Abstract
We examined the effects of single and multiple maternal glucocorticoid courses on apoptosis in the cerebral cortices of ovine fetuses (CC). Ewes received single dexamethasone or placebo courses at 104–106 or 133–135 days or multiple courses between 76–78 and 104–106 days gestation. In the single-course groups, ewes received four 6 mg dexamethasone or placebo injections every 12 hr for 48 hr. Multiple-course groups received the same treatment once per week for 5 weeks. Neuronal and nonneuronal apoptotic cell numbers per square millimeter were determined with TUNEL and NeuN staining and with caspase-3 enzyme activity on CC tissues harvested at 106–108 (70%) or 135–137 (90%) days of gestation. Apoptotic cell numbers and caspase-3 activity were 50% lower (P < 0.02) after single placebo courses at 90% than 70% gestation; 90% of apoptotic cells were (P < 0.01) nonneuronal at both ages. Nonneuronal apoptotic cells and caspase-3 activity were 40% and 20% lower (P < 0.02) after single dexamethasone than placebo courses at 70%, but not 90%, gestation. Caspase-3 activity was 20% lower (P < 0.01) after multiple dexamethasone than placebo courses, but apoptotic cell number did not differ. We conclude that nonneuronal apoptosis represents the major form of apoptosis in the CC at both 70% and 90% of gestation. Apoptosis in nonneuronal cells decreases with maturity and after a single course of dexamethasone at 70%, but not at 90%, gestation and not after multiple courses at 70% gestation. We speculate that a single course of glucocorticoids exerts maturational changes on the rate of apoptosis in the cerebral cortex of preterm ovine fetuses.
Keywords: apoptosis, brain, caspase, development, fetus, maternal, sheep, steroids
Cerebral cortical development involves coordinated processes that include removal of cells that are no longer necessary (Rakic, 2006). Apoptosis is an essential mechanism for cellular death normally occurring during brain development (Lossi and Merighi, 2003), which varies with the developmental stages of the brain (Blaschke et al., 1996). DNA breakdown is a key process in apoptosis (Saraste and Pulkki, 2000). Caspases promote apoptosis by targeting important cellular structural proteins (Saraste and Pulkki, 2000; Sastry and Rao, 2000). Caspase-3 is a key executioner of apoptosis (Khurana et al., 2002), which mediates cell death in both neurons and oligodendrocyte progenitors (Rothstein and Levison, 2005). However, apoptosis of proliferating precursors and young premigratory cells can occur without caspase-3 cleavage in the brain (Lossi et al., 2004).
Central nervous system (CNS) development is dependent on many factors including the hormonal environment. Glucocorticoid increases during fetal development affect the maturation of many organs, including the brain (Challis and Brooks, 1989; NIH, 1995). Glucocorticoids regulate cellular proliferation and survival (Alonso, 2000; Wong and Herbert, 2004) and apoptotic cell death in the CNS (Almeida et al., 2000). The effects of glucocorticoids on CNS apoptotic cell death vary with age, dose, and cell type (Hassan et al., 1996; Almeida et al., 2000; Haynes et al., 2001). Dexamethasone increases apoptotic CNS cell death in young and old adult rats (Almeida et al., 2000), but the affect is greater in the older rats (Almeida et al., 2000). Although dexamethasone increases apoptosis in some studies (Almeida et al., 2000; Baud et al., 2001), this affect is not consistent (Tan et al., 2002). Therefore, the effects of dexamethasone on apoptosis in the CNS remain controversial.
A single course of glucocorticoids has been widely used to treat women at risk of premature birth (Newnham et al., 2002). Exposure of the sheep fetus to glucocorticoid levels similar to those to which human fetuses are exposed affects cytoskeletal proteins, cerebral blood flow, and neuronal activity (Schwab et al., 2000, 2001a,2001b; Adams et al., 2001). A single course of maternal glucocorticoids reduces blood–brain barrier permeability in fetuses at 60% and 80%, but not 90%, of gestation (Stonestreet et al., 1999, 2000). These studies suggest that glucocorticoid exposure in doses similar to those used to treat women in premature labor affects the fetal brain.
An NIH Panel recommended that weekly courses of glucocorticoids should be reserved for controlled trials (NIH, 2001). Recent trials comparing repeated and single courses of glucocorticoids demonstrated better shortterm outcomes after repeated than single courses of glucocorticoids (Crowther et al., 2006; Wapner et al., 2006). However, these trials raised concerns about lower birth weights (Crowther et al., 2006; Wapner et al., 2006), and one study reported smaller head circumferences after repeated courses (Crowther et al., 2006). Long-term outcomes (Crowther et al., 2007; Wapner et al., 2007) suggest that children exposed to repeated courses do not differ in physical or neurocognitive measures compared with a single course (Wapner et al., 2007). However, a trend in one study toward higher rates of cerebral palsy after repeated courses continues to raise concern (Wapner et al., 2007).
Given these considerations, we tested the hypotheses that 1) maternal glucocorticoid treatment in doses similar to those used to treat women in premature labor modifies the amount of apoptotic cell death in the cerebral cortex early (70%) and late (90%) in fetal development and 2) multiple glucocorticoid courses accentuate these perturbations compared with a single course. We examined apoptosis in neuronal and nonneuronal cells, because glucocorticoids affect both of these cell lines (Avola et al., 2004; Baud, 2001).
MATERIALS AND METHODS
This study was conducted after approval by the Institutional Animal Care and Use Committees of Brown University and Women and Infants’ Hospital of Rhode Island and according to the NIH Guide for the care and use of laboratory animals (National Institutes of Health Publication No. 85-23, 1985).
Study Groups
Forty-three fetuses of mixed-breed ewes were randomly assigned to six groups. Six fetuses at 104–106 days of gestation, hereafter designated as 70% of gestation, were assigned to a single-course placebo group; five fetuses at 70% of gestation were assigned to a single-course dexamethasone group; seven fetuses at 70% of gestation were assigned to a multiple-course placebo group; eight fetuses at 70% of gestation were assigned to a multiple-course dexamethasone group; nine fetuses at 133–135 days of gestation, hereafter designated as 90% of gestation, were assigned to a single-course placebo group; and eight fetuses at 90% of gestation were assigned to a single-course dexamethasone group. Term gestation in the ovine fetus is approximately 145 days. At 70% and 90% of gestation, the fetal sheep brain is approximately equivalent to human fetuses at 25–28 and 33–36 weeks of gestation, respectively (Back et al., 2006). The blood and cerebral cortical samples for this study were obtained from animals enrolled in a series of studies to examine the effects of antenatal glucocorticoids on blood–brain barrier permeability and regional tissue water contents (Stonestreet et al., 2000, 2003, 2004; Sadowska et al., 2006).
Animal Preparation
The details of the surgical preparation for the study groups summarized above have been previously reported (Stonestreet et al., 1999). Briefly, surgery was performed with animals under halothane anesthesia on time-dated Eastern mixed breed pregnant ewes at 98–101 days or 128–129 days of gestation as previously described (Stonestreet et al., 1999). The thoracic aorta was cannulated for blood sample withdrawal. Fetuses of ewes assigned to receive multiple courses of placebo or dexamethasone were catheterized at 98–101 days of gestation after the ewes had received four courses of placebo or dexamethasone. The fifth and last course was started on the fourth day of recovery from surgery at 105 days of gestation.
