Abstract
Objectives
To examine racial/ethnic disparities in older women’s health-related quality of life (QOL) and type of breast cancer treatment as mediated by physician level and individual level variables.
Methods
A cross-sectional survey of a population-based, consecutive sample identified through the Los Angeles Cancer Surveillance Program of Latina (n = 99), African-American (n = 66), and White (n = 92) women aged 55 years or older (N = 257) between 3 and 9 months after primary breast cancer diagnosis and at least one month post treatment. An exploratory, empirically-developed latent variable model tested the relationships among demographic and physician-related variables, patient attitudes, and health-related outcomes. Health-related outcomes included QOL measures and receipt of breast conserving surgery (BCS).
Results
Latinas reported less BCS and poorer QOL compared to Whites. Physician communication that can empower patients, in terms of patient efficacy in patient-physician interactions and breast cancer knowledge, mitigated racial/ethnic disparities in receipt of BCS. Physician emotional support was not related to patient cognitive empowerment and treatment outcomes. Medical mistrust in minority women was related to less self-efficacy and less positive coping, as well as, both directly and indirectly, to reduced QOL. Latinas reported poorer QOL in the tested model.
Conclusions
Physician communication style, specifically information-giving and participatory decision-making, may empower older women with breast cancer and help mitigate racial/ethnic disparities in surgical treatment received.
INTRODUCTION
The U.S. population is rapidly aging and becoming more ethnically diverse (Institute for the Future, 2000). Among older women, breast cancer is the most common cancer and the second leading cause of cancer death (Jemal et al., 2007; Landis, Murray, Bolden, & Wingo, 1998). Because of its prevalence among older women, breast cancer may serve as a valuable model for examining sources of health care disparities in vulnerable populations. Older breast cancer patients have well documented disparities in breast cancer care (Busch et al., 1996; Guadagnoli et al., 1998). In addition, studies report racial/ethnic group disparities in incidence, treatment, and survival among breast cancer patients (Institute of Medicine, 2003). Correlates of disparities include patient sociodemographic characteristics, physician and system-related factors, and patient cognitive factors (Institute of Medicine, 2003).
There are two disparity-related outcomes of breast cancer care assessed in this study: type of surgical treatment received, breast conserving surgery versus mastectomy, and health-related quality of life. Health-related quality of life (QOL), including physical and emotional health and functioning, is widely recognized as an important outcome of breast cancer care (American Society of Clinical Oncology, 1996). However, examination of racial/ethnic differences in post-surgical QOL for breast cancer patients is sparse, conflicting, and typically limited by small minority sample sizes, low minority group response rates, and/or non-population based samples (Ashing-Giwa, Ganz, & Petersen, 1999; Culver, Arena, Antoni, & Carver, 2002; Spencer et al., 1999). A significant factor in QOL may be type of surgical treatment received.
Breast conserving surgery (BCS) followed by radiation has been shown to have equivalent rates of breast cancer survival as mastectomy (A. D. Morris et al., 1997). Recent research indicates that mastectomy patients may experience poorer outcomes in physical functioning than do BCS patients after breast cancer treatment is complete (Ganz et al., 2004). Furthermore, older women appear to benefit from breast conserving therapy rather than mastectomy as much as younger women in terms of better body image, less disruption of daily habits, and less sense of insecurity (Engel, Kerr, Schlesinger-Raab, Sauer, & Holzel, 2004). The role of race/ethnicity in receipt of BCS has been more extensively studied than in QOL, but findings are also inconsistent (Gilligan, Kneusel, Hoffmann, Greer, & Nattinger, 2002; Mandelblatt et al., 2002; Michalski & Nattinger, 1997; C. R. Morris, Cohen, Schlag, & Wright, 2000; Roetzheim et al., 2000).
Ultimately, it is at the level of the patient-physician interaction that disparities in health care and outcomes, whether originating socially, organizationally, or individually, are either effected or mitigated. From the physician side of this relationship, physician participatory style and information-giving may enhance patient positive coping with breast cancer (McWilliam, Brown, & Stewart, 2000) and patient QOL (Kerr, Engel, Schlesinger-Raab, Sauer, & Holzel, 2003), as well as be associated with greater patient breast cancer knowledge and receipt of BCS (R. C. Maly, Leake, & Silliman, 2004). However, minority patients are reported to experience less participatory visits and receive less information than white patients (Cooper-Patrick et al., 1999; R. C. Maly, Leake, & Silliman, 2003). Racial/ethnic subgroups may also perceive less positive affect from their physicians than white patients (Johnson, Roter, Powe, & Cooper, 2004). Physician emotional support has been reported to alleviate patient distress and increase patient perceived efficacy in coping with cancer (Zachariae et al., 2003), which may, in turn, enhance patient QOL. However, such support does not appear to affect patient receipt of BCS (R. C. Maly, Leake et al., 2004).
In terms of patient elements in the patient-physician relationship, patient self-efficacy in communicating with physicians is reported to predict better QOL (Maliski et al., 2004) and is also associated with greater participation in treatment decision-making (R. C. Maly, Umezawa, Leake, & Silliman, 2004), which in turn may affect receipt of BCS (S. J. Katz et al., 2005) and QOL (Deadman, Leinster, Owens, Dewey, & Slade, 2001; Hack, Degner, Watson, & Sinha, in press). A patient cognitive factor that can be a product of the patient-physician interaction is knowledge about breast cancer and its treatment, which may lead to increased receipt of BCS (Whelan et al., 2004) and be associated with better QOL (Stiegelis et al., 2004). However, minority women are reported to have less breast cancer knowledge compared to white women (Paskett et al., 2004). In terms of other patient cognitive factors that may impact on QOL, patients’ “active” coping styles (e.g., positive reframing, acting/planning to change situations) have been shown to be related to better mental health outcomes in cancer patients (Carver et al., 1993; Dunkel-Schetter, Feinstein, Taylor, & Falke, 1992). However, as one possible contributing factor in this regard, it has been suggested that minority women may have more “fatalistic” attitudes towards cancer’s impact on their lives (Powe & Finnie, 2003).
