Abstract
Distinct human neurodegenerative diseases share remarkably similar temporal emergence patterns, even though different toxic proteins are involved in their onset. Typically, familial neurodegenerative diseases emerge during the fifth decade of life, whereas sporadic cases do not exhibit symptoms earlier than the seventh decade. Recently, mechanistic links between the aging process and toxic protein aggregation, a common hallmark of neurodegenerative diseases, have been revealed. The insulin/insulin-like growth factor 1 (IGF1) signalling pathway — a lifespan, metabolism and stress-resistance regulator — links neurodegeneration to the aging process. Thus, although a reduction of insulin signalling can result in diabetes, its reduction can also increase longevity and delay the onset of protein-aggregation-mediated toxicity. Here we review this apparent paradox and delineate the therapeutic potential of manipulating the insulin/IGF1 signalling pathway for the treatment of neurodegenerative diseases.
For decades aging was thought to be a stochastic, uncontrolled deterioration process with little or no genetic regulation1. This view has changed as scientific advances have revealed that at least three independent metabolic pathways regulate the aging process and have critical roles in the determination of lifespan (BOX 1). First, dietary restriction has been reported to extend the lifespan of worms, flies, rats and a large variety of other species2,3. Second, the insulin/insulin-like growth factor 1 (IGF1) signalling (IIS) pathway was found to be an active regulator of the lifespan and youthfulness of worms4, flies5,6 and mice7–9 (BOX 2). Third (and most recently), it was discovered that the rate of mitochondrial respiration is also a lifespan determinant10,11. Among these three pathways, the most prominent and thus far best studied is the IIS pathway, which is highly conserved across phyla. As the mammalian IIS pathway and its roles in the regulation of aging have been reviewed elsewhere12, here we focus on the IIS pathway components that are important for maintaining protein homeostasis (proteostasis)13 and countering toxic protein aggregation (proteotoxicity) in neurons and other tissues, mainly in the nematode Caenorhabditis elegans (C. elegans) and the mouse.
Box 1 | Insulin-signalling-independent lifespan-regulating pathways
Lifespan is regulated by at least three different mechanisms, one of which is the insulin/insulin-like growth factor 1 signalling (IIS) pathway. Dietary restriction (DR) was the first metabolic treatment found to extend the lifespan of an organism, after it was carried out first on rats in 1935 (REF. 90). Ever since, DR has been reported to have similar longevity effects on many other species2, including yeast91, worms92 and flies93. When available food quantities are reduced below the ad libitum level (the amount that an animal consumes when food is unlimited), lifespan increases steadily until an optimal food-intake level (typically ~60% of the ad libitum) is reached. Further reduction of dietary intake leads to starvation and shortens lifespan. Although it was originally termed ‘calorie restriction’, it seems that the longevity effect associated with reduced dietary intake cannot be explained by calorie consumption94. Recent studies performed in worms revealed that the transcription factors PHA-4 (REF. 95) (also known as forkhead 1) and SKN-1 (REF. 54) have critical roles in enabling DR to mediate longevity. An additional metabolic pathway that regulates longevity in Caenorhabditis elegans is the mitochondrial electron transport chain (ETC). Decreasing the activity of the ETC with RNA interference directed towards the ETC complexes I, III, IV and V resulted in ~40% life extension10. Unlike the IIS pathway, which executes its longevity functions during early adulthood89, decreasing ETC activity only extends lifespan if it is carried out during larval development10.
Box 2 | Biological functions of the IIS pathway
Besides lifespan, the insulin/insulin-like growth factor 1 (IGF1) signalling (IIS) pathway regulates several other biological functions. In worms, IIS regulates the developmental switch that determines whether the developing larva will arrest as a dauer larva or become an adult worm. Because the regulation of lifespan by IIS occurs during the worm’s reproductive adulthood89, it is temporally distinct from the dauer switch that occurs during development. A reduction in IIS also modifies the reproductive profile of worms. Worms grown on bacteria that knock-down the worms’ daf-2 mRNA through RNA interference lay fewer eggs per day than their control counterparts but continue to lay eggs for more days. Thus, the cumulative number of eggs laid by the two worm groups is similar96. Elevated oxidative-stress resistance is an additional outcome of a reduction in IIS. Worms that harbour a weak daf-2 allele97, as well as mice that harbour only one copy of the gene that encodes the IGF1 receptor (Igf1r)7, exhibit extended survival compared with their control counterparts after oxidative treatment. Similarly, daf-2-mutant worms are relatively heat resistant98. Recently, daf-2 mutations were reported to protect worms from tumour-like uncontrolled germline cell proliferation99. This protective effect is mediated by DAF-16 and a subset of its target genes, some of which share similarities with identified human tumour-suppressor genes100. Lastly, and as discussed comprehensively in this Review, a reduction in IIS protects from toxic protein aggregation32–34. Reduced stress resistance, tumour development and protein-aggregation-mediated disorders are associated with aging. Thus, the IIS pathway seems to be a comprehensive regulator that actively promotes aging by reducing cellular capabilities to prevent harmful processes.