Two to eight days after recovery from surgery, the ewes received a 6-mg intramuscular injection of dexamethasone (Fujisawa, Deerfield, IL; concentration = 4 mg/ml, 1.5 ml was given to each ewe) or placebo (0.9% NaCl, 1.5 ml) every 12 hr for 48 hr starting at 104–106 or 133–135 days of gestation in the single-course groups, and at days 76, 84, 91, 98, and 105 of gestation in the multiple-course groups. The dose of dexamethasone used in these studies was based on recommendations for fetal maturation in pregnant women with premature labor (NIH, 1995).
The ewes in single- and multiple-course treatment groups remained in the animal care facility for different time intervals, i.e., approximately 2 weeks in the single- and 6 weeks in the multiple-course groups. Therefore, two groups of ewes received slightly different diets and potentially had different levels of activity, because the single-course groups remained on the farm for longer than the multiple-course groups.
Experimental Protocol, Methodology, and Tissue Collection
Samples were obtained from the fetal sheep at least 5 days after recovery from surgery. The ewes were monitored for premature labor by changes in amniotic fluid pressures as previously described (Stonestreet et al., 2000). On days 106–108 or 135–137 of gestation, 18 hr after the last dose of placebo or dexamethasone was given to the ewes, fetal arterial pH, blood gases, and plasma cortisol concentrations were obtained. Then, the ewes were given intravenous pentobarbital (15–20 mg/kg) to achieve a surgical plane of anesthesia, and a hysterotomy was performed. The fetuses were removed and the fetal brains weighed. A standardized portion of fetal frontal cerebral cortex was dissected, immediately frozen in liquid nitrogen, and stored at −80°C until histological preparation. The ewes were given an overdose of pentobarbital (100–200 mg/kg).
Arterial pH, blood gases, mean arterial blood pressures, and total cortisol concentrations were measured as described by Sadowska et al. (2006). Total plasma cortisol concentrations were measured using the Clinical Assays GammaCoat Cortisol 125I-radioimmunoassay (DiaSorin, Stillwater, MN). The GammaCoat antiserum exhibits 100% cross-reactivity with cortisol. The observed coefficient of variation for interand intraassay precision was 10.1% and 7.9% respectively.
Histological Methods
Frozen cerebral cortex from each fetal brain was homogenously sectioned with a microtome into six-µm sections with a cryostat (Leica Cryocut 1800). Sections were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburg, PA) and postfixed in acetone for 5 min. The slides were stored at −80°C until required for analysis. Four slides were prepared from each fetal sheep cerebral cortex. Two slides were stained with hematoxylin and eosin to determine the entire surface area of the cerebral cortical tissue for each fetal sheep. Two slides were used to measure the amount of apoptosis in the neuronal and nonneuronal cellular populations using the methods described below.
Immunohistochemical Labeling of Sheep Neurons for Neuronal Nuclear Antigen (NeuN)
NeuN is a sensitive and specific neuronal marker that identifies postmitotic neurons for diagnostic histopathology (Wolf et al., 1996). This nuclear antigen first appears at developmental time points corresponding to the withdrawal of the neuron from the cell cycle and/or initiation of terminal differentiation of the neuron (Mullen et al., 1992; Sarnat et al., 1998). The antigen is not expressed in young neuroblasts or in nonneuronal cell types such as oligodendrocytes, astrocytes, or glia.
The primary mouse monoclonal antineuronal-specific nuclear protein (NeuN) antibody has been characterized for a variety of vertebrate species (Chemicon, Temecula, CA) and has been used as a neuronal marker in sheep (Mullen et al., 1992; Sliwowska et al., 2004; Anderson et al., 2008). We confirmed that mouse anti-NeuN monoclonal antibody identified the sheep NeuN antigen by simultaneously examining positive and negative controls from sheep and rat cerebral cortex. The mouse anti-NeuN exhibited comparable staining in both rat and sheep cerebral cortical neurons. The negative controls did not have the addition of the primary antibody and as a result did not exhibit any staining in either the rat or the sheep brain.
For immunostaining, the slides were incubated overnight at 4°C in 4% normal horse serum suspended in Fluorescent Treponema Antigen Buffer (FTA)-0.1% Triton X blocking solution (Vectastain Kit; Vector Laboratories, Burlingame, CA). The sections were washed with FTA-0.1% Triton-X three times for 10 min each. After blocking, the slides were incubated for 2 hr in the dark at room temperature with primary mouse monoclonal anti-NeuN antibody (180 µl; Chemicon, Temecula, CA; MAB377, 1 mg/ml) at a dilution of 1:50 suspended in the blocking solution described above (Vectastain Kit; Vector Laboratories). The sections were rinsed again in FTA-0.1%Triton-X, three times for 10 min each and incubated for 1 hr in the dark at room temperature with a secondary donkey anti-mouse IgG affinity-purified rhodamine-conjugated antibody absorbed for dual labeling (180 µl; Chemicon; AP192R, 1 mg/ml) at a dilution 1:100 in FTA-0.1% Triton X. The slides were then rinsed and prepared for the ApopTag protocol described below.
Determination of DNA Fragmentation
DNA fragmentation indicative of apoptosis within cells was determined using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method with a commercial kit (ApopTag Fluorescein In Situ Apoptosis Detection Kit; Chemicon). We initially used the ApopTag peroxidase in situ apoptosis staining method (Chemicon) and brightfield microscopy of sheep cerebral cortical tissue to validate adequate staining of sheep cerebral cortical nuclei. We validated the ApopTag assay for use in sheep by examining sections of sheep cerebral cortex along with a set of negative and positive controls provided by the manufacturer (Chemicon). This analysis demonstrated adequate staining of sheep cerebral cortical tissue with cells containing nuclei with the typical morphologic characteristics of apoptosis and minimal background staining of the negative control sheep sections in which the active antibody was not applied. A similar procedure has been previously used to detect apoptosis in the fetal sheep brain (Falkowski et al., 2002).
We also compared the peroxidase in situ apoptosis detection staining method with the fluorescein method by demonstrating equivalent staining of the same nuclei on consecutive sections. We decided to use the fluorescein in situ detection method because this assay provided the advantage of detecting more apoptotic nuclei against the dark background, which is important given the low occurrence of apoptosis on the sheep cerebral cortical sections (Falkowski et al., 2002). The fluorescein in situ assay also allowed for double-fluorescent labeling of the samples to detect simultaneously the NeuN-labeled and apoptotic neurons.
Double-Label Immunofluorescence Microscopy and Morphometric Analysis: Neuronal, Nonneuronal, and Apoptotic Cellular Counts
After labeling of the neuronal nuclei with the fluorescent rhodamine-conjugated antibody for NeuN antigen visualization and the fluorescein ApopTag marker for apoptotic nuclei, the slides were rinsed in buffer three times and then coverslipped in Vectashield (Vector Laboratories) mounting medium containing 4′,6-diamidino-2-phenylindole stain (DAPI; Vector Laboratories). DAPI forms fluorescent complexes with natural double-stranded DNA, strongly enhancing its fluorescence. Consequently, all nuclei in the section acquire a blue fluorescent color when viewed through the blue channel of the fluorescent microscope. Nuclei positive for the NeuN antigen exhibit colocalization of the rhodamine red with the DAPI blue fluorescence, when the pictures are merged through dual channel fluorescent microscopy. Similarly, nuclei positive for the apoptosis marker exhibit colocalization of the green fluorescein with the blue DAPI fluorescence.
