Abstract
Background
National cholesterol education program (NCEP) guidelines recommended development of direct assays for LDL cholesterol (LDL-C) measurement, but it is unclear how these compare with Friedewald calculation in predicting cardiovascular disease (CVD).
Methods
In a prospective study of 27,331 healthy women with triglycerides ≤400 mg/dL, baseline fasting Friedewald LDL-C was compared with fasting and nonfasting direct homogenous measurement (Roche) for incident CVD over an 11-year follow-up period.
Results
LDL-C by the two methods was highly correlated (r=0.976 in fasting samples, P<0.001). Compared with fasting Friedewald LDL-C, mean fasting direct LDL-C was lower by 5.6 mg/dL, and nonfasting direct LDL-C lower by 11.5 mg/dL, both P<0.0001. The adjusted hazard ratio (HR) per 1-standard deviation (1-SD) increment in fasting direct LDL-C was 1.23 (95% CI 1.15–1.32; 1-SD, 34.1 mg/dL), almost identical to fasting Friedewald HR, 1.22 (95% CI 1.14–1.30; 1-SD, 34.9 mg/dL). Nonfasting LDL-C was not associated with CVD by either method. Fasting participants fell into the same NCEP risk category with either method in 79.3% of participants, while 20.7% of participants differed by one NCEP category, with most of these participants classified into a lower risk category by direct LDL-C.
Conclusions
The association of LDL-C with CVD by the two methods was nearly identical in fasting samples. However, the lower direct LDL-C concentrations may misclassify a substantial proportion of individuals into a lower NCEP category. Moreover, the lack of association of nonfasting direct LDL-C with CVD questions the clinical utility of a direct assay for LDL-C measurement on nonfasting blood samples.
Keywords: Lipids, Lipoproteins, Cardiovascular Diseases, Women
According to the third report of the National Cholesterol Education Program Adult Treatment Panel (NCEP), low-density lipoprotein cholesterol (LDL-C) is the primary target for the diagnosis and treatment of hypercholesterolemia.(1) The common approach for determining LDL-C concentration in the clinical laboratory is the Friedewald calculation,(2) which derives LDL-C from total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides in the fasting state. Although this method is routinely used and convenient for clinical practice, it is not recommended for use in nonfasting blood samples or the presence of hypertriglyceridemia (>400 mg/dL) or type III hyperlipoproteinemia. For these reasons, an expert panel of NCEP recommended in 1995 developing direct methods for the measurement of LDL-C.(3, 4) In addition, the Friedewald calculation of LDL-C requires three primary measurements (total cholesterol, HDL cholesterol, and triglycerides), potentially decreasing the accuracy and precision of the derived cholesterol concentration. Direct assays are currently used in clinical laboratories, but evaluations of these assays were done in small cross-sectional or retrospective studies, with scarce information comparing the association of LDL-C measured directly compared with Friedewald estimation in association with clinical events.(5)
Therefore, we conducted this study to evaluate the association of baseline LDL-C concentrations as determined by a direct assay compared with Friedewald calculation in predicting incident CVD events in a prospective cohort of more than 27,000 initially healthy women. We also tested whether the direct assay provides useful information when performed on nonfasting blood samples.
Materials and Methods
Study Population
Study participants were enrolled in the Women’s Health Study, a randomized, double-blinded, placebo-controlled clinical trial of low-dose aspirin and vitamin E in the primary prevention of CVD and cancer in US female healthcare professionals.(6–8) Eligible participants were apparently healthy women, ages 45 years or older, who were free of self-reported CVD or cancer at study entry (1992–1995), with follow-up for incident CVD through February 2006. At the time of enrollment, participants gave written informed consent, completed questionnaires on demographics, medical history, medications, and lifestyle factors. They were also asked to provide a blood sample. Participants were requested, but not required, to have the sample drawn in the morning before eating, and reported the number of hours since their last meal before the blood draw and the time of day for the blood draw. Of the 28,023 women with baseline blood samples, we excluded 86 with missing baseline lipid values, one whose calculated Friedewald LDL-C was less than 0, and another 605 with triglyceride concentrations >400 mg/dL, resulting in 27,331 women for analysis. Participants whose last meal was 8 hours or more prior to their blood draw comprised the fasting sample (N=19,777), those who had eaten within 8 hours of their blood draw comprised the nonfasting sample (N=6,165), and another 1,389 participants had unknown fasting status. The study was approved by the institutional review boards of the Brigham and Women’s Hospital (Boston, Mass).