Finally, a particularly salient issue for minority patients in obtaining health care and in health outcomes may be perceived racism and mistrust of the medical system (LaVeist, Nickerson, & Bowie, 2000) These may be system-level attitudinal variables, rather than patient or patient-physician communication characteristics, because they represent perceived inequitable or oppressive treatments that are collectively experienced by socially disempowered groups. Such perceptions may have a spillover effect on the patient-physician relationship and trust in one’s personal physician, which, in turn, may lead patients to question the medical information provided and the efficacy of recommended treatment (e.g., the choice between mastectomy and BCS with radiation). Less adherence to the physician’s recommendations and greater dissatisfaction with care may result as well. Additionally, literature suggests that that racism in society at large may have a direct adverse impact on QOL (Clark, Anderson, Clark, & Williams, 1999).
Most recent studies addressing racial/ethnic differences in breast cancer treatment and outcomes adjust for some patient characteristics, including demographics and socioeconomic status (SES). Despite the cumulative findings reported above, to our knowledge, no study has simultaneously adjusted for a wide variety of patient measures such as those described, including race/ethnicity, SES, and cognitive factors, physician communication, as well as racism in/mistrust of the health care system. Also of necessity is controlling for stage of cancer and other comorbid conditions. Using the empirically suggested associations between the various types of variables from our literature review, we developed an exploratory, comprehensive conceptual model to examine these relationships heuristically.
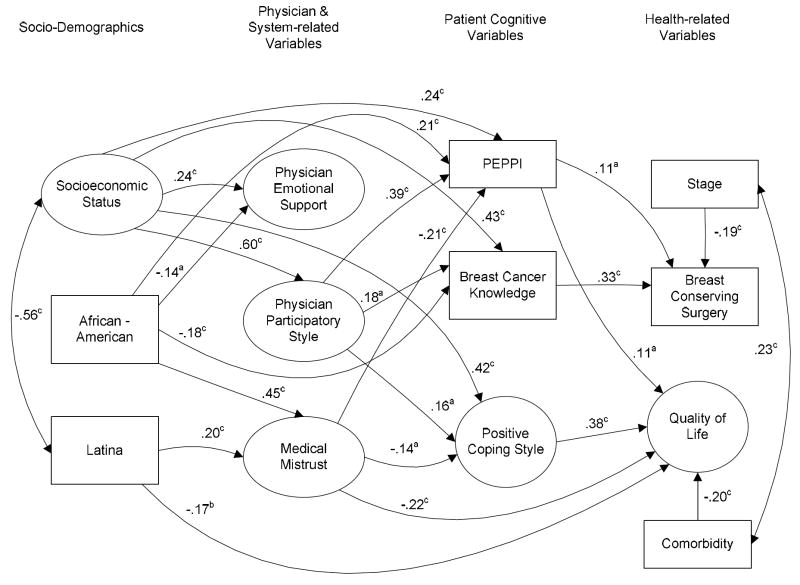
In the process of model development for this research, incorporating the evidence cited above, we posited a logical order that sociodemographic variables would would have an influence on physician communication (e.g., ethnic minority status associated with less information giving) (R. C. Maly et al., 2003), which in turn would influence patient cognitive variables (e.g., information-giving resulting in more breast cancer knowledge) (R. C. Maly, Leake et al., 2004), and lastly, that these latter variables would directly have an effect on the outcomes of treatment received (e.g., greater knowledge influencing treatment choices) (Whelan et al., 2004) and quality of life (e.g., positive coping resulting in better functioning) (Carver et al., 1993; Dunkel-Schetter et al., 1992) (see Figure 1 for framework and flow of the model). Assumptions of directionality were based on prior research and reasonable plausibility, as well as potential for intervention through education and training of health personnel. For example, although physician-patient communication is inherently bidirectional, physicians have been shown to largely control and dominate the physician-patient interaction (Del Piccolo, Mazzi, Saltini, & Zimmermann, 2002). Further, the influence of physician on patient is of particular interest because any one physician has a much broader reach than any one patient, hence interventions to improve outcomes may be most effectively targeted on physicians.
Figure 1.
Final structural equation model depicting the effect of physician communication and patient cognitive factors on treatment received and quality of life. Note: All estimated parameters are standardized. The large circles designate latent variables; the rectangles represent measured variables. One-headed arrows represent regression paths, two-headed arrows represent correlations. ap < .05; bp < .01; cp < .001. (PEPPI = Perceived Efficacy in Patient-Physician Interactions).
In addition to assessing overall relationship patterns within our heuristic model, the following three hypotheses were specifically examined. First, there would be racial/ethnic disparities in older women’s QOL and breast cancer treatment. Second, patient-empowerment through physician communication, as reported by patients, would predict better QOL and receipt of BCS. Third, relationships among race/ethnicity and the outcomes of QOL and BCS would be mediated by physician- and system-level factors (physician communication and perceived racism), and patient cognitive factors (self-efficacy, knowledge, and coping).