Aging, proteostasis and neurodegeneration
The ability to ensure proper protein folding is critical for cellular function and organismal viability13. In cells undergoing division, damaged and oxidized proteins can be sequestered and retained in mother cells, enabling daughter cells to have a pristine, undamaged proteome14. However, in post-mitotic cells, such as most neurons, protein quality control must be maintained by other, possibly more complex, mechanisms13. Although many proteins fold spontaneously, subsets of nascent polypeptides — mainly those that are processed in the secretory pathway — require assistance to attain their correct three-dimensional structures. Highly conserved, specialized sets of chaperones assist these nascent polypeptides in folding correctly and perform quality-control processes to ensure their integrity. Polypeptides that fail to fold properly, along with damaged and oxidized mature proteins, are targeted for degradation by specialized cellular degradation machineries15. Despite this cellular effort, subsets of aggregation-prone proteins challenge the cellular machinery, which often fails to handle them. A failure to prevent the misfolding and aggregation of one protein can destabilize the proteome, resulting in uncontrolled aggregation of other polypeptides16. In some cases protein aggregation leads to the development of maladies, collectively termed ‘conformational diseases’ (REF. 17). Among the conformational diseases are the human neurodegenerative diseases, such as Alzheimer’s (AD), Parkinson’s (PD)18 and Huntington’s (HD)19. In AD, dual digestion of amyloid precursor protein (APP) releases the aggregation-prone peptides that are collectively termed amyloid-β (Aβ). The accumulation and aggregation of Aβ (particularly the highly aggregative Aβ1–42) is associated with AD. Similarly, aberrant aggregation of α-synuclein is associated with the emergence of PD, and the aggregation of mutated huntingtin (which bears abnormally long poly-glutamine (polyQ) stretches) initiates HD. The mechanisms that lead to the development of neurodegenerative diseases are largely unknown; however, recent data suggest that small oligomeric aggregative structures (sometimes termed protofibrils) are the underlying cause20,21, rather than high-molecular-mass aggregates as previously assumed22. Impairment of long-term potentiation (LTP), a hallmark of AD23, was induced by Aβ dimers24 and trimers25. Similarly, small polyQ aggregates mediate neuronal death, whereas large polyQ fibrils are thought to be neuroprotective26. Moreover, molecular chaperones that are known to disrupt aggregates actually promoted active aggregation when the concentration of the aggregating protein was high27,28. Therefore it has been proposed that, although protein aggregation is hazardous under certain circumstances, the creation of apparently less-toxic large aggregates is protective.
The major risk factor for the development of neurodegenerative diseases is aging29. Typically, individuals that carry neurodegeneration-linked mutations develop the disease during their fifth decade, whereas sporadic neurodegenerative maladies appear during the seventh decade or later29. Furthermore, both polyQ- and Aβ-expressing worms and certain Aβ-expressing mouse strains exhibit constant expression of aggregation-prone proteins, yet protein-aggregation-mediated toxicity is not apparent until late stages of life30,31. Why do neurodegenerative disorders occur late in life? And why do distinct disorders share similar temporal emergence patterns? These questions are still puzzling researchers. It is plausible that the aging process actively suppresses cells’ ability to clear toxic protein aggregates, enabling the accumulation of these aggregates and initiating neurodegeneration late in life. Recent studies32–34 point to the IIS pathway as the major candidate to link aging, proteotoxicity and late-onset neurodegenerative diseases (BOX 3).
Box 3 | Proteotoxicity, neurotoxicity and neurodegeneration
The toxic effects that stem from the misassembly or aggregation of proteins or peptides (in any cell type) are collectively termed proteotoxicity101, whereas neurotoxicity is a term that refers to general toxic effects observed in neurons. Owing to methodological practicalities, such as short lifespan and the need for a detectable toxic phenotype, many studies on the possible links between aging and neurodegeneration are performed in invertebrates, in which the neurodegeneration-linked aggregation-prone proteins are mostly expressed in non-neuronal tissues32–34,66,102. What is the relevance of proteotoxicity to neurotoxicity and to neurodegeneration? To answer this question, a comprehensive understanding of the mechanisms that lead to the development of neurodegenerative diseases is required. To date these mechanisms are poorly understood103; however, it is clear that protein aggregation is tightly linked to the emergence and development of neurodegenerative diseases18,21. Moreover, the mechanisms that have been found to counter toxic protein aggregation in different cell types and different organisms are highly conserved104. Thus, it is likely that proteotoxicity that is associated with the expression of neurodegeneration-linked aggregation-prone proteins in any tissue can provide insights into the neuronal defence mechanisms. Many of the insights that have been obtained from invertebrate-based or non-neuronal studies await confirmation in mammalian neuronal systems and clinical observations.
The IIS pathway determines longevity
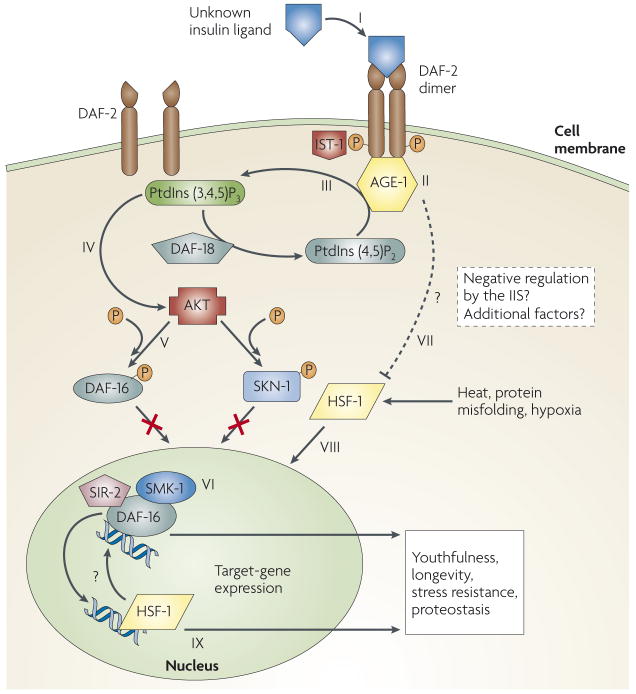
The worm IIS cascade (FIG. 1), which is nearly identical to that in mammals12, is initiated when an as-yet-undefined insulin-like ligand binds to DAF-2, the sole worm insulin/IGF-1 receptor4,35. Subsequently, DAF-2 recruits IST-1 (an insulin receptor substrate 1 orthologue) and AGE-1, a phosphatidylinositol 3-kinase36 that mediates the production of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3). In turn, PtdIns(3,4,5)P3 activates members of the AKT kinase family in a pyruvate dehydrogenase kinase (PDK)-dependent manner37,38. The IIS pathway has an internal negative regulator: the phosphatase DAF-18 (a PTEN orthologue), which acts in opposition to AGE-1, mediates PtdIns(3,4,5)P3 dissociation and reduces AKT activation39.