Baseline Lipid Measurements
EDTA blood samples were obtained at the time of enrollment and stored in vapor phase liquid nitrogen (−170° C). Subsequently, in a laboratory (N. Rifai) certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program, baseline samples were thawed and analyzed for lipid concentrations. Direct determination of concentrations of total cholesterol, HDL-C, LDL-C, and triglycerides was simultaneously performed on the Hitachi 917 analyzer using reagents and calibrators from Roche Diagnostics (Indianapolis, IN). LDL-C was determined by a homogenous direct method from Roche Diagnostics. The day-to-day coefficients of variation (CVs) for direct LDL-C concentrations of 90, 106, and 129 mg/dL were 3.01, 2.34 and 2.18%, respectively. Total cholesterol was assayed enzymatically. At total cholesterol concentrations of 132.8 and 280.4 mg/dL, the CVs were 1.7% and 1.6%, respectively. The concentration of HDL-C was determined using a direct enzymatic colorimetric assay. HDL-C at the concentrations of 27.0 and 54.9 mg/dL had CVs of 3.3% and 1.7%, respectively. Triglycerides were measured enzymatically with correction for endogenous glycerol. Triglycerides at concentrations of 84.0 and 201.8 mg/dL had CVs of 1.8% and 1.7%, respectively. The Friedewald equation was used to calculate LDL-C from total cholesterol, HDL cholesterol, and triglycerides.(2) The CV for Friedewald LDL-C was 5.1% over a broad range of LDL-C concentrations.
Ascertainment of CVD Events
The primary endpoint of interest was a composite endpoint of incident CVD (nonfatal myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, nonfatal stroke, or cardiovascular death). During the 11-year follow-up period, women reported the endpoints of interest on follow-up questionnaires every 6 or 12 months. All events were adjudicated by an end points committee.
Statistical Analysis
Statistical analyses were performed using STATA version 8.2 (STATA Corporation, College Station, Texas). The distributions of LDL-C concentrations by direct method (fasting and nonfasting) were compared with Friedewald calculation on fasting samples (reference method). P values were obtained from Student’s t-tests for means and Kruskal-Wallis tests for medians. Pearson’s correlation coefficients, scatterplots, and Bland-Altman graphs were used to compare Friedewald and direct LDL-C concentrations. We also examined direct LDL-C concentrations according to time since last meal (2-hour intervals) using boxplot graphs.
Following guidelines from the Department of Health and Human Services,(9) LDL-C concentrations by Friedewald calculation and direct method were divided into quintiles based on their distributions among women not taking hormone therapy. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for quintiles of fasting and nonfasting direct LDL-C, which were then compared with hazard ratios for quintiles of fasting Friedewald LDL-C. Tests for linear trend were performed using the median value for each quintile.
We also performed regression models with LDL-C coefficients expressed per 1-SD and per 10 mg/dL. Comparing lipid effects on a 1-SD basis or per quintile relates their effects to their population distributions, whereas the 10 mg/dL analysis compares their effects directly, without taking into account differences in their distributions. Finally, in order to determine the concordance of the two methods in classifying risk, we used NCEP categories of risk to classify participants based on their fasting LDL-C concentrations as determined by Friedewald calculation or direct measurement. All P-values were two-tailed.