METHODS
Participants
The study was a cross-sectional survey of Latina, African-American and White women living in Los Angeles County, aged 55 or older, who were recently diagnosed with and treated for breast cancer (N = 257; 99 Latinas, 66 African Americans, and 92 Whites). Medical records were obtained on all participants and abstracted for stage of cancer and surgical treatment. The participant pool was drawn from the LA County Cancer Surveillance Program’s (CSP) rapid ascertainment program. Study eligibility criteria were: English- or Spanish-speaking Latina, African-American, or White women aged 55 and older, diagnosed for the first time with stage one or higher breast cancer. It was not possible to sample other racial/ethnic groups due to CSP prohibitions against simultaneous, multi-study solicitation of the same population group. All participants had to be between three and nine months of diagnosis and at least one month post treatment, i.e., after surgery, chemotherapy, or radiation, whichever occurred last. The details of the recruitment, study flow and design, are published elsewhere (Maly, Umezawa, Ratliff, & Leake, 2006). The response rate was 64% and was equivalent across racial/ethnic groups. Responders were somewhat younger than non-responders (mean (SD): 68.0 (8.5) versus 71.3 (1.0) years of age, p < 0.001). The average time from date of diagnosis to interview was 8.2 months (SD = 2.7).
Study participants engaged in telephone or face-to-face in-depth interviews and completed short supplemental self-administered questionnaires; all respondents were contacted by telephone to assist with any difficulties completing the self-administered questionnaires. Sixty-eight interviews were conducted in Spanish (26.5% of the total sample). Spanish interviews were conducted by bilingual as well as bicultural interviewers. English-Spanish translation procedures for the instruments included forward and backward translation to assure the accuracy of translations. The study received University of California, Los Angeles Human Subjects Protection Committee approval.
Measures
Measures consisted of both multiple-indicator latent variables (which are error-free constructs in structural equation modeling and represent the shared variance of multiple measured variables, explained further below) and measured variables.
Predictor variables
Socioeconomic Status was a latent variable representing education and income. Education was assessed on a 1-8 categorical scale ranging from less than or equal to 4 years of grade school to more than a college graduate. Income was measured by self-reported total household income before taxes. Race/ethnicity was based on patient self-identification. White participants were the reference category.
Physician Emotional Support was a latent variable comprised of 4 parcels of means created from 8 items assessing patient perception of emotional support from the surgeon who gave or confirmed the breast cancer diagnosis. Surgeons were the specific focus of all physician measures in this study, as surgeons are almost always the first breast cancer care provider with whom newly diagnosed patients consult regarding treatment. We combined the emotional support items into parcels to avoid too many indicators for the size of the sample (Little, Cunningham, Shahar, & Widaman, 2002). This procedure is acceptable when the coefficient alpha among all of the items is high (in this case, coefficient α = .88) and no clear factor structure can separate the items into meaningful sub-factors (first eigenvalue = 4.77, all other eigenvalues less than 1). Parcels were labeled Emot1 to Emot4. The 8 items representing surgeon emotional support were based on a qualitative study on older women with breast cancer in which participants described aspects of physician emotional support that were important to them (Feher & Maly, 1999). A typical item included “at the time of your breast cancer diagnosis your surgeon was extremely compassionate,” with a Likert-style response set.
Physician Participatory Style reflected the degree to which the surgeon had provided the women with information about breast cancer and encouraged them to participate in their own treatment decision-making process. This latent variable was represented by 1) physicians’ interactive information-giving as measured by a previously published index, 2) physicians’ participatory decision-making style scores, and 3) whether surgeons had specifically asked about the patients’ preferred treatment for their breast cancer. A summed index derived from 13 breast cancer topics that patients considered important for providers to discuss, with a score range of 0-13, was used to measure information giving; details on the topics and the construction of this index are available elsewhere (R. C. Maly et al., 2003).
The participatory decision-making style of patients’ surgeons, i.e., their propensity to involve patients in diagnostic and treatment decisions, was measured by patient self-report using the 3-item Participatory Decision Making (PDM) Style Scale from the Medical Outcomes Study (Kaplan, Greenfield, Gandek, Rogers, & Ware, 1996), and an individual item inquiring about the degree of surgeon solicitation of patient input. The PDM scale has a range of 0 to 100; a higher score indicates a more participatory decision-making style and was rescaled for analytic purposes by dividing by 10. Cronbach’s alpha for the PDM scale in this study was 0.93 and did not differ across racial/ethnic groups. The single additional item has a range of 1-5, with a higher score indicating greater solicitation of input from the patient.
The Racism/Medical Mistrust measure, a latent variable obtained from the self-administered questionnaire, contained 2 components: 1) the mean of 4 items related to racism in medical care (e.g., “Doctors treat ethnic minorities and White people the same,” coefficient α = .85) and 2) the mean of 4 items related to general medical mistrust (e.g., “Patients have sometimes been deceived or misled at hospitals”; coefficient α = .67) (LaVeist et al., 2000); each item had a 5 point Likert-type response set with a range of 1-5, higher numbers indicating greater perceived racism and medical mistrust. This last measure was utilized as a system level variable, in contrast to measures of individual-specific physician behavior or patient cognitive factors, because the items ask about bias in both institutions and providers in general.
Perceived Efficacy in Patient-Physician Interactions (PEPPI), a measured variable, consisted of the mean of 5 items assessing patients’ confidence and feelings of efficacy in their interactions with their physicians (R. C. Maly, Frank, Marshall, DiMatteo, & Reuben, 1998). For instance, patients were asked about their confidence in their ability in knowing what questions to ask a doctor. The range of scores is 0-50, 50 indicating greater self-efficacy, and scores were rescaled for the purposes of the analysis by dividing by 10. Coefficient alpha was high among the 5 items (.97).
Breast cancer knowledge was the sum of correct responses to 5 questions (Street, Voigt, Geyer, Manning, & Swanson, 1995) and ranged from 0-5. A typical question was: “Radiation therapy typically follows which form of treatment?”