Comparable areas of the cerebral cortex were identified on the sections from all fetal sheep in the different study groups by a microscopist who was not aware of the group assignments. Six nonoverlapping high-power fields (HPF) viewed with the same ×40 power optical were randomly sampled from the cerebral cortex of each fetus on each slide. The numbers of nuclei stained with the DAPI blue fluorescence were counted in each HPF and averaged for the six fields. This represented the average of the total number of nuclei per HPF, reflecting the cellular density in the cerebral cortical sample from each fetus.
Nuclei that exhibited colocalization of the red rhodamine with the blue DAPI fluorescence were designated as nuclei containing the NeuN antigen, i.e., neuronal nuclei. Nuclei that exhibited only the DAPI fluorescence when viewed through the double red and blue channels represented nuclei that did not contain the NeuN antigen and were designated as nonneuronal nuclei. Similarly, nuclei that exhibited colocalization of the three stains, the green fluorescein apoptosis marker, red NeuN antigen marker, and the blue DAPI nuclear DNA marker were considered apoptotic neuronal nuclei. Nuclei that exhibited colocalization of the green fluorescein and blue fluorescence, but not the red fluorescence, were considered nonneuronal apoptotic nuclei.
Stained slides were observed with a Nikon Eclipse E800 microscope (Nikon Inc., Melville, NY) equipped with a Spot II CCD camera (Diagnostic Instruments, Sterling Heights, MI). In each cerebral cortical section, the six randomly selected nonoverlapping gray-scale HPFs per slide (eight bit) were acquired with a Spot II CCD camera using the ×40 PlanApo objective (Nikon E800). In each field, separate images were acquired with the DAPI, rhodamine, and fluorescein filters with the Spot II digital camera. Image processing and analysis was performed in NIH Image (NIH, Springfield VA). Positive cellular staining was defined through intensity thresholding. The resulting binary images were analyzed for cell counts for NeuN staining (rhodamine) and total cell counts as determined by the DAPI stain.
The entire surface area of each cerebral cortical section was used to detect the number of apoptotic cells present because of the low occurrence of apoptosis in the fetal sheep cerebral cortex (Falkowski et al., 2002). The cerebral cortex cellular density of the sections was determined to be relatively homogeneous and similar among the different groups of fetal sheep by the microscopist not aware of group assignments. The entire surface area of each cerebral cortical sample was calculated in NIH Image 1.62. All apoptotic cells stained with fluorescein (Chemicon) present on each slide were visually counted and recorded.
The slides stained with hematoxylin and eosin were viewed under light microscopy and used for the area measurements. The slides were scanned with Polaroid SprintScan 35 Plus (Polaroid, Waltham, MA) using a Pathscan Enabler (Meyer Instrument, Houston, TX). Area measurements were performed with NIH Image 1.62. Images were calibrated so that results were expressed as number of apoptotic cells per square millimeter. The number of green fluorescein apoptotic nuclei and the number of the double-labeled and red rhodamine apoptotic neurons per square millimeter were determined on the sections. All values represent the average of two slides per fetal sheep.
Caspase-3 Enzyme Activity
A portion of the fresh frozen cerebral cortex adjacent to the area used for the microscopic analyses was obtained for caspase-3 analysis. Caspase-3 is a key executioner of apoptosis (Khurana et al., 2002). The larger the proportion of caspase-3 mediated apoptotic cellular death in a tissue, the greater the quantity of active caspase-3 enzyme that can be detected in the same tissue (Hughes and Gobe, 2007).
We measured caspase-3 enzyme activity according to previously described methods (Khurana et al., 2002; Chiang et al., 2007) with some modifications. The relative amount of the active caspase-3 enzyme was measured using the Biovision caspase-3/32-kDa cysteine protease (CPP32) fluorometric assay (Bio Vision, Mountain View, CA). The assay is based on the detection of cleavage of the substrate Asp-Glu-Val-Asp-Amino-4-trifluoromethyl coumarin (DEVD-AFC) by the caspase-3 enzyme. DEVD-AFC emits blue light (λmax = 400 nm) upon cleavage of the substrate by the CPP32 or related caspases, whereas free AFC emits a yellow-green fluorescence (λmax = 505 nm). Comparison of the fluorescence emission of AFC from an experimental sample containing apoptotic cells with a control sample permits the determination of the quantity of increase or decrease in caspase-3 activity relative to a standard (Bio Vision; Hall et al., 2000).
The samples were thawed and homogenized in a cell lysis buffer (Bio Vision). Protein concentrations were determined on an aliquot of the extracted sample from each fetus by a bicinchoninic acid protein assay (BCA; Pierce, Rockford, IL). The average protein concentration of the extract was 12.8 ± 2.5 mg/ml. A four-position cell changer luminescence spectrometer (LS50B; Perkin Elmer, Waltham, MA) operated by the FL Win Lab 4.0 software system (Perkin Elmer) was used for the caspase-3 determinations. Each cell in the changer has a 10-mm path length of light.
We designed a plastic adapter to align a 3.0 × 3.0 mm (outside diameter) four-sided optically clear polystyrene (350 µl capacity; Versa Fluor Microcurette, Bio-Rad Laboratories, Hercules, CA) along the optical path of the spectrometer to ensure maximal exposure of the microcuvette contents to the light path when filled with a 110-µl solution. All equipment was preheated to 37°C, and the four-position cell changer was thermostated to 37.5°C using circulating warm water. The four cuvettes of the adapter were used in each assay as follows: 1) position one was fitted with a standard size cuvette filled with water and used for monitoring the internal temperature of the apparatus at the beginning and end of each assay to ensure consistency of temperatures throughout each assay and between assays; 2 and 3) the next two cuvette positions were used for the two duplicate aliquots of the extract from each fetal brain homogenate; and 4) the last cuvette position was used to load an equal amount of induced Jurkat cytosolic extract (1,000-µg vials; Bio Vision; catalog No. 1107-1000, lot No. 7067). The cytosolic Jurkat extract, prepared from the Jurkat cells induced with 2 µM camptothecin, was used as a positive control and reference standard. We used the Jurkat lysate extract as a standard because it was reproducible and similar in magnitude to the relatively low values that we obtained in our experimental samples.
Aliquots were adjusted for equal loading ratios of 500 µg protein in 50 µl cell lysis buffer, mixed with 50 µl reaction buffer (Bio Vision), and 5 µl of the DEVD substrate per the manufacturer’s protocol. Five hundred micrograms was selected after testing serial dilution curves of 200-, 500-, and 1,000-µg quantities from representative experimental samples of cerebral cortex of fetal sheep, because this quantity produced the optimal signal. We performed serial dilutions on the induced Jurkat cytosolic extracted samples to determine the dilution, which was in the same range as the experimental samples. We determined that 15.5 µg of the induced Jurkat cytosolic extracted sample diluted to a volume of 60 µl in cell lysis buffer was in a range similar to that of the experimental samples. We used the same Jurkat lot number for the cell lysate for consistency in all experiments.