Results
Study Population and Distributions of LDL Cholesterol
As shown in Table 1, the mean (SD) age of study participants at baseline was 54.7 (7.1) years. Fasting blood samples were available in 19,777 participants (72.4%) and nonfasting samples in 6,615 participants (22.6%). The mean lipid concentrations were as expected for a healthy cohort of middle-aged women.
Table 1.
Mean (SD) values or percentage distributions of clinical and lipid variables
| Baseline Characteristic | N=27,331 |
|---|---|
| Age, y | 54.7 (7.1) |
| Current smoking, % | 11.5 |
| Hypertension, % | 24.7 |
| Diabetes, % | 2.5 |
| Postmenopausal status, % | 54.3 |
| Postmenopausal hormone use, % | 43.5 |
| Fasting Status*, % | |
| Fasting | 72.4 |
| Nonfasting | 22.6 |
| Unknown | 5.1 |
| Body mass index, kg/m2 | 25.9 (5.0) |
| Friedewald LDL cholesterol, mg/dL | 129.7 (34.8) |
| Direct LDL cholesterol, mg/dL | 124.2 (33.9) |
| Total cholesterol, mg/dL | 210.8 (40.9) |
| HDL cholesterol, mg/dL | 54.1 (14.9) |
| Triglycerides, mg/dL | 135.2 (70.8) |
Percentages may not add up due to rounding
Compared with fasting Friedewald LDL-C (Table 2), mean concentrations of fasting direct LDL-C were lower by 5.6 mg/dL, and nonfasting direct LDL-C lower by 11.5 mg/dL, both P<0.0001. Mean nonfasting Friedewald LDL-C (124.7, SD 34.1 mg/dL) was also lower than fasting Friedewald by 6.7 mg/dL. In participants with triglycerides ≤200 mg/dL, a similar pattern was observed, with fasting and nonfasting direct LDL-C being lower than fasting Friedewald LDL-C. In participants with triglycerides between 200 and 400 mg/dL, there was a small difference between fasting concentrations of direct and Friedewald LDL-C (−1.3 mg/dL, respectively, P<0.0001), while nonfasting direct LDL-C was lower by 7.1 mg/dL compared with fasting Friedewald (P<0.0001).
Table 2.
Distribution of LDL cholesterol values according to Friedewald calculation and direct assay
| N | Mean (SD) | Median | 25th, 75th Percentile | P fasting Friedewald vs fasting direct* | P fasting Friedewald vs nonfasting direct* | |
|---|---|---|---|---|---|---|
| All participants | ||||||
| Friedewald, fasting | 19,777 | 131.4 (34.9) | 128.4 | 107.3, 152.1 | <0.0001 | |
| Direct, fasting | 19,777 | 125.8 (34.1) | 123.0 | 102.3, 145.9 | ||
| Direct, nonfasting | 6,165 | 119.9 (33.0) | 117.1 | 96.9, 139.6 | <0.0001 | |
| Participants with triglycerides ≤200 mg/dL | ||||||
| Friedewald, fasting | 16,840 | 129.9 (33.8) | 127.3 | 106.4, 149.9 | <0.0001 | |
| Direct, fasting | 16,840 | 123.5 (32.9) | 121.0 | 101.0, 143.2 | ||
| Direct, nonfasting | 4,899 | 116.5 (31.4) | 113.9 | 94.6, 135.9 | <0.0001 | |
| Participants with triglycerides of 201–400 mg/dL | ||||||
| Friedewald | 2,937 | 140.0 (39.8) | 136.5 | 113.2, 163.6 | <0.0001 | |
| Direct, fasting | 2,937 | 138.7 (37.8) | 135.6 | 113.1, 160.5 | ||
| Direct, nonfasting | 1,266 | 132.9 (35.9) | 129.8 | 108.9, 152.8 | <0.0001 | |
P values were obtained from Student’s t-tests comparing mean concentrations of fasting Friedewald (reference) with either fasting direct (paired t test) or nonfasting direct (unpaired t test).