Figure 1. The Caenorhabditis elegans IIS pathway.
The binding of an as-yet-undefined ligand to DAF-2, the sole insulin/insulin-like growth factor 1 (IGF-1) receptor of C. elegans (I), triggers the insulin/IGF-1 signalling (IIS) pathway. This binding leads to DAF-2 self-phosphorylation and dimerization and to the recruitment of the phosphatidylinositol 3-kinase AGE-1 (II) and the insulin receptor substrate 1 orthologue IST-1. AGE-1 catalyses the generation of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) (III), a molecule that activates kinases of the AKT family (IV). The phosphatase DAF-18 (a PTEN orthologue) opposes AGE-1 activity by converting PtdIns(3,4,5)P3 to phospatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2); this leads to reduced AKT activation. Activated AKT phosphorylates the FOXO transcription factor DAF-16 (V), preventing it from entering the nucleus, where it would otherwise interact with its cofactors, including SMK-1 and SIR-2, and regulate its target genes (VI), which mediate longevity and stress resistance. Similarly, IIS prevents the transcription factor SKN-1 from entering the nucleus and executing its gene-expression functions. Heat-shock factor 1 (HSF-1) is also critical for the longevity functions of IIS (VII) and might be negatively regulated by the IIS pathway (dashed line). HSF-1 regulates the expression of key gene networks (IX) that are required for longevity and stress resistance, some of which are probably also regulated by DAF-16.
Activated AKT phosphorylates the downstream protein DAF-16 (REFS 40,41), which is the sole C. elegans FOXO forkhead transcription factor. Phosphorylated DAF-16 is prevented from entering the nucleus and thus cannot regulate the expression of its target genes, such as the small heat-shock protein (HSP) chaperones42. Therefore, the IIS pathway negatively regulates the activity of DAF-16 by modifying its intracellular localization. Accordingly, a reduction in IIS hyper-activates DAF-16, alters the expression of the DAF-16 transcriptome, extends youthfulness and lifespan and elevates stress resistance43. Analogously, deleting one copy of Igf1r, the murine daf-2 orthologue, increases longevity and stress resistance in mice (particularly in females)7. Although AKT activation is reduced in these animals7, it has not been determined whether this increased longevity is dependent on one of the three mouse FOXO transcription factors. In addition, mice with fat-specific insulin receptor knockout (FIRKO mice) are long-lived44, as are mice that have reduced insulin signalling as a result of having low amounts of insulin receptor substrate 2 (IRS2), an insulin signalling mediator9. Interestingly, human centenarians carrying mutated IGF1R were also found to exhibit reduced IGF1 signalling compared with a control group45, suggesting that the effects of IGF1 signalling on longevity are conserved in humans.
Another key transcription factor that is required for the worm lifespan extension that is facilitated by reduced IIS is heat-shock factor 1 (HSF-1)33,46. HSF-1 is a highly conserved47, leucine-zipper-containing transcription factor that, on activation, trimerizes, enters the nucleus and regulates the expression of its target genes48. HSF-1 has critical roles in various cellular and organismal processes, including development49, the stress response50, hypoxia51, circadian rhythmicity52 and innate immunity53. The molecular interactions, if any, between the IIS pathway and HSF-1 are largely unknown. It is plausible that a reduction in IIS promotes elevated stress resistance by activating HSF-1 and thus helping to regulate the expression of its target genes, which also include a subset of small HSPs. This enables the maintenance of proper protein homeostasis with age33. In this view, IIS activity negatively regulates HSF-1. However, to date, direct regulation of HSF-1 by the insulin/IGF-1 receptor or its downstream kinases has not been established (FIG. 1).
Similar to DAF-16, the cellular localization of the lifespan-regulating transcription factor SKN-1 (REF. 54) was shown to be regulated by the IIS pathway in the worm55. SKN-1 is thought to modulate longevity by regulating sets of genes that are involved in countering oxidative stress55.
Together, the data that have been generated during the past decade in both invertebrate and vertebrate model organisms indicate that the IIS pathway regulates lifespan, stress resistance and health by controlling the activity of specific gene-expression networks (transcriptomes).