Positive Coping Style was a latent variable derived from six positive coping subscales from the Brief COPE questionnaire (Carver, 1997). The women were asked to respond with ways they had been coping with general stress and life changes since learning they had breast cancer. The coping subscales included active coping (α = .84), planning (α = .84), seeking instrumental support (α = .71), seeking emotional support (α = .81), positive reframing (α = .71), and acceptance (α = .79). The six subscales were collapsed into 3 parcels to avoid too many measured variables.
Control variables
Stage of breast cancer and significant comorbid illnesses were variables that could potentially confound QOL and which surgical treatment was chosen. Stage of breast cancer was confirmed by medical record review. Comorbidity was assessed by an adaptation of the Charlson Comorbidity Index for patient self-report (J. N. Katz, Chang, Sangha, Fossel, & Bates, 1996). The variable was dichotomized for analysis as “any” versus “none.”
Outcome variables
BCS versus mastectomy was confirmed by medical record review. QOL was a latent variable obtained from the self-administered questionnaire that consisted of four measures of physical and emotional health. 1) The SF-36 Health Survey (Stewart, Hays, & Ware, 1988) which includes 36 items with 8 subscales (physical functioning, role functioning-physical, bodily pain, general health, vitality, social functioning, role functioning-emotional, and mental health). Items were reverse-scored, where appropriate, and the sum was used as an indicator (alpha = .89). 2) A total-item mean score from the 16-item Center for Epidemiological Studies Depression (CES-D) scale (Radloff, 1977) that assessed the frequency of depressive symptoms in the previous week on a 4-point response scale ranging from 0 (rarely or none of the time) to 3 (most of the time). The items were reverse-scored, as appropriate, and summed. Higher scores indicate less depression (alpha = .93). 3) Generalized anxiety was measured by the 20-item State subscale of the State-Trait Anxiety Inventory (STAI-S) (Spielberger, Gorsuch, & Lushene, 1970). The anxiety items assess transitory emotional states such as apprehension and worry (alpha = .96). 4) Breast cancer-specific anxiety was assessed by the mean of 4 items such as worry about the breast cancer recurring (Silliman, Dukes, Sullivan, & Kaplan, 1998, alpha = .89).
Analyses
Confirmatory factor analyses
Initial confirmatory factor analyses (CFA) were performed with each latent construct predicting its hypothesized manifest indicators. This analysis tested the sufficiency of the measurement model and examined associations among the latent and measured variables. The Lagrange Multiplier (LM) test was used to determine additional relationships to add to models for fit improvement (Bentler, 2006).
Data were then analyzed with latent variable structural equation models (Bentler, 2005). Latent variables are error-free constructs that represent the shared variance among multiple measured variables. Goodness-of-fit of the models was assessed with the maximum-likelihood χ2 statistic, the Comparative Fit Index (CFI), the Satorra-Bentler χ2 (S-B χ2), the Robust Comparative Fit Index (RCFI), and the root mean squared error of approximation (RMSEA) (Bentler, 2005). The Robust S-B χ2 was used in addition to the maximum likelihood χ2 because it is more appropriate when the data depart from multivariate normality. Mardia’s normalized multivariate kurtosis estimate was high (z-statistic = 32.2). The CFI and RCFI range from 0 to 1 and reflect the improvement in fit of a hypothesized model over a model of complete independence among the measured variables. Values approaching 0.95 or greater are desirable for the CFI and RCFI. The RMSEA is a measure of fit per degrees of freedom, controlling for sample size; values less than .06 indicate a relatively good fit.
Latent variable path analysis
As posited in the Introduction above, a path model (Figure 1) positioned sociodemographic variables, specifically, SES, African-American and Latina race/ethnicity as foundational to patient-reported physician and system-related variables, i.e., physician emotional support, physician participatory style, and medical mistrust. These three perceptions of physician behaviors, in particular, and the health care system, in general, were then positioned as contributing to patient self-efficacy in patient-physician interactions, breast cancer knowledge, and positive coping style. All variables were used to determine their relationships to the outcome variables of QOL and BCS. Stage of breast cancer and comorbidity were also included as important controls of QOL and BCS. Non-significant paths were dropped in successive model iterations as suggested by MacCallum (1986).
Results
Demographics
The average age of the overall sample was 68.7 years (S.D., 8.5), with no significant differences between racial/ethnic groups. Latinas were much more likely than African Americans and Whites to have less than a high school education (57.6%, 25.8%, 5.4%, respectively, p ≤ .001). The African-American women were least likely to be married compared to Latinas and Whites (27.3%, 46.5%, and 58.7%, respectively, p ≤ .001). Both Latinas and African Americans were much more likely than Whites to have an income less than $20,000 (51.5%, 27.3%, 10.9%, respectively, p ≤ .001). Only 8% of the overall sample had breast cancer stage greater than II, with no significant racial/ethnic group differences. Over half of Latinas and African-Americans had at least one comorbid medical condition, compared to one third of Whites (55.6%, 54.5%, 32.7%, respectively, p = .02). Latinas and African-Americans were less likely than Whites to receive BCS (51% versus 59% for the African-American women and 71% for the White women; p ≤ .02).
Confirmatory Factor Analysis (CFA)
Fit indexes for the CFA model testing the adequacy of the measurement model were acceptable: Maximum-Likelihood χ2 (201, N = 257) = 350.58, CFI = .95, RMSEA = .05; S-B χ2 (201, N = 257) = 336.52, RCFI = .95, RMSEA = 0.05. All measured variables hypothesized to be indicators of the latent variables had significant factor loadings (p ≤ .001). Table 1 presents the factor loadings of the measured variables on their hypothesized latent variables in the CFA model and reports the means and standard deviations of all of the measured variables used in the model. For an improved fit, one correlated error residual suggested by the LM test was added between two of the indicators of Physician Emotional Support.
Table 1.