We used the following parameters to set up the LS50B luminescence spectrometer: excitation wavelength 400 nm, excitation slit 15.0 mm, emission wavelength 505 nm, and emission slit 10.0 mm. We set up the software to subtract the background signal at time zero from all subsequent measures for each position. The intensity of emission was recorded in the time drive mode over a 20-min period, and the units of intensity were plotted against time in seconds, with sampling time every 4 sec. The larger the quantity of active caspase-3 molecules in the solution, the more DEVD molecules degraded per unit time and the larger the number of fluorescence-emitting particles produced per unit time. The rate of the reaction was represented by the slope of the graph calculated over the middle portion of the graph that was most linear. The slopes of the experimental samples were divided by the slopes of the Jurkat standard samples. The resultant intensity ratio was used to express the standardized relative caspase-3 enzyme activity in each experimental sample. The relative enzyme activity for each experimental animal was expressed as the average of the ratios for the two aliquots from each cerebral cortical homogenate, which had been loaded along with the Jurkat standard in each assay. A similar technique for normalization of caspase activity recently has been reported (Park et al., 2007). The intra- and interassay coefficients of variability of the experimental assay used at these dilutions were 4.3% and 3.5%, respectively.
Statistical Analysis
Two-way ANOVA was used to compare values among fetuses of the ewes treated with single or multiple placebo or dexamethasone courses and the fetuses at 70% and 90% of gestation. The factors were treatment (placebo or dexamethasone), course frequency (single or multiple), and gestational age (70% or 90% of gestation). We also included gender (male or female) in the ANOVA as a factor, because previous work has suggested that there are innate gender-based differences in outcome for programmed cell death pathways of neurons after exposure to environmental stressors (Du et al., 2004). If a significant difference was found by ANOVA, the Fisher least significant difference test was used to detect specific differences among the fetuses of the ewes treated with dexamethasone or placebo. Differences are considered statistically significant at P < 0.05. Values are expressed as means ± SEM.
RESULTS
Physiological, Biochemical, Hormonal, Gender, and Anthropometric Variables in the Ovine Fetuses
The physiological, biochemical, hormonal, and anthropometric results from the fetuses of the placebo- and dexamethasone-treated ewes have previously been reported and will be briefly summarized here for this subset of animals (Stonestreet et al., 2000, 2003, 2004; Sadowska et al., 2006). The pH, blood gas, and mean arterial blood pressure values in each age group were within the physiological range for our laboratory (Stonestreet et al., 2003; Sadowska et al., 2006) and similar to values reported from other laboratories (Unno et al., 1999). The fetuses of ewes at 70% of gestation treated with a single course of dexamethasone had significantly higher arterial oxygen tensions (37.0 ± 2.3 mmHg) than those of the placebo-treated ewes (24.5 ± 4 mmHg). There were no significant differences between the corresponding groups of fetuses of the placebo- and dexamethasone-treated ewes at each gestational age within the single- and multiple-course groups with respect to fetal pH, arterial carbon dioxide tension, or mean arterial blood pressures (Sadowska et al., 2006).
Both male and female fetal sheep were included in the studies. At 70% of gestation, there were four male and two female fetuses of the ewes exposed to a single course of the placebo and one male and four female fetuses of the ewes exposed to a single course of the dexamethasone. There were five male and no female fetuses of the ewes exposed to multiple courses of the placebo, but two fetuses in this group did not have gender recorded. There were five male and three female fetuses of the ewes exposed to multiple courses of the dexamethasone. At 90% of gestation, there were three male and two female fetuses of ewes exposed to a single course of placebo, but four fetuses in this group did not have gender recorded. There were four male and two female fetuses of the ewes at 90% of gestation exposed to a single course of the dexamethasone, but two fetuses in this group also did not have gender recorded.
The fetal brain weights have been reported previously (Stonestreet et al., 2004). Analysis of the subgroup of fetal sheep at 70% of gestation reported here showed that the brain weights of the fetuses of the ewes exposed to a single course of the placebo (30.9 ± 3.0 g) were heavier (P < 0.05) than those exposed to dexamethasone (27.3 ± 1.8 g). The brain weights of the fetuses of the ewes exposed to multiple placebo courses (30.7 ± 3.6 g) were also heavier (P < 0.05) than those exposed to multiple dexamethasone courses (28.8 ± 2.1 g). Fetal brain weights from the fetuses of the ewes at 90% of gestation were not available. The fetal cortisol values have been reported elsewhere (Stonestreet et al., 2000; Sadowska et al., 2006). The analysis of the subgroup reported here at 70% of gestation showed that the plasma cortisol values of fetuses of the ewes exposed to the single and multiple courses of placebo did not differ significantly from those exposed to similar courses of dexamethasone. However, as expected, the fetal plasma cortisol values were higher (P < 0.01) in the fetuses of the placebo-treated ewes at 90% of gestation (16.9 ± 2.3 ng/ml) than those of the placebo-treated ewes at 70% of gestation (8.3 ± 0.8 ng/ml). There were no differences between the plasma cortisol levels of the fetuses of the placebo- and dexamethasone-treated ewes at 90% of gestation. None of the treated ewes developed premature labor.
Apoptotic Cell Number, Total Cell Number, and Caspase-3 Activity in Fetuses of Ewes Treated With the Single Courses at 70% and 90% of Gestation
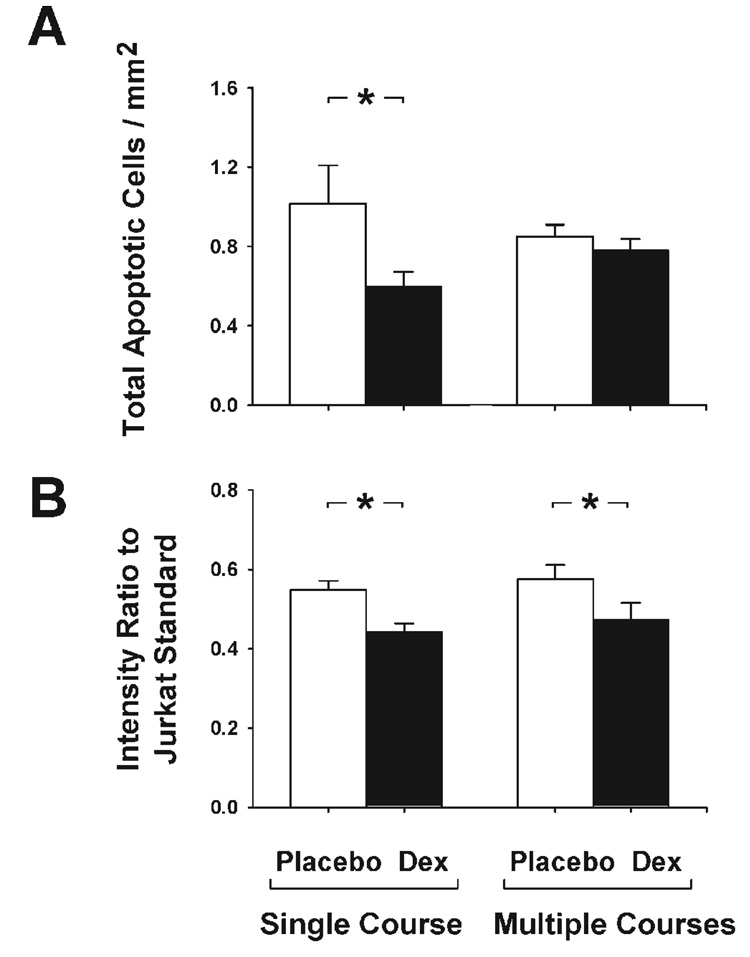
The total number of apoptotic cells in the cerebral cortex of the fetuses of the ewes exposed to a single course of placebo was 50% lower (P < 0.02) at 90% than at 70% of gestation (Fig. 1A). The number of apoptotic cells in the cerebral cortex of the fetuses of the ewes exposed to a single course of dexamethasone was 40% lower (P < 0.02) than in those of the placebo-treated ewes at 70% of gestation but did not differ between the fetuses of the placebo- and dexamethasone-treated ewes at 90% of gestation (P: NS; Fig. 1A). Maternal treatment with dexamethasone did not differentially affect fetal apoptosis as a function of gender (P = 0.59). Similarly, the activity of caspase-3 measured as the intensity ratio to the Jurkat standard was 50% lower (P < 0.01) in the fetuses of the placebo-treated ewes at 90% than 70% of gestation and 20% lower (P < 0.01) in the fetuses of the dexamethasone- vs. the placebo-treated ewes at 70% but not at 90% of gestation (P: NS; Fig. 1B).