The DAF-16 and HSF-1 transcriptomes
Although the details of how the IIS pathway regulates aging and lifespan are largely obscure, the identification of DAF-16- and HSF-1-regulated transcriptomes seems to be critical for understanding the underlying mechanism. Thus far, four distinct approaches have been adopted to characterize the DAF-16 transcriptome. DNA microarrays have been used to compare the gene-expression patterns of worms lacking daf-2 or daf-16 with those of wild-type worms42,56. Serial analysis of gene expression (SAGE)57 and chromatin immunoprecipitation (CHIP)58 have been used to search for DAF-2- and DAF-16-regulated genes. Finally, the differences in soluble protein content between worms with reduced IIS and wild-type worms have also been analysed by quantitative mass spectrometry59. Several gene subsets were identified by each technique; however, the most consistently and prominently identified subset was the HSP family, in particular the small HSPs, such as the HSP-16 family. Intriguingly, in various vertebrate cell types, as well as in nematode neurons, HSPs are also regulated by HSF-1 (REF. 60), indicating that DAF-16 and HSF-1 have overlapping target genes. The roles of HSPs in assisting proper protein folding61, in maintaining protein integrity and in mediating the clearance of misfolded, oxidized and aggregated proteins62 point to unsuccessful protein homeostasis as a pivotal aspect of aging. Accordingly, knock-down of HSPs partially decreases the long lifespan of reduced-IIS animals33,46.
IIS and toxic protein aggregation
Several studies performed in C. elegans proteotoxicity models indicate that the IIS pathway directly links aging to the onset of toxic protein aggregation. A purely post-mitotic proteotoxicity cell model was created in transgenic worms by expressing a fluorescently tagged (with yellow fluorescent protein (YFP)) PolyQ repeat in body-wall muscle cells34. At least 40 glutamine repeats were required for efficient aggregation in young (day 2 of adulthood) worms. The threshold number of repeats that was needed for aggregation decreased as the animals aged. In worms expressing polyQ lengths of 35 repeats (polyQ35-YFP), aggregates were detected by day 4 of adulthood, whereas polyQ29-YFP aggregates were not detected earlier than day 9 of adulthood. An assessment of motility impairments (an indirect measure of toxicity in muscle cells) showed that they were associated with the aggregation levels of the tagged polyQ constructs. In accordance with the relationship between age and aggregation, worms that expressed polyQ lengths of 33–35 repeats exhibited no motility impairments at young ages but exhibited decreasing motility later in life. Furthermore, an RNA interference (RNAi)-mediated reduction of AGE-1 (a central IIS-pathway component, see FIG. 1) protected worm embryos from polyQ82-YFP aggregation and reduced the motility impairment of young polyQ82-YFP-expressing worms. These protective effects were dependent on daf-16, as they were abolished by RNAi-mediated depletion of daf-16.
Resveratrol, an activator of sirtuins that extends the lifespan of yeast63, worms, flies64 and fish65, alleviates the toxicity of polyQ in worm neurons by inhibiting sirtuin 2 (sir-2)66, a gene that is thought to act immediately upstream of daf-16 to regulate longevity 67 (FIG. 1).
The direct link between aging and the onset of polyQ aggregation was further established when HSF-1 was found to be essential not only for lifespan extension owing to compromised IIS, but also for the reduction of polyQ40-YFP aggregation in worm muscle cells33,34. This protective effect of reduced IIS was also daf-16-dependent. In addition, these studies indicate that the small HSP members of the crystalline family, the transcription of which is regulated by DAF-16 and HSF-1, are important players in both lifespan extension and protection from proteotoxicity. Interestingly, hsp-90 (also known as daf-21 in C. elegans) and members of the hsp-70 family are also vital for the increased longevity that results from reduced IIS46.
Collectively these studies indicate that a reduction in IIS can reduce the proteotoxicity that is associated with the aggregation of polyQ stretches in the worm, but they leave several fundamental questions unanswered. What are the mechanistic details of this protective effect? can a reduction in IIS protect from the proteotoxicity that is associated with other disease-linked aggregative proteins? To address these queries, a worm model of AD that expresses the AD-linked human protein Aβ1–42 in body-wall muscles (Aβ worm) was examined. The expression and subsequent aggregation of Aβ1–42 in worms results in progressive paralysis68. Similar to its effect on polyQ toxicity, a reduction in IIS protected the worms from Aβ1–42-mediated proteotoxicity32. Therefore, it seems that reduced IIS can protect cells from both polyQ- and Aβ-mediated proteotoxicity.
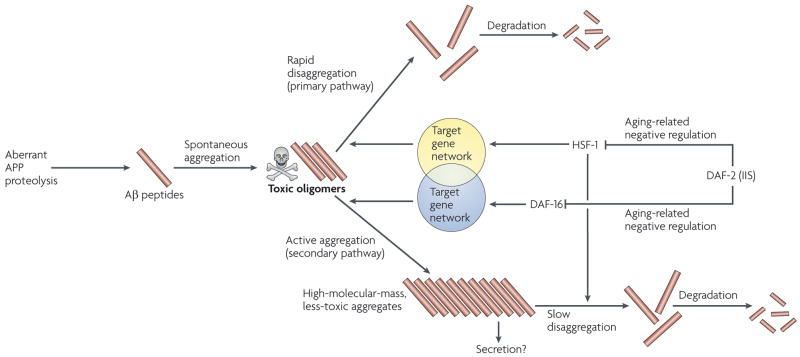
Using genetic, biochemical, in-vitro and microscopic techniques, it has become apparent that the amount of high-molecular-mass Aβ1–42 aggregates does not correlate with toxicity. For example, a reduction of IIS (by RNAi against daf-2 mrnA) resulted in reduced toxicity but caused a small increase in the levels of high-molecular-mass Aβ1–42aggregates, whereas a reduction of DAF-16 (by RNAi against daf-16 mrnA) reduced the amount of high-molecular-mass Aβ1–42aggregates but enhanced toxicity32. Furthermore, RNAi against hsf-1 mrnA led to a drastic elevation of both toxicity and high-molecular-mass Aβ1–42 aggregates32. These observations indicate that the IIS pathway is pivotal to aging and proteotoxicity because it regulates two distinct counter proteotoxic activities: disaggregation that is regulated by HSF-1 and protective active aggregation that is regulated by DAF-16 (FIG. 2).