Means, Standard Deviations, and Factor Loadings in the Confirmatory Factor Analysis
| Variables | M (SD) | Factor Loading* |
|---|---|---|
| Socioeconomic Status | ||
| Education | 5.25 (1.49) | .77 |
| Income | 4.50 (2.06) | .72 |
| African American | 0.26 (0.44) | — |
| Latina | 0.39 (0.49) | — |
| Physician Emotional Support | ||
| Emot 1 | 8.12 (2.18) | .82 |
| Emot 2 | 4.50 (0.73) | .75 |
| Emot 3 | 3.39 (1.10) | .68 |
| Emot 4 | 3.56 (1.02) | .74 |
| Physician Participatory Style | ||
| Information-giving | 5.68 (2.75) | .71 |
| Decision-making style | 5.22 (3.53) | .68 |
| Soliciting patient input | 3.53 (1.53) | .65 |
| Medical Mistrust | ||
| Perceived racism | 2.61 (1.00) | .93 |
| General mistrust | 2.89 (0.85) | .81 |
| PEPPI | 3.93 (1.30) | — |
| Breast cancer knowledge | 2.37 (1.45) | — |
| Positive Coping Style | ||
| Posi 1 | 4.73 (1.15) | .89 |
| Posi 2 | 4.08 (1.33) | .65 |
| Posi 3 | 4.63 (1.03) | .60 |
| Stage of breast cancer | 1.72 (0.69) | — |
| Comorbidity | 0.98 (1.64) | — |
| Breast conserving surgery | 1.61 (0.49) | — |
| Quality of Life | ||
| Breast cancer emotional health | 7.07 (2.49) | .77 |
| SF-36 | 4.90 (1.75) | .82 |
| CES-D | 3.58 (1.10) | .94 |
| State anxiety | 6.43 (1.30) | .89 |
All loadings significant p ≤.001.
PEPPI = Perceived Efficacy in Patient-Physician Interactions.
Table 2 presents the correlations among the latent and measured variables. Of note, African-American race/ethnicity was significantly and negatively associated with physician emotional support (-.14) and Latina ethnicity with self-efficacy (-.32). Minority status was significantly and negatively associated with BC knowledge (-.17 for African Americans, -.16 for Latinas), as well as with positive coping (-.17 for African Americans, -.16 for Latinas). Latina ethnicity was significantly and negatively related to both BCS and QOL (-.15, -.30, respectively).
Table 2.
Correlations among Latent Variables and Single Items
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SES | — | |||||||||||
| 2. African American | -.06 | — | ||||||||||
| 3. Latina | -.58c | NA | — | |||||||||
| 4. Physician Emotional Support | .28c | -.14a | -.08 | — | ||||||||
| 5. Physician Participatory Style | .53c | -.07 | -.10 | .63c | — | |||||||
| 6. Medical Mistrust | -.17 b | .35c | -.01 | -.33c | -.22c | — | ||||||
| 7. PEPPI | .54c | .13a | -.32c | .38c | .51c | -.26c | — | |||||
| 8. Breast cancer knowledge | .58c | -.17b | -.19c | .22c | .42c | -.17b | .32c | — | ||||
| 9. Positive Coping Style | .58c | -.13a | -.16a | .21b | .45c | -.24c | .46c | .45c | — | |||
| 10. Stage of breast cancer | -.05 | .05 | .01 | -.17b | -.24c | .15a | -.10 | -.07 | -.01 | — | ||
| 11. Comorbidity | -.26c | .05 | .05 | .00 | -.20b | -.01 | -.03 | -.18b | -.12 | .23c | — | |
| 12. Breast conserving surgery | .30c | .00 | -.15c | -.01 | .17a | -.03 | .23c | .37c | .23c | -.22c | -.15a | — |
| 13. Quality of Life | .51c | .02 | -.30c | .26c | .35c | -.34c | .40c | .27c | .54c | -.08 | -.25c | .12 |
p ≤.05;
p ≤.01;
p ≤.001.
PEPPI = Perceived Efficacy in Patient-Physician Interactions, NA = not applicable.
Path Model
Figure 1 presents the final path model after non-significant paths were successively eliminated, as described above. The final model has acceptable goodness-of-fit statistics: Maximum-Likelihood χ2 (245, N = 257) = 404.90, CFI = .95, RMSEA = .05; S-B χ2(245, N = 257) = 393.72, RCFI = .95, RMSEA = 0.05. Based on the degrees of freedom in the model, sample size power was adequate for a close fit of greater than .90 (α = .05; MacCallum, Browne, & Sugawara, 1996). The structural equation model explained 43% of the variance in QOL and 18% in BCS. Direct predictors of better QOL included positive coping style, greater self-efficacy, less mistrust of the medical system, non-Latina race/ethnicity and less comorbidity. Direct statistical predictors of receipt of BCS included greater breast cancer knowledge, a lower stage of cancer, and greater perceived him to self-efficacy in interacting with physicians.
Physicians’ participatory style (p ≤.01) had positive indirect effects on reporting better QOL and having had BCS; these effects were mediated by patient coping, breast cancer knowledge, and feelings of self-efficacy in interacting with physicians. Latina and African-American race/ethnicity had negative indirect effects on QOL (p ≤.01); these ethnic effects were partially mediated by medical mistrust, which was also a mediator of the indirect effects of Latina and African-American race/ethnicity (p ≤.05 and p ≤.01, respectively) on the intermediate domain of patient self-efficacy. There were also other significant indirect effects on the outcome measures. Higher SES (p ≤.001) had significant indirect effects on BCS, QOL and Patient Self-Efficacy. Physician participatory style had a significant indirect effect on BCS (p ≤.01) and on QOL (p ≤.05), mediated through patient empowerment variables.
