL-13 and IL-10. Eur. J. Immunol. 1999;29:1275–1285. doi: 10.1002/(SICI)1521-4141(199904)29:04<1275::AID-IMMU1275>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 49.Azim AC, Barkalow K, Chou J, Hartwig JH. Activation of the small GTPases, rac and cdc42, after ligation of the platelet PAR-1 receptor. Blood. 2000;95:959–964. [PubMed] [Google Scholar]
- 50.Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y, Rohn WM, Benveniste EN. IFN-gamma regulation of the type IV class II transactivator promoter in astrocytes. J. Immunol. 1999;162:4731–4739. [PubMed] [Google Scholar]
- 52.Ma Z, Shah RC, Chang MJ, Benveniste EN. Coordination of cell signaling, chromatin remodeling, histone modifications, and regulator recruitment in human matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 2004;24:5496–5509. doi: 10.1128/MCB.24.12.5496-5509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]