Figure 2. The IIS pathway links aging and proteotoxicity.
Serial digestion of amyloid precursor protein (APP) releases aggregation-prone amyloid-β (Aβ) peptides, which spontaneously form small toxic oligomers. Heat-shock factor 1 (HSF-1)-regulated disaggregation machinery disrupts the Aβ aggregates and prepares them for degradation. When the disaggregation pathway is overtaxed, a DAF-16-regulated active aggregation activity creates high-molecular-mass aggregates of lower toxicity that might subsequently be secreted from the cell. If they are not secreted, the high-molecular-mass aggregates undergo slow disaggregation and subsequent degradation. The insulin/insulin-like growth factor 1 signalling (IIS) pathway compromises the activity of both protective mechanisms in an age-dependent manner by negatively regulating HSF-1 and DAF-16.
Reduced IIS protects cells from both Aβ1–42 and polyQ toxicity; however, the source and identity of the toxic macromolecular structures of Aβ1–42 and polyQ repeats seem to be distinct. A reduction in IIS results in more high-molecular-mass Aβ1–42 aggregates, whereas in polyQ models a reduction in IIS results in less polyQ aggregates. This apparent inconsistency between Aβ1–42 and polyQ proteins might be resolved if hyper-aggregation of polyQ is protective: this would make large polyQ aggregates markers of proteotoxicity, not the source of proteotoxicity. In agreement with this hypothesis, diffuse polyQ aggregates were found to be toxic and led to striatal neuron death in rats, whereas polyQ-containing inclusion bodies were protective26. In this view, the primary HSF-1-regulated disaggregation-and-degradation mechanism might be sufficient to clear polyQ aggregates in the worm model but not Aβ aggregates. Thus, in the case of Aβ the cell needs the secondary DAF-16-regulated active-aggregation pathway for protection.
How does IIS couple aging and proteotoxicity?
On the basis of the above findings, we propose a model to explain the relationship between the IIS pathway, its downstream transcription factors (DAF-16 and possibly HSF-1), toxic protein aggregation and aging (FIG. 2). According to this model, in Aβ worms Aβ peptides spontaneously aggregate to form small toxic oligomers. Cells have two opposing detoxifying machineries. The primary mechanism, which is regulated by HSF-1 but not by DAF-16 (REF. 32), disaggregates the toxic species to enable their degradation. Under severe aggregation load (such as the high expression of Aβ in the worm model), when the disaggregation-and-degradation mechanism is overloaded, a secondary detoxification mechanism that is regulated by DAF-16 actively aggregates peptides to create high-molecular-mass aggregates of lower toxicity. The regulation of this mechanism by DAF-16 explains why worms that lacked daf-16 contained low amounts of high-molecular-mass aggregates and yet were hypersensitive to Aβ toxicity. The idea that hyper-aggregation can protect cells from proteotoxicity is supported by discoveries that the protective chaperones HSP104 (REF. 28) and TriC27 disrupt protein aggregates when the aggregative protein is present in low concentrations but accelerate protein aggregation when the protein’s concentration exceeds a certain threshold.
Which is protective: increased or decreased IIS?
In contrast to the results from the invertebrate models described above, in some cases researchers have found that an apparent increase of IIS protects against proteotoxicity in vertebrate models. A mammalian cell-culture-based report69 suggested that activation of the IGF/AKT pathway protected neurons from polyQ-mediated toxicity. This protection involved the phosphorylation of hunting-tin by the kinase AKT and its clearance by autophagy. Consistent with this observation, autophagy-mediated clearance of huntingtin is triggered by the activation of insulin receptor substrate 2 (IRS2)70, one of the known IIS mediators in mammals9. The authors proposed that the polyQ-toxicity-protective effect was AKT-independent. This contradiction suggests that, in this context, signals downstream of IRS2 diverge and might counter proteotoxicity through more than one mechanism.
Infusion of IGF1 into aging rats was reported to enhance the clearance of brain Aβ and achieve levels similar to those that are found in the brains of young rats71. Recently the same group reported that injection of IGF1 into AD-model mice reduced the typical behavioural impairments that are associated with increased Aβ levels72. The researchers concluded that an increased amount of circulating IGF1 protects from Aβ, and suggested that hyper-activation of the IIS pathway is neuroprotective. On the basis of these and other publications, it has been proposed that IIS induction might be an effective AD treatment73.
Several observations challenge the idea that increased IIS protects from proteotoxicity. First, aging is the major risk factor for the development of neurodegenerative diseases, including AD29, and reduced IIS slows aging in various species, including mice. Moreover, mice with reduced growth-hormone production and consequent reduced levels of circulating IGF1 and insulin are long-lived74, as are animals with tissue-specific knockout of the insulin receptor44 or of its downstream adaptor protein IRS2 (REF. 9).
One of the major questions in the field is thus whether it is increased or decreased IIS that protects from proteotoxicity. On the one hand, insulin and IGF1 are important beneficial hormones; for instance, lack of insulin leads to severe conditions such as juvenile diabetes. Moreover, reduced IIS can lead to high glucose levels, which are neurotoxic75. On the other hand, reduced IIS is beneficial because it extends lifespan and elevates stress resistance. Therefore, insulin resistance, which seems to be analogous to reduced insulin signalling, is expected to be beneficial despite the fact that it leads to type II diabetes in humans and mice76. Additionally, individuals that carry mutations in AKT2 exhibit severe insulin resistance and develop diabetes77. Yet, a recent study shows that total insulin resistance is less toxic to mice than partial insulin resistance78. Collectively these studies raise the insulin paradox: is it increased or decreased IIS that provides health and longevity?