Figure 1.
Total number of apoptotic cells per square millimeter in the cerebral cortex plotted for the fetuses of the ewes exposed to a single course of placebo (open bars) and dexamethasone (solid bars) at 70% and 90% of gestation (A); Fetuses of placebo (n = 6)- and dexamethasone (n = 5)-treated ewes at 70% of gestation; fetuses of placebo (n = 7)- and dexamethasone (n = 8)-treated ewes at 90% of gestation. B: The quantity caspase-3 measured as the intensity ratio to the Jurkat standard for the fetuses of the ewes exposed to a single course of placebo and dexamethasone at 70% and 90% of gestation. Numbers and bars as for A. *P < 0.02.
There were no differences in the total number of cells or number of neuronal or nonneuronal cells per HPF between the fetuses of the placebo- and dexamethasone-treated ewes exposed to the single courses at 70% or 90% of gestation (Table I). The total number of cells and number of neuronal cells per HPF were significantly lower at 90% than 70% of gestation in the fetuses of the placebo-treated ewes. The differences between the total numbers of cells per HPF were due mainly to differences in the number of neuronal cells per HPF in the fetuses between 70% and 90% of gestation, because differences were not observed in the number of nonneuronal cells per HPF. The neuronal and nonneuronal cells were approximately equally abundant, with the ratio of the nonneuronal to the total number of cells ranging from 45% to 55% within each of the four groups. In contrast, double-labeling immunohistochemistry for the NeuN marker and TUNEL staining demonstrated that more (P < 0.01) than 90% of the total number of apoptotic cells were nonneuronal cells within each of the four groups. Significant differences in the number of neuronal apoptotic cell counts per square millimeter were not observed among the four groups exposed to the single courses (P: NS; Fig. 2A). In contrast, nonneuronal apoptotic cells per square millimeter were 50% lower (P < 0.01) in the fetuses of the placebo-treated ewes at 90% than at 70% of gestation, and they were 40% lower (P < 0.01) in the fetuses of the dexamethasone- vs. the placebo-treated ewes at 70% of gestation but not 90% of gestation (P: NS; Fig. 2B).
TABLE I.
Total Number, Neuronal and Nonneuronal Cells per HPF, and Neuronal and Nonneuronal Apoptotic Cells as Percentage of Total Apoptotic Cells in the Fetuses of Ewes Exposed to Single Placebo and Dexamethasone Courses at 70% and 90% of Gestation†
| Placebo | Dexamethasone | |
|---|---|---|
| Total cells, #HPF | ||
| 70% Gestation | 308 ± 18 | 281 ± 20 |
| 90% Gestation | 255 ± 13* | 253 ± 12 |
| Neuronal cells, #HPF | ||
| 70% Gestation | 164 ± 12 | 134 ± 22 |
| 90% Gestation | 113 ± 8* | 115 ± 8 |
| Non-neuronal cells, #HPF | ||
| 70% Gestation | 139 ± 8 | 147 ± 18 |
| 90% Gestation | 142 ± 10 | 138 ± 10 |
| Neuonal apoptotic cells | ||
| % Total apoptotic cells | ||
| 70% Gestation | 7.0 ± 1.5 | 7.6 ± 2.9 |
| 90% Gestation | 9.8 ± 2.5 | 10.7 ± 3.1 |
| Non-neuronal cells | ||
| % Total apoptotic cells | ||
| 70% Gestation | 93.0 ± 1.5** | 92.4 ± 2.9** |
| 90% Gestation | 90.2 ± 2.5** | 89.3 ± 3.1** |
Values mean ± SEM.
P < 0.01 vs. 70% gestation for the same treatment group.
P < 0.01 vs. neuronal apoptotic cell as for the same age group and treatment.
Figure 2.
Number of apoptotic neuronal cells times 10−2 per square millimeter in the cerebral cortex plotted for the fetuses of the ewes exposed to a single course of placebo and dexamethasone at 70% and 90% of gestation (A). Fetuses of placebo (n = 6)- and dexamethasone (n = 5)-treated ewes at 70% of gestation. Fetuses of placebo (n = 7)- and dexamethasone (n = 8)- treated ewes at 90% of gestation. The number of nonneuronal apoptotic cells per square millimeter is plotted for the fetuses of the ewes exposed to a single course of placebo and dexamethasone at 70% and 90% of gestation (B). Numbers and bars as for A and Figure 1. *P < 0.01.
Apoptotic Cell Number, Total Cell Number, and Caspase-3 Activity in Fetuses of Ewes Treated With the Single And Multiple Courses at 70% of Gestation
In contrast to maternal treatment with a single course of dexamethasone at 70% of gestation, which was associated with a 40% reduction in the total number of apoptotic cells per square millimeter, maternal treatment with five courses of dexamethasone was not associated with a reduction in the total number of apoptotic cells when compared with placebo treatment (P: NS, Fig. 3A). Maternal treatment with multiple courses of dexamethasone also did not differentially affect fetal apoptosis as a function of gender (P = 0.69). The quantity of total apoptotic cells in the cerebral cortex of fetuses of the ewes exposed to the single course of placebo was 16% higher than those exposed to the multiple courses of placebo; however, the differences did not reach statistical significance (P = 0.3). Fetal cerebral cortical capase-3 activity measured as a ratio to the Jurkat standard was 20% lower in the fetuses of the dexamethasone- than the placebo-treated ewes after both single- and multiple-course treatments (Fig. 3B). The data from the single-course group are reproduced here from Figure 1 for the purpose of comparison with the multiple-course groups.
Figure 3.
The total number of apoptotic cells per square millimeter in the cerebral cortex plotted for the fetuses of the ewes exposed to the single and multiple courses of placebo and dexamethasone at 70% of gestation (A). Fetuses of ewes exposed to a single course of placebo (n = 6)- and dexamethasone (n = 5)-treated ewes; fetuses of ewes exposed to multiple courses of placebo (n = 9) and dexamethasone (n = 8) at 70% of gestation. The quantity of caspase-3 measured as the intensity ratio to the Jurkat standard for the fetuses of the ewes exposed to the single and multiple courses of placebo and dexamethasone at 70% (B). Numbers and bars as for A and Figure 1. *P < 0.01.