Discussion
This study is among the first to comprehensively examine the role of physician communication and patient cognitive factors, along with socioeconomic status, on racial/ethnic disparities in breast cancer treatment and QOL within a single exploratory model derived from empirical findings from the extant literature. Compared to the other women, African Americans reported equally good QOL, whereas Latinas reported worse QOL, concurring with the results of previous studies (Spencer et al., 1999). Further, both African-American women and Latinas received less BCS compared to Whites, which is also supported by previous literature (Mandelblatt et al., 2002; C. R. Morris et al., 2000). Thus, the first hypothesis that there would be racial/ethnic disparities in older women’s QOL and breast cancer treatment was confirmed, especially among Latinas.
Greater patient perception of interactive information-giving and shared treatment decision-making by physicians correlated with increased QOL and receipt of BCS in the bivariate analysis, confirming the study’s second hypothesis. However, the path model showed that the influence of patient-reported physician participatory style was indirect, operating through patient cognitive variables. The positive association of these patient-empowering communications with patient QOL was mediated by patients’ positive breast cancer coping strategies and their feelings of self-efficacy in patient-physician interactions. The latter finding is consistent with other cancer research supporting the role of self-efficacy in predicting better QOL (Maliski et al., 2004), but expands on those results by highlighting the powerful role of physician communication in mediating these effects. Similarly, the model takes previous work demonstrating the relationship of formation of patient-physician partnerships through physician communication to BC patients’ coping (McWilliam et al., 2000) one step further to show its positive influence on QOL.
In terms of BCS, the model links earlier findings that better physician communication in the form of information giving improves BC knowledge (R. C. Maly, Leake et al., 2004; Whelan et al., 2004) and that knowledge increases BCS rates (Whelan et al., 2004). Overall, the model provides a comprehensive picture that illustrates how BC treatment decisions may be influenced by patient empowering communication. Self-efficacy posited to be engendered by physicians in the model, also was associated with that increased BCS rates, showing its independent effect on treatment. Moreover, since physicians’ emotional support had no effects on the outcomes, it appears that physician communication that may specifically empower patients, in terms of self-efficacy, knowledge, and coping, was a key statistical predictor of patient QOL and treatment received, controlling for sociodemographic characteristics, comorbidity, and BC stage. However, the role of participatory decision-making in breast cancer treatment merits further inquiry since some authors report a negative association between patient involvement and receipt of BCS (S. J. Katz et al., 2005), while this study found that inclusion of patients in their treatment plans was associated indirectly with greater receipt of BCS.
The third study hypothesis that relationships between race/ethnicity and the outcomes of QOL and BCS would be mediated by provider- and system-level factors and patient cognitive factors was partially confirmed. Racial/ethnic disparities in breast cancer treatment were, in fact, mitigated to some degree by these factors, but Latina ethnicity was a direct statistical predictor of poorer QOL in the path model, bypassing all intermediate pathways. The direct link between Latina ethnicity and QOL indicates that, beyond mistrust and poor educational and financial resources included in the model, additional unmeasured factors likely played a part in older Latinas’ poorer reported QOL. Interestingly, although there was a positive and statistically significant association between the two study outcomes in bivariate analysis, QOL and receipt of BCS, as supported by recent research (Engel et al., 2004), this relationship did not persist in the path model. Factors beyond those examined in the other studies, such as patient cognitive variables and medical mistrust, were incorporated in our comprehensive model and may have attenuated this association and thus suggest that further study is required.
The path model indicates that compared to Whites, African Americans expressed substantial mistrust of the health care system and Latinas reported somewhat less mistrust. Medical mistrust, in turn, was associated with less positive coping with breast cancer, leading to reduced QOL in the path model. Medical mistrust also predicted decreased feelings of self-efficacy in interacting with physicians, thus reducing both QOL and the likelihood of receiving BCS. These findings support the contention that medical mistrust accounts for at least some of the racial/ethnic disparities in medical outcomes generally (Bogart, Bird, Walt, Delahanty, & Figler, 2004; LaVeist et al., 2000; Matthews, Sellergren, Manfredi, & Williams, 2002; O’Malley, Sheppard, Schwartz, & Mandelblatt, 2004).
African-American race did not predict surgeon participatory style and information-giving, although it was a negative predictor of surgeon emotional support and breast cancer knowledge. Because perception of surgeons’ emotional support was highly correlated with report of their participatory style, it may be that even if adequate information had been provided, it could have been met with suspicion on the part of African-American patients because of their greater mistrust of the health care system.
SES had a profound relationship to patient report of surgeon participatory style and was significantly correlated with all of the provider-and system-level variables, including surgeon communication and patient self-efficacy, breast cancer knowledge and positive coping. While the life circumstances patients bring to a diagnosis of breast cancer cannot be altered, policy makers and providers can take steps to offset, or at least mitigate the effects of fewer educational and economic resources by increasing information-giving and providing low SES patients, in particular, with opportunities to participate in decisions about their treatment (Institute of Medicine, 2003).
This study has several limitations. First, much of the data were collected by patient self-report and are subject to recall and social response biases. However, people who have undergone a major, life-threatening health crisis often manifest very clear recall of the details surrounding the event (Brown & Kulik, 1982). For example, breast cancer patients can recall when they first noticed their symptoms (Burgess, Ramirez, Richards, & Love, 1998), and can also accurately report details of their treatment (Maunsell, Drolet, Ouhoummane, & Robert, 2005). A second limitation is that study participants were from the Los Angeles County area and may not reflect the greater population of older women in the United States, especially Latinas. The majority of Latinas in Los Angeles County are of Mexican origin (California Health Interview Survey, 2005) and their cultural beliefs, preferences and attitudes may vary from those of Latinas in other parts of the country. A third limitation is that participants with low health literacy may not have been able to adequately complete the self-administered portion of the survey instrument. However all forms were intended for an eighth grade education or less and telephone consultation was available to all respondents. Finally, the predictive model is cross-sectional and causality cannot be assumed. Other models and directionality of influences might be equally reasonable. In particular, patient-level attributes may induce different physician-level communication styles, so influences may flow in a bidirectional manner. However, we have provided a rationale for the directionality of our particular model that influence normally flows from the physician to the individual patient. Future studies should obtain observational data on patient-physician communication style and/or longitudinal data over the course of diagnosis and treatment to better resolve such issues and provide for model improvement.