What models might be able to settle the insulin paradox? It has been proposed that insulin signalling has different metabolic effects in different tissues79. For instance, loss of the insulin receptor in adipose tissues promotes longevity, whereas its loss in hepatic tissues causes diabetes80. Perhaps in conditions of systemic IIS reduction the metabolic syndromes (such as diabetes) that stem from the liver mask the potential health benefits of reduced IIS in other tissues, such as the brain. The observation that patients with AD exhibit abnormally low insulin levels in their cerebrospinal fluid but high insulin levels in their plasma81 supports the tissue-specificity model and suggests a possible interplay between insulin levels in different tissues. In this model, IIS regulatory feedback loops42, which sense the rate of insulin signalling, would regulate the amount of circulating insulin and induce a negative-feedback response that reduces IIS in the brain. Similar mechanisms might regulate IGF1 levels and signalling in different tissues. The finding that female offspring of human centenarians exhibit higher than normal IGF1 plasma levels but lower than normal IGF1 signalling45 also supports the model that lower IGF1 signalling is key to neuroprotection and organismal survival.
Optimal IIS levels?
We propose a model that accommodates the insulin paradox in relation to proteotoxicity. According to this model, each organism has an optimal level of IIS that maximizes its reproduction, health and longevity. IIS levels either higher or lower than the optimal rate interfere with metabolism, can cause disease and eventually shorten lifespan (FIG. 3). For instance, insulin dysregulation is a key cause of polycystic ovarian syndrome, one of the major causes of infertility in women82. Nevertheless, the rate of insulin signalling was not evolutionarily tuned to fit the needs of the individual; rather, it was tuned to fit the needs of the species83. For example, worms that are grown on an unlimited food supply exhibit relatively short lifespans but reproduce quickly. Owing to their short lifespan, they consume fewer of the resources that are needed for the next generation to reach maturity. Both a high reproductive profile and more available resources for the progeny are clearly beneficial for the survival of the species. When food is restricted, the IIS pathway mediates the transformation of young larvae into developmentally arrested dauer larvae, which can live up to six times as long as adult animals, again in favour of the species’ survival in harsh environmental conditions84. The idea of optimal IIS rates seems to be consistent with the regulation of lifespan by dietary restriction. Progressive reductions in the amount of food that is provided to animals promotes lifespan extension until the animal reaches an optimal food-consumption level; thereafter the animal is starved and lifespan shortens3. It has largely been assumed that any reduction of IIS in invertebrates is beneficial, whereas reduction in vertebrates results in insulin resistance and diabetes-like states. This striking contradiction in the effects of a reduction in IIS in invertebrate and vertebrate models introduces additional complexity to the insulin paradox. However, a thorough comparison reveals that the invertebrate literature of IIS almost mirrors the vertebrate literature. In worms and flies, null mutations in the gene that encodes the insulin/IGF-1 receptor result in embryonic and larval lethality, much like homozygous null mutations in the mouse85. Furthermore, invertebrate work has identified an allelic series of mutations in the gene that encodes the insulin/IGF-1 receptor, and it is only the weak alleles that allow an organism to live longer: stronger alleles arrest development, block reproduction and result in lethality86.
Figure 3. Optimal-IIS-rate model.
a | Each organism and each tissue has an optimal insulin/insulin-like growth factor 1 (IGF1) signalling (IIS) level that ensures maximal health and longevity. IIS rates that are either lower or higher than optimal will cause disease and reduce lifespan. b | The natural IIS rate of well-fed Caenorhabditis elegans is tuned to be at the high zone. Thus, feeding them with bacteria that knock down their daf-2 RNA through RNA interference (RNAi) reduces the rate of IIS and extends their health and lifespan. Mutated daf-2 alleles further reduce IIS and provide maximal longevity, whereas daf-2 inactivation or over-reduction causes lethality. c | In mice that harbour only one copy of Igf1r, the reduction in brain IIS provides stress resistance and extends lifespan. Similarly, the optimal IIS model proposes that IGF1 infusion activates feedback loops that reduce IIS in the brain, leading to similar outcomes. Igf1r-null mice are embryonic lethal.
Can a specific tissue’s IIS be controlled in humans to provide benefit without deleterious side effects? Although individuals with lower-than-optimal IIS rates will develop diabetes, it is interesting to speculate that those with higher-than-optimal IIS will develop AD owing to reduced counter-proteotoxic activities. But why then is type II diabetes a risk factor for the development of AD87,88? This can be explained by loss of tissue-specific regulation of IIS: the high plasma-insulin levels that are typical in diabetic patients increase IIS levels in the brain, compromising protective activity and leading to aggregate accumulation and disease. Moreover, work in worms and flies has discovered that temporal inactivation of the IIS pathway can result in profound increases in longevity without affecting development and reproduction5,89. It is not clear whether longevity and proteotoxicity can be separated in humans; however, as the IIS pathway is tightly conserved from the worm to humans, it might be possible to temporally and spatially reduce IIS in the brain to help treat late-onset neurodegenerative diseases.
Clearly, more research is required to clarify the insulin paradox. In the future it will be essential to test whether a direct reduction of IIS in a bona fide AD-mouse model can protect the animals from Aβ-associated toxicity. Additional approaches should focus on deciphering the positive- and negative-feedback loops that affect the IIS pathway in mammals when exogenous IGF1 is administered.