Maternal treatment with multiple courses of dexamethasone also was not associated with changes in the total number of cells or number of neuronal or nonneuronal cells per HPF compared with the fetuses of the placebo treated ewes (P: NS; Table II). Although the number of nonneuronal cells remained the predominant population of apoptotic cells in the multiple-course groups, there were no differences between the number of apoptotic neuronal or nonneuronal nuclei between the fetuses of ewes exposed to the multiple courses of placebo and dexamethasone (Table II).
Table II.
Total Number, Neuronal and Nonneuronal Cells per HPF and Neuronal and Nonneuronal Apoptotic Cells as % of Total Apoptotic Cells in the Fetuses of Ewes Exposed to the Multiple Courses†
| Multiple placebo | Multiple dexamethasone | |
|---|---|---|
| Total cells, #HPF | 290 ± 20 | 285 ± 16 |
| Neuronal cells, #HPF | 153 ± 13 | 162 ± 8 |
| Nonneuronal cells, #HPF | 137 ± 18 | 122 ± 13 |
| Neuronal apoptotic cells, mm2 | 0.13 ± 0.02 | 0.07 ± 0.02 |
| Nonneuronal apoptotic cells, #/mm2 | 0.72 ± 0.08 | 0.71 ± 0.1 |
| Neuronal apoptotic cells, % total apoptotic cells | 12.9 ± 6.0 | 9.4 ± 2.8 |
| Nonneuronal apoptotic cells, % total apoptotic cells | 87.1 ± 6.0* | 90.6 ± 2.8* |
Values mean ± SEM.
P < 0.01 vs. neuronal apoptotic cells as % total apoptotic cells.
DISCUSSION
There are four major findings of our study. First, the amount of apoptosis in the ovine fetal cerebral cortex decreases between 70% and 90% of gestation. Second, nonneuronal apoptotic cells constitute the majority of apoptotic cells in the fetal cerebral cortex at 70% and 90% of gestation. Third, maternal treatment with a single course of glucocorticoids reduced the activity of the caspase-3 enzyme and apoptosis in nonneuronal cells at 70%, but not 90%, of gestation. Fourth, maternal treatment with multiple courses of glucocorticoids decreased the quantity of the caspase-3 enzyme but not the number of apoptotic cells at 70% of gestation.
We determined the amount of apoptosis in cerebral cortical tissue of ovine fetuses with the fluoresceintagged ApopTag TUNEL assay. We used rhodaminetagged secondary antibody to mouse monoclonal primary anti-NeuN antibody to label the apoptotic nuclei of the postmitotic neurons. Consistently with previous findings, we confirmed the suitability of the ApopTag TUNEL assay for use in ovine brain and mouse monoclonal NeuN antibody to recognize ovine NeuN-positive neuronal nuclei (Mullen et al., 1992; Sliwowska et al., 2004; Anderson et al., 2008). We also used a realtime assay to measure the intensity of emissions from the cleavage products of caspase-3 substrate to estimate the amount of caspase-3-related apoptosis present in the cerebral cortical tissue. We modified the caspase-3 assay from a previous method (Chiang et al., 2007) to detect low levels of caspase-3 in the cerebral cortex of normoxic fetuses. Changes in the quantity of the caspase-3 observed after most of the fetal exposures were consistent with the changes in apoptosis determined by the TUNEL assay.
Several studies have examined changes in apoptosis during normal cerebral cortical maturation and demonstrated dual waves of apoptotic activity during the later stages of fetal development. The first wave consists of apoptotic cell death of proliferating precursors and young neuroblasts and is linked to cell cycle regulation (Blaschke et al., 1996). This is observed at about 70% of gestation in rodents (Blaschke et al., 1996). The second wave affects postmitotic neurons at later stages of cerebral cortical development. Both caspase-3-dependent and -independent pathways contribute to the naturally occurring cellular death in the developing brain (Lossi et al., 2004). Our findings in well-oxygenated ovine fetuses during the last one-third of gestation are consistent with these concepts and further suggest that the amount of naturally occurring apoptotic cellular death is greater in the cerebral cortex at 70% than at 90% of gestation (Fig. 1). The greater amount of caspase-3 detected at 70% than at 90% of gestation further substantiates this finding. Consistent with previous reports (Falkowski et al., 2002), the level of apoptotic cell death in the ovine fetal cerebral cortex is relatively low at both ages that we examined. Furthermore, the greatest decrease in apoptosis was in the cells that did not stain with the NeuN antigen (Fig. 2; Hu et al., 2002). The population of nonneural apoptotic cells that did not express NeuN was presumably precursor cells, glial cells, or young neuroblasts (Magavi et al., 2000; Hu et al., 2002). The reduction in the number of apoptotic cells as well as in the caspase-3 activity in the fetuses of the ewes treated with a single course of placebo at 90% than 70% of gestation most likely represents a normal ontogenic decrease in apoptosis with maturation.
This is the first study to examine the modulatory effects of maternal glucocorticoid treatment on the quantity of naturally occurring apoptotic cell death in the brain of a large mammalian fetus during the last one-third of gestation. There is little information on the effects of glucocorticoids on apoptotic cell death in the developing fetal brain in spite of widespread glucocorticoid use to treat women in premature labor (Newnham et al., 2002; Baud, 2004; Cavalieri and Cohen, 2006). Evidence suggests that the prevailing corticosteroid environment before and after cell division regulates survival of premitotic progenitor cells and postmitotic neurons (Wong and Herbert, 2004). Although low levels of glucocorticoids enhance progenitor cell survival (Warringa et al., 1987), high levels reduce survival of progenitor cells and postmitotic neurons (Wong and Herbert, 2004). Consistent with these concepts, we detected a decrease in apoptosis and potentially increased survival of nonneuronal cells at 70% of gestation after a single low-dose course of glucocorticoids but not after prolonged exposure to the multiple glucocorticoid courses (Fig. 1–Fig. 3). The 40% decrease in apoptosis along with only a 20% decrease in the quantity of caspase-3 at 70% of gestation after the single course of glucocorticoids suggests that some of the glucocorticoid-related apoptosis could have been via caspase-3-independent mechanisms (Fig. 1). In contrast, the same maternal treatment at 90% of gestation most likely did not affect the amount of apoptosis because most of the progenitor cells could have been mature and/or removed by this late time in gestation (Fig. 1).
The fetuses of the ewes exposed to a single glucocorticoid course at 70% of gestation had higher arterial oxygen tensions than those of placebo-treated ewes. However, it is not likely that the higher oxygen tensions could have contributed to the smaller amount of apoptosis in the cerebral cortex, because the arterial oxygenation in both groups was well within the normoxic range (Unno et al., 1999; Stonestreet et al., 2000).