Clearly, medical mistrust, positive coping with breast cancer, knowledge about breast cancer and its treatment options, and feelings of self-efficacy derived from patients’ personal backgrounds and physician communication, played important roles in QOL and surgical treatment selection, independent of SES, stage of breast cancer, and comorbidity. Other factors not considered in this study may also have crucial influences. Further studies are needed to understand patient preferences and their origins, preconceptions about breast cancer treatment, and competing needs that may compel some women to select mastectomy as a shorter form of therapy, and the role of family and friends during decision-making. In addition, extraneous factors that were not measured can affect QOL, such as living circumstances, family caretaking responsibilities, and other sources of stress found among older women.
The study is encouraging in that neither minority ethnicity nor medical mistrust were directly associated with rates of BCS, but rather operated through the potentially malleable constructs of self-efficacy, coping, and knowledge, which, in turn, were associated with physician communication styles, also potentially malleable through education and training. However, the negative relationship of Latina ethnicity with QOL may be more difficult to address since it was independent of the patient-cognitive factors that were related to physician communication. Future studies should address medical mistrust among older minority breast cancer patients, overcoming gaps in breast cancer knowledge, enhancing self-efficacy in patient-provider discussions, and supporting positive coping, so that appropriateness of treatment decision-making and quality of life can be maximized in all older patients.
Acknowledgments
This research was funded by the California Breast Cancer Research Program (grant # 4PB-0161) and by the Robert Wood Johnson Foundation (grant # 036833). Dr. Maly was also supported by the American Cancer Society (grant # TURSG-02-081-01-PBP).
Dr. Stein was supported by Grant NIDA DA 01070-34 from the National Institute on Drug Abuse.
Ms. Umezawa was funded by the Diversity Supplement to Grant 7PB-0070 from the California Breast Cancer Research Program.
The authors gratefully acknowledge the secretarial and administrative assistance of Gisele Pham and Shannon Jason.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/hea/.
References
- American Society of Clinical Oncology. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. Journal of Clinical Oncology. 1996;14(2):671–679. doi: 10.1200/JCO.1996.14.2.671. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999;85(2):418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS 6 structural equations program manual. Encino, CA: Multivariate Software, Inc; 2005. [Google Scholar]
- Bogart LM, Bird ST, Walt LC, Delahanty DL, Figler JL. Association of stereotypes about physicians to health care satisfaction, help-seeking behavior, and adherence to treatment. Social Science & Medicine. 2004;58(6):1049–1058. doi: 10.1016/s0277-9536(03)00277-6. [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. In: Neisser U, editor. Memory observed. San Francisco: Freeman and Company; 1982. pp. 23–40. [Google Scholar]
- Burgess CC, Ramirez AJ, Richards MA, Love SB. Who and what influences delayed presentation in breast cancer? British Journal of Cancer. 1998;77:1343–1348. doi: 10.1038/bjc.1998.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch E, Kemeny M, Fremgen A, Osteen RT, Winchester DP, Clive RE. Patterns of breast cancer care in the elderly. Cancer. 1996;78(1):101–111. doi: 10.1002/(SICI)1097-0142(19960701)78:1<101::AID-CNCR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- California Health Interview Survey. California Health Interview Survey 2005. 2005 Retrieved December 10, 2007, from http://www.chis.ucla.edu.
- Carver CS. You want to measure coping but your protocol’s too long?: consider the brief COPE. International Journal of Behavioral Medicine. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: a study of women with early-stage breast-cancer. Journal of Personality and Social Psychology. 1993;65(2):375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans. A biopsychosocial model. American Psychologist. 1999;54(10):805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Powe NR, Nelson C, et al. Race, gender, and partnership in the patient-physician relationship. Jama. 1999;282(6):583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic Whites. Psycho-Oncology. 2002;11(6):495–504. doi: 10.1002/pon.615. [DOI] [PubMed] [Google Scholar]
- Deadman JM, Leinster SJ, Owens RG, Dewey ME, Slade PD. Taking responsibility for cancer treatment. Social Science & Medicine. 2001;53(5):669–677. doi: 10.1016/s0277-9536(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Del Piccolo L, Mazzi M, Saltini A, Zimmermann C. Inter and intra individual variations in physicians’ verbal behaviour during primary care consultations. Social Science & Medicine. 2002;55(10):1871–1885. doi: 10.1016/s0277-9536(01)00314-8. [DOI] [PubMed] [Google Scholar]
- Dunkel-Schetter C, Feinstein LG, Taylor SE, Falke RL. Patterns of coping with cancer. Health Psychology. 1992;11(2):79–87. doi: 10.1037//0278-6133.11.2.79. [DOI] [PubMed] [Google Scholar]
- Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. The Breast Journal. 2004;10(3):223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- Feher S, Maly RC. Coping with breast cancer in later life: the role of religious faith. Psycho-Oncology. 1999;8(5):408–416. doi: 10.1002/(sici)1099-1611(199909/10)8:5<408::aid-pon409>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. Journal of the National Cancer Institute. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- Gilligan MA, Kneusel RT, Hoffmann RG, Greer AL, Nattinger AB. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Medical Care. 2002;40(3):181–189. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Guadagnoli E, Weeks JC, Shapiro CL, Gurwitz JH, Borbas C, Soumerai SB. Use of breast-conserving surgery for treatment of stage I and stage II breast cancer. Journal of Clinical Oncology. 1998;16(1):101–106. doi: 10.1200/JCO.1998.16.1.101. [DOI] [PubMed] [Google Scholar]
- Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psycho-Oncology. doi: 10.1002/pon.907. in press. [DOI] [PubMed] [Google Scholar]
- Institute for the Future. Health and health care 2010. Princeton, NJ: Robert Wood Johnson Foundation; 2000. [Google Scholar]
- Institute of Medicine. Unequal treatment: confronting racial/ethnic disparities in health care. Washington, D.C.: The National Academic Press; 2003. [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94(12):2084–2090. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE., Jr Characteristics of physicians with participatory decision-making styles. Annals of Internal Medicine. 1996;124(5):497–504. doi: 10.7326/0003-4819-124-5-199603010-00007. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, et al. Patient involvement in surgery treatment decisions for breast cancer. Journal of Clinical Oncology. 2005;23(24):5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- Kerr J, Engel J, Schlesinger-Raab A, Sauer H, Holzel D. Communication, quality of life and age: results of a 5-year prospective study in breast cancer patients. Annals of Oncology. 2003;14(3):421–427. doi: 10.1093/annonc/mdg098. [DOI] [PubMed] [Google Scholar]
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA: A Cancer Journal for Clinicians. 1998;48(1):6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Medical Care Research and Review. 2000;57(Supplement 1):146–1461. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- Little TD, Cunningham WA, Shahar G, Widaman KF. To parcel or not to parcel: exploring the question, weighing the merits. Structural Equation Modeling. 2002;9:151–173. [Google Scholar]
- Maliski SL, Kwan L, Krupski T, Fink A, Orecklin JR, Litwin MS. Confidence in the ability to communicate with physicians among low-income patients with prostate cancer. Urology. 2004;64(2):329–334. doi: 10.1016/j.urology.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. Journal of the American Geriatrics Society. 1998;46(7):889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Maly RC, Leake B, Silliman RA. Health care disparities in older patients with breast carcinoma: informational support from physicians. Cancer. 2003;97(6):1517–1527. doi: 10.1002/cncr.11211. [DOI] [PubMed] [Google Scholar]
- Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. Journal of the American Geriatrics Society. 2004;52(7):1138–1145. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- Maly RC, Umezawa Y, Leake B, Silliman RA. Determinants of participation in treatment decision-making by older breast cancer patients. Breast Cancer Research and Treatment. 2004;85(3):201–209. doi: 10.1023/B:BREA.0000025408.46234.66. [DOI] [PubMed] [Google Scholar]
- Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast cancer patients. Cancer. 2006;106(4):957–965. doi: 10.1002/cncr.21680. [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Kerner JF, Hadley J, Hwang YT, Eggert L, Johnson LE, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer. 2002;95(7):1401–1414. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- Matthews AK, Sellergren SA, Manfredi C, Williams M. Factors influencing medical information seeking among African American cancer patients. Journal of Health Communication. 2002;7(3):205–219. doi: 10.1080/10810730290088094. [DOI] [PubMed] [Google Scholar]
- Maunsell E, Drolet M, Ouhoummane N, Robert J. Breast cancer survivors accurately reported key treatment and prognostic characteristics. Journal of Clinical Epidemiology. 2005;58(4):364–369. doi: 10.1016/j.jclinepi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- McWilliam CL, Brown JB, Stewart M. Breast cancer patients’ experiences of patient-doctor communication: a working relationship. Patient Education and Counseling. 2000;39(23):191–204. doi: 10.1016/s0738-3991(99)00040-3. [DOI] [PubMed] [Google Scholar]
- Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79(2):314–319. [PubMed] [Google Scholar]
- Morris AD, Morris RD, Wilson JF, White J, Steinberg S, Okunieff P, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. The Cancer Journal from Scientific American. 1997;3(1):6–12. [PubMed] [Google Scholar]
- Morris CR, Cohen R, Schlag R, Wright WE. Increasing trends in the use of breast-conserving surgery in California. American Journal of Public Health. 2000;90(2):281–284. doi: 10.2105/ajph.90.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Preventive Medicine. 2004;38(6):777–785. doi: 10.1016/j.ypmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Paskett ED, Tatum C, Rushing J, Michielutte R, Bell R, Foley KL, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004;101(11):2650–2659. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26(6):454–465. doi: 10.1097/00002820-200312000-00005. quiz 466-457. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Silliman RA, Dukes KA, Sullivan LM, Kaplan SH. Breast cancer care in older women: sources of information, social support, and emotional health outcomes. Cancer. 1998;83(4):706–711. [PubMed] [Google Scholar]
- Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychology. 1999;18(2):159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Counseling Psychologists Press; 1970. [Google Scholar]
- Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Medical Care. 1988;26(7):724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Stiegelis HE, Hagedoorn M, Sanderman R, Bennenbroek FT, Buunk BP, van den Bergh AC, et al. The impact of an informational self-management intervention on the association between control and illness uncertainty before and psychological distress after radiotherapy. Psycho-Oncology. 2004;13(4):248–259. doi: 10.1002/pon.738. [DOI] [PubMed] [Google Scholar]
- Street RL, Jr, Voigt B, Geyer C, Jr, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer. 1995;76(11):2275–2285. doi: 10.1002/1097-0142(19951201)76:11<2275::aid-cncr2820761115>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA: the Journal of the American Medical Association. 2004;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- Zachariae R, Pedersen CG, Jensen AB, Ehrnrooth E, Rossen PB, von der Maase H. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. British Journal of Cancer. 2003;88(5):658–665. doi: 10.1038/sj.bjc.6600798. [DOI] [PMC free article] [PubMed] [Google Scholar]