The therapeutic potential of manipulating IIS
The apparent regulation of a protective cellular mechanism by the IIS makes a reduction of this signalling pathway an attractive target for the development of treatments against protein-aggregation-mediated diseases, including neurodegenerative diseases. It is plausible that a threshold amount of toxic protein species is required to initiate disease. Early in life, efficient aggregate clearance maintains low levels of toxic aggregative species, preventing them from reaching the disease-initiation threshold. With age, an IIS-mediated decline in protective activities (including disaggregation, active aggregation, proteolysis and transport) causes the amounts of toxic structures to increase to the levels that are required for disease to commence. We propose that IIS is a determinant of the age-of-onset of neurodegenerative diseases (FIG. 4). Patients that harbour neurodegeneration-linked mutated genes produce relatively high amounts of aggregation-prone toxic polypeptides and therefore develop the disease early in life. Individuals who do not carry neurodegeneration-linked mutated genes produce less toxic aggregates and, thus, sporadic cases emerge only later in life, if at all.
Figure 4. Age-associated decline of counter-proteotoxic activities leads to late-onset neurodegeneration.
The balance between the rate of toxic protein aggregation and the cellular detoxification capacity determines the age at which the amount of toxic aggregates will cross the threshold level that is required for disease onset. A higher aggregation load and lower protective activities will lead to early disease onset, such as in familial neurodegenerative diseases, whereas lower aggregation load and higher protective capabilities will postpone the age of disease onset. This model proposes that the similar age-of-onset of different neurodegenerative diseases stems from one phenomenon, the age-related decline in natural counter-proteotoxic activities.
Perhaps reducing IIS in the brain will enable cells to maintain the activity of protein quality-control mechanisms and clearance capabilities to a later age. This would postpone the onset of neurodegenerative diseases and perhaps other conformational diseases. Several key questions have to be addressed to evaluate this therapeutic potential: can a reduction in IIS protect from proteotoxicity when induced late in life (at the age at which neurodegenerative diseases emerge and are diagnosed)? will a reduction in IIS late in life extend the lifespan, or can the counter-proteotoxic and longevity functions of the IIS pathway be uncoupled? Finally, and most importantly, can the deleterious effects of reduced IIS in peripheral tissues be uncoupled from the beneficial effects in the brain?
Conclusions and future directions
The significant advances in aging research and the establishment of neurodegeneration models in short-lived invertebrates have enabled the discovery of mechanistic links between aging and the emergence of late-onset proteotoxicity maladies. These insights have set a foundation for the development of new therapeutic approaches aimed at alleviating aging-associated disorders. This is particularly significant as it might be possible to treat various late-onset human disorders, such as AD, PD and HD, with a single therapeutic entity. In the near future we foresee a scientific effort to elucidate the mechanistic details of the links between IIS and neurodegenerative diseases in mammalian systems. The effect of IIS manipulation on neurodegeneration and diabetes mouse models, accompanied with comprehensive behavioural and biochemical assessment, will determine whether the benefits of reducing IIS are conserved in mammals.
- Youthfulness
The physical capabilities and conditions, such as agility, speed of crawling, rate of food intake and tissue integrity, that are typical of young but not old animals
- Proteostasis
The favourable protein-homeostasis condition, in which the protein’s composition, distribution, integrity, folding and protein–protein interactions are properly maintained, enabling optimal function at the cellular level
- Protein folding
The post-translational process that a polypeptide has to complete in order to attain its optimal three-dimensional structure. Protein folding is often assisted by chaperones. Proteins that fail to achieve their correct folding are termed ‘misfolded proteins’
- Proteome
All of the functional proteins in a cell at a certain time point. The proteome can change as a function of age, stress conditions, et cetera
- Amyloid-β
(Aβ). A highly aggregative peptide that is associated with and causative of AD
- PolyQ
A stretch of glutamine repeats in a protein sequence. Abnormally long polyQ stretches are associated with various diseases, including HD
- Protofibril
A small, soluble protein aggregate that is thought to be the cause of various neurodegenerative maladies
- Long-term potentiation (LTP)
A long-lasting form of synaptic plasticity that results in an increase in the strength of synaptic transmission and is thought to be critical for memory function
- Transcriptome
The gene network that is regulated by an activated transcription factor under certain conditions. Different transcriptomes can be regulated by the same transcription factor under distinct conditions
- Protein clearance
A set of cellular activities aimed at identifying misfolded proteins, targeting them to specialized degradation complexes and mediating their digestion by specialized degradation machineries, such as proteasomes or lysosomes
- RNA interference (RNAi)
A technique used to knock down the expression of a specific gene by introducing a double stranded RNA molecule that complements the gene of interest and triggers the degradation of the gene’s mRNA molecules
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
α-synuclein | AGE-1 | AKT2 | APP | DAF-2 | DAF-16 | DAF-18 | IGF1 | Igf1r | IRS2 | IST-1 | PHA-4 | sir-2 | SKN-1
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
AD | HD | PD
Competing interests statement
The authors declare competing financial interests: see web version for details.