Contrary to the effects of a single glucocorticoid course at 70% of gestation and to our original hypothesis, five courses of maternal glucocorticoids did not reduce the quantity of apoptosis in the fetal cerebral cortex. However, the quantity of the total apoptotic cells appeared higher in the cerebral cortex of fetuses exposed to the single vs. multiple courses of placebo (Fig. 3). Although the differences were not statistically significant, we cannot rule out the possibility that the lower value for the quantity of apoptosis in the fetuses exposed to multiple courses of placebo could have contributed to the lack of differences between the fetuses exposed to the multiple courses of placebo and dexamethasone. Even though we cannot be certain of the reason for the slightly higher values in the fetuses exposed to the single than the multiple placebo courses, the ewes in the multiple-course groups remained in the animal care facility longer than the single-course groups. However, it is not clear how potential small differences in diet and/or activity level could have affected the amount of apoptosis in the fetal cerebral cortex.
Although there were no differences in the quantity of apoptosis between the fetuses of the multiple-course placebo and dexamethasone-treated ewes, the quantity of caspase-3 was lower in the fetuses after the exposure to the multiple dexamethasone courses (Fig. 3). We cannot be certain of the etiology for the discrepancy between lack of change in apoptosis and decrease in caspase-3 after the multiple treatment courses. However, one possibility is that an increase in apoptosis occurred through pathways that do not involve caspase-3 activation, after the first course and during the subsequent dexamethasone courses. This potential increase in apoptosis via caspase-3-independent mechanisms could have offset the expected decrease in the caspase-3-related apoptosis that we observed after the single course of dexamethasone. Consequently, these changes could result in similar amounts of histological apoptosis but a decrease in caspase-3 after the multiple courses. Nonetheless, contrary to our hypothesis, the amount of apoptosis in the fetal cerebral cortex remained similar to that in the fetuses of the placebo-treated ewes after exposure to five courses of maternal glucocorticoids.
In contrast to previous work suggesting that there are innate gender-based differences in outcome for programmed cell death pathways in neurons after exposure to environmental stressors (Du et al., 2004), maternal treatment with dexamethasone also did not differentially affect apoptosis as a function of gender in the cerebral cortex of the ovine fetus in the present studies. However, we were not able to study a large number of fetal sheep, and gender determination had not been recorded in some fetal sheep in the previous studies. Therefore, we cannot rule out the possibility that dexamethasone could have affected apoptosis as a function of gender in the cerebral cortex of the fetuses if we had been able to study a larger number of sheep.
Consistent with the known developmental decrease in brain cell number (Heinsen, 1978; Sturrock, 1979), we detected a lower number of total and neuronal cells per HPF in the fetuses of the placebo-treated ewes at 90% than at 70% of gestation (Table I). The decrease in total cell number between 70% and 90% of gestation could be attributed to neuronal rather than nonneuronal cell loss, because the number of cells that did not express the NeuN antigen was similar at 70% and 90% of gestation. Although we cannot be certain of the reason for the decrease in the total and neuronal cell number 70% and 90% of gestation, we speculate that a smaller number of neurons might have been detected per each cross-sectional area examined in a given HPF as the cerebral cortex enlarges and convolutes (Rakic, 2006).
Our current findings deserve comparison with our previous work (Stonestreet et al., 2003, 2004). We have shown that a single course of antenatal glucocorticoids results in small but significant decreases in cerebral cortical water content in normoxic ovine fetuses at 60% but not at 70%, 80%, or 90% of gestation (Stonestreet et al., 2003, Stonestreet et al., 2004). Therefore, it is not likely that changes in cerebral cortical water content could have affected the observed rate of apoptosis in the current study. Although exposure to the multiple courses of antenatal glucocorticoids reduced water content in some brain regions, the small reduction in cerebral cortical water content was not statistically significant and therefore most likely could not have affected the rate of apoptosis in the fetuses of the ewes exposed to the multiple courses of dexamethasone (Stonestreet et al., 2004). In addition, if the decrease in the brain water content had affected the rate of apoptosis, it would most likely have increased the cellular tissue density and, consequently, the apparent rate of apoptosis. In contrast, we observed decreases in the rate of apoptosis in the fetuses of the glucocorticoid-treated sheep at 70% of gestation.
There are some potential limitations to our study. The NeuN labeling distinguished apoptotic postmitotic neuronal nuclei from other apoptotic nuclei of nonneuronal glial cells and/or neuroblasts. However, further characterization of the non-NeuN-staining apoptotic nuclei was not feasible, insofar as we did not have enough sample adequately preserved to measure oligodendrocyte progenitor and/or other cells (Back et al., 2001).
In conclusion, the magnitude of apoptotic cellular death was higher in the cerebral cortex of the fetuses at 70% than at 90% of gestation. The majority of apoptotic nuclei did not stain for the postmitotic neuronal marker NeuN. Maternal treatment with a single course of glucocorticoids reduced apoptotic cell death in the cerebral cortex at 70%, but not 90%, of gestation. Multiple courses of glucocorticoids at 70% of gestation did not decrease the number of apoptotic cells in the fetal cortex. The findings of our study can be interpreted to suggest that the modulatory effects of maternally administered glucocorticoids on apoptotic cellular death in the normally developing cerebral cortex are a function both of the duration of exposure and of the developmental stage of the brain at the time of fetal exposure.
PERSPECTIVES
We speculate that antenatal glucocorticoid exposure could affect the degree of apoptosis and regulate caspase-3 activity in the fetal cerebral cortex by modulating specific cellular pathways that are activated during apoptosis by both genomic and nongenomic mechanisms and by inducing maturational changes in neuronal and nonneuronal cells in the developing CNS (Almeida et al., 2000; Cheng and de Vellis, 2000; Lossi et al., 2005). These mechanisms could include direct signal transduction from cell surface receptors, intracellular molecules and receptors that affect mitochondrial regulation and molecules involved in cell cycle regulation. After an appropriate cellular stimulus and activation of specific pathways, key effector caspase enzymes, such as caspase-3, are activated, and the cell undergoes an organized degradation of cellular organelles, culminating in cell death and removal. Occasionally, a specific apoptosis-inducing factor accumulates in the cytoplasm and induces the apoptotic cascade independently of caspase-3 activation (Lossi et al., 2005). The effects of glucocorticoids on apoptosis at the molecular level cannot be determined from our study. However, our findings suggest that a single course of antenatal glucocorticoids potentially exerts maturational changes on rate of apoptosis in the cerebral cortex of physiologically stable, normoxic, preterm ovine fetuses.
ACKNOWLEDGMENTS
The authors acknowledge the valuable suggestions provided by Edward Stopa, MD, and the assistance provided by Teddy Youn and Paul Monfils on this project.
Contract grant sponsor: NIH; Contract grant number: R01-HD34618; Contract grant number: 1R01-HD-057100.