References
- 1.Reichel W. The biology of aging. J Am Geriatr Soc. 1966;14:431–436. doi: 10.1111/j.1532-5415.1966.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 2.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nature Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 3.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 5.Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 7.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. This study established that the longevity and stress-resistance effects of reduced IIS are conserved from invertebrates to mammals. [DOI] [PubMed] [Google Scholar]
- 8.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nature Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 10.Dillin A, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nature Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- 13.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 14.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 15.Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 16.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. In this article the authors reported that the expression of one aggregation-prone polyQ protein can destabilize the genome and lead to the aggregation of other proteins. [DOI] [PubMed] [Google Scholar]
- 17.Kopito RR, Ron D. Conformational disease. Nature Cell Biol. 2000;2:E207–E209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 18.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 19.Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- 20.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 21.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nature Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 22.Forloni G. Neurotoxicity of beta-amyloid and prion peptides. Curr Opin Neurol. 1996;9:492–500. doi: 10.1097/00019052-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Med. 2008 Jun 22; doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 27.Behrends C, et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol Cell. 2006;23:887–897. doi: 10.1016/j.molcel.2006.08.017. Along with reference 28, this work indicated that chaperones, which are known to disrupt protein aggregates, switch to actively create aggregates of high molecular mass when the concentration of the aggregating protein is high. These papers thus established the idea that active aggregation can be protective. [DOI] [PubMed] [Google Scholar]
- 28.Shorter J, Lindquist S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science. 2004;304:1793–1797. doi: 10.1126/science.1098007. [DOI] [PubMed] [Google Scholar]
- 29.Amaducci L, Tesco G. Aging as a major risk for degenerative diseases of the central nervous system. Curr Opin Neurol. 1994;7:283–286. doi: 10.1097/00019052-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Brignull HR, Morley JF, Morimoto RI. The stress of misfolded proteins: C. elegans models for neurodegenerative disease and aging. Adv Exp Med Biol. 2007;594:167–189. doi: 10.1007/978-0-387-39975-1_15. [DOI] [PubMed] [Google Scholar]
- 31.Jankowsky JL, et al. Transgenic mouse models of neurodegenerative disease: opportunities for therapeutic development. Curr Neurol Neurosci Rep. 2002;2:457–464. doi: 10.1007/s11910-002-0073-7. [DOI] [PubMed] [Google Scholar]
- 32.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 33.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. This paper showed that HSF-1 is crucial for enabling reduced IIS to extend lifespan. [DOI] [PubMed] [Google Scholar]
- 34.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. This seminal study indicated that the minimal number of polyQ repeats that is needed to enable aggregation declines with age. It also showed that a reduction in IIS protects worms from polyQ aggregation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 36.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 37.Kops GJ, et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 38.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- 40.Henderson ST, Johnson T. E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 41.Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 43.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 45.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. This report indicated that reduced IGF signalling is typical in the offspring of centenarians and suggested that the regulation of lifespan by IGF signalling is conserved in humans. It also showed that high IGF plasma levels can lead to reduced IGF signalling, supporting the idea that feedback loops regulate the IIS pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu XD, Liu PC, Santoro N, Thiele DJ. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997;16:6466–6477. doi: 10.1093/emboj/16.21.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 49.Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J. 2003;17:1960–1962. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- 50.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baird NA, Turnbull DW, Johnson EA. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem. 2006;281:38675–38681. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- 52.Reinke H, et al. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008;22:331–345. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh V, Aballay A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci USA. 2006;103:13092–13097. doi: 10.1073/pnas.0604050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 55.Tullet JM, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 57.Halaschek-Wiener J, et al. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh SW, et al. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nature Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- 59.Dong MQ, et al. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–663. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- 60.Tonkiss J, Calderwood SK. Regulation of heat shock gene transcription in neuronal cells. Int J Hyperthermia. 2005;21:433–444. doi: 10.1080/02656730500165514. [DOI] [PubMed] [Google Scholar]
- 61.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 62.Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin Cell Dev Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 64.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 65.Valenzano DR, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 66.Parker JA, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genet. 2005;37:349–350. doi: 10.1038/ng1534. This study showed that altering aging with the drug Resveratrol protects PolyQ-expressing neurons from protein aggregation. It thus linked the aging process and protein-aggregation neurotoxicity. [DOI] [PubMed] [Google Scholar]
- 67.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 68.Link C. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Humbert S, et al. The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves Huntingtin phosphorylation by Akt. Dev Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol. 2006;172:719–731. doi: 10.1083/jcb.200510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nature Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 72.Carro E, et al. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol Aging. 2006;27:1250–1257. doi: 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 73.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci. 2003;26:404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 74.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nature Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 76.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE. 2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 77.George S, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biddinger SB, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 80.Michael MD, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 81.Craft S, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. This study indicated that insulin levels can vary in the plasma and in the CSF, and it correlated these levels with severity of dementia in Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 82.Teede HJ, Hutchison S, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. 2006;30:45–53. doi: 10.1385/ENDO:30:1:45. [DOI] [PubMed] [Google Scholar]
- 83.Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ. Evolution of lifespan in C. elegans. Nature. 2000;405:296–297. doi: 10.1038/35012693. [DOI] [PubMed] [Google Scholar]
- 84.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 85.Holzenberger M, et al. Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology. 2001;142:4469–4478. doi: 10.1210/endo.142.10.8461. [DOI] [PubMed] [Google Scholar]
- 86.Patel DS, et al. Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics. 2008;178:931–946. doi: 10.1534/genetics.107.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leibson CL, et al. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann NY Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 88.Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 89.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 90.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 5:155–171. discussion 172 (1989) [PubMed] [Google Scholar]
- 91.Lin SJ, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 92.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 93.Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 94.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 96.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 98.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- 100.Pinkston-Gosse J, Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nature Genet. 2007;39:1403–1409. doi: 10.1038/ng.2007.1. [DOI] [PubMed] [Google Scholar]
- 101.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 102.Outeiro TF, et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 103.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nature Med. 2004;10(Suppl):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 104.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nature Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]