REFERENCES
- Adams DF, Ment LR, Vohr B. Antenatal therapies and the developing brain. Semin Neonatol. 2001;6:173–183. doi: 10.1053/siny.2001.0046. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Mondares RL, Born DE, Gleason CA. The effect of binge fetal alcohol exposure on the number of vasoactive intestinal peptide-producing neurons in fetal sheep brain. Dev Neurosci. 2007;30:276–284. doi: 10.1159/000110349. [DOI] [PubMed] [Google Scholar]
- Avola R, Di Tullio MA, Fisichella A, Tayebati SK, Tomassoni D. Glial fibrillary acidic protein and vimentin expression is regulated by glucocorticoids and neurotrophic factors in primary rat astroglial cultures. Clin Exp Hypertens. 2004;26:323–333. doi: 10.1081/ceh-120034137. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- Baud O. Antenatal corticosteroid therapy: benefits and risks. Acta Paediatr Suppl. 2004;93:6–10. doi: 10.1111/j.1651-2227.2004.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Baud O, Laudenbach V, Evrard P, Gressens P. Neurotoxic effects of fluorinated glucocorticoid preparations on the developing mouse brain: role of preservatives. Pediatr Res. 2001;50:706–711. doi: 10.1203/00006450-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Cavalieri RL, Cohen WR. Antenatal steroid therapy: have we undervalued the risks? J Matern Fetal Neonatal Med. 2006;19:265–269. doi: 10.1080/14767050600676075. [DOI] [PubMed] [Google Scholar]
- Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev. 1989;10:182–204. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- Cheng JD, de Vellis J. Oligodendrocytes as glucocorticoids target cells: functional analysis of the glycerol phosphate dehydrogenase gene. J Neurosci Res. 2000;59:436–445. doi: 10.1002/(SICI)1097-4547(20000201)59:3<436::AID-JNR19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Ashraf QM, Ara J, Mishra OP, Delivoria-Papadopoulos M. Mechanism of caspase-3 activation during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett. 2007;421:67–71. doi: 10.1016/j.neulet.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. E-pub 32004 Jul 38561. [DOI] [PubMed] [Google Scholar]
- Falkowski A, Hammond R, Han V, Richardson B. Apoptosis in the preterm and near term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Brain Res Dev Brain Res. 2002;136:165–173. doi: 10.1016/s0165-3806(02)00361-9. [DOI] [PubMed] [Google Scholar]
- Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.res.87.7.574. [DOI] [PubMed] [Google Scholar]
- Hassan AH, von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF. Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol. 1996;140:43–52. doi: 10.1006/exnr.1996.0113. [DOI] [PubMed] [Google Scholar]
- Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ. Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience. 2001;104:57–69. doi: 10.1016/s0306-4522(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Heinsen H. Postnatal quantitative changes in the cerebellar uvula of albino rats. Anat Embryol. 1978;154:285–304. doi: 10.1007/BF00345658. [DOI] [PubMed] [Google Scholar]
- Hu X, Johansson IM, Brannstrom T, Olsson T, Wester P. Long-lasting neuronal apoptotic cell death in regions with severe ischemia after photothrombotic ring stroke in rats. Acta Neuropathol. 2002;104:462–470. doi: 10.1007/s00401-002-0579-8. [DOI] [PubMed] [Google Scholar]
- Hughes J, Gobe G. Identification and quantification of apoptosis in the kidney using morphology, biochemical and molecular markers. Nephrology. 2007;12:452–458. doi: 10.1111/j.1440-1797.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- Khurana P, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochem Res. 2002;27:931–938. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- Lossi L, Merighi A. In vivo cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. Prog Neurobiol. 2003;69:287–312. doi: 10.1016/s0301-0082(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Lossi L, Tamagno I, Merighi A. Molecular morphology of neuronal apoptosis: analysis of caspase 3 activation during postnatal development of mouse cerebellar cortex. J Mol Histol. 2004;35:621–629. doi: 10.1007/s10735-004-2189-3. [DOI] [PubMed] [Google Scholar]
- Lossi L, Cantile C, Tamagno I, Merighi A. Apoptosis in the mammalian CNS: lessons from animal models. Vet J. 2005;170:52–66. doi: 10.1016/j.tvjl.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- National Institute of Health Consensus Conference. Antenatal corticosteroids revisited: repeat courses. Obstet Gynecol. 2001;98:144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel. NIH Consensus Development Panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Moss TJ, Nitsos I, Sloboda DM. Antenatal corticosteroids: the good, the bad and the unknown. Curr Opin Obstet Gynecol. 2002;14:607–612. doi: 10.1097/00001703-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim HJ, Lee JY, Cho BK, Gallo RL, Cho DH. Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J Invest Dermatol. 2007;127:1210–1216. doi: 10.1038/sj.jid.5700703. E-pub 2007 Jan 1218. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16 Suppl 1:i3–i17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Rothstein RP, Levison SW. Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev Neurosci. 2005;27:149–159. doi: 10.1159/000085987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Patlak CS, Petersson KH, Stonestreet BS. Effects of multiple courses of antenatal corticosteroids on blood–brain barrier permeability in the ovine fetus. J Soc Gynecol Invest. 2006;13:248–255. doi: 10.1016/j.jsgi.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- Schwab M, Roedel M, Anwar MA, Muller T, Schubert H, Buchwalder LF, Walter B, Nathalielsz W. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol. 2000;528:619–632. doi: 10.1111/j.1469-7793.2000.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Antonow-Schlorke I, Kuhn B, Muller T, Schubert H, Walter B, Sliwka U, Nathanielsz PW. Effect of antenatal betamethasone treatment on microtubule-associated proteins MAP1B and MAP2 in fetal sheep. J Physiol. 2001a;530:497–506. doi: 10.1111/j.1469-7793.2001.0497k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Schmidt K, Roedel M, Mueller T, Schubert H, Anwar MA, Nathaniels PW. Non-linear changes of electrocortical activity after antenatal betamethasone treatment in fetal sheep. J Physiol. 2001b;531:535–543. doi: 10.1111/j.1469-7793.2001.0535i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Billings HJ, Goodman RL, Coolen LM, Lehman MN. The premammillary hypothalamic area of the ewe: anatomical characterization of a melatonin target area mediating seasonal reproduction. Biol Reprod. 2004;70:1768–1775. doi: 10.1095/biolreprod.103.024182. E-pub 2004 Feb 1718. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood–brain barrier permeability in the ovine fetus. Am J Physiol. 1999;276:R283–R289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood–brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–R477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Elitt CM, Markowitz J, Petersson KH, Sadowska GB. Effects of antenatal corticosteroids on regional brain and nonneural tissue water content in the ovine fetus. J Soc Gynecol Invest. 2003;10:59–66. doi: 10.1016/s1071-5576(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Watkins S, Petersson KH, Sadowska GB. Effects of multiple courses of antenatal corticosteroids on regional brain and somatic tissue water content in ovine fetuses. J Soc Gynecol Invest. 2004;11:166–174. doi: 10.1016/j.jsgi.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol. 1979;5:433–456. doi: 10.1111/j.1365-2990.1979.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Tan CK, Yan J, Ananth C, Kaur C. Dexamethasone induces dendritic alteration but not apoptosis in the neurons of the hippocampus in postnatal rats. Neurosci Lett. 2002;326:206–210. doi: 10.1016/s0304-3940(02)00349-x. [DOI] [PubMed] [Google Scholar]
- Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am J Physiol. 1999;276:H248–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, Mercer B, Thorp JM, Moawad A, O’sullivan MJ, Ramin S, Carpenter MW, Rouse DJ, Sibai B, Gabbe SG. Single vs. weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- Warringa RA, Hoeben RC, Koper JW, Sykes JE, van Golde LM, Lopes-Cardozo M. Hydrocortisone stimulates the development of oligodendrocytes in primary glial cultures and affects glucose metabolism and lipid synthesis in these cultures. Brain Res. 1987;431:79–86. doi: 10.1016/0165-3806(87)90197-0. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]