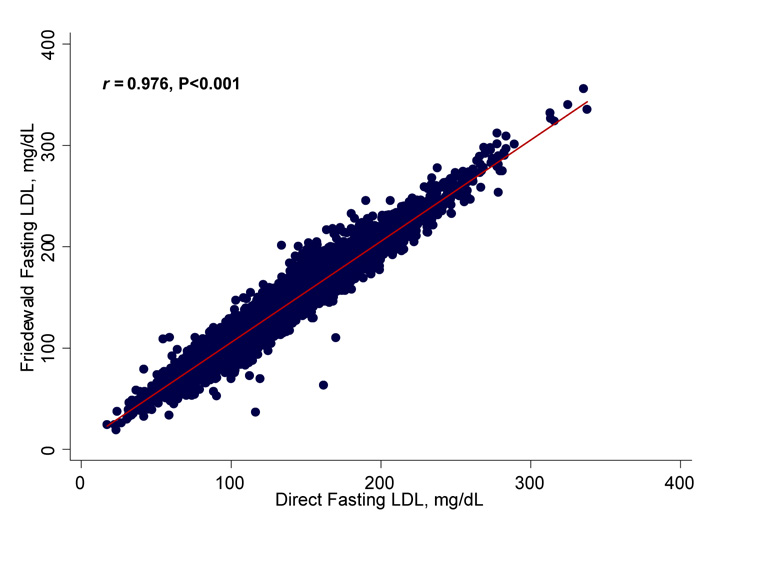
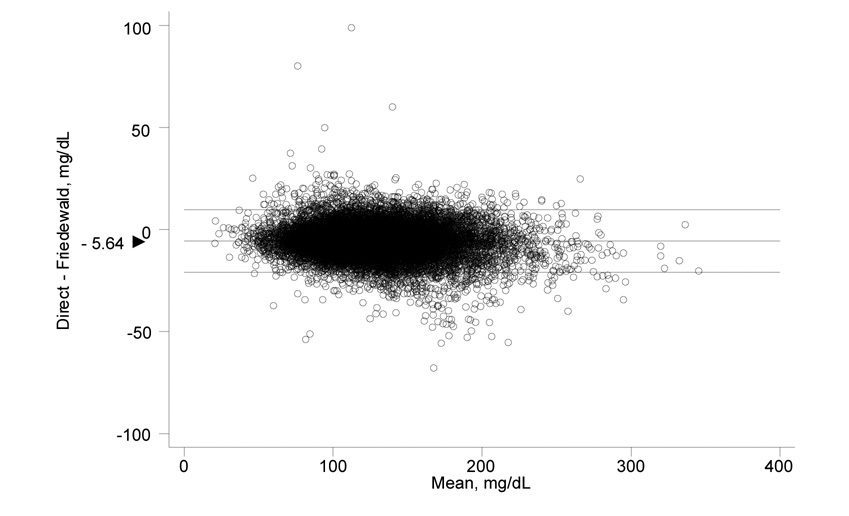
As shown in Figure 1, fasting direct LDL-C correlated highly with fasting Friedewald in a linear manner (Pearson’s correlation coefficient r = 0.976, P<0.001). Nonfasting direct LDL-C also correlated highly with nonfasting Friedewald LDL-C (r = 0.972, P<0.001). The difference between fasting concentrations of direct and Friedewald LDL-C is plotted against their mean in the Bland-Altman graph shown in Figure 1, right panel. The mean difference in LDL-C between the two methods (direct – Friedewald) was −5.64 mg/dL (95% CI −5.75, −5.53) in fasting samples. In nonfasting samples, the mean difference was −4.84 (95% CI −5.04, −4.64).
Figure 1.
Scatterplot showing the correlation of Friedewald fasting and direct fasting LDL-C values, Pearson correlation coefficient 0.976, P<0.001 (left); and Bland-Altman plot showing the difference between direct fasting and Friedewald fasting values vs the mean of the two (right). The mean difference (direct minus Friedewald) was − 5.64 (95% CI − 5.75, − 5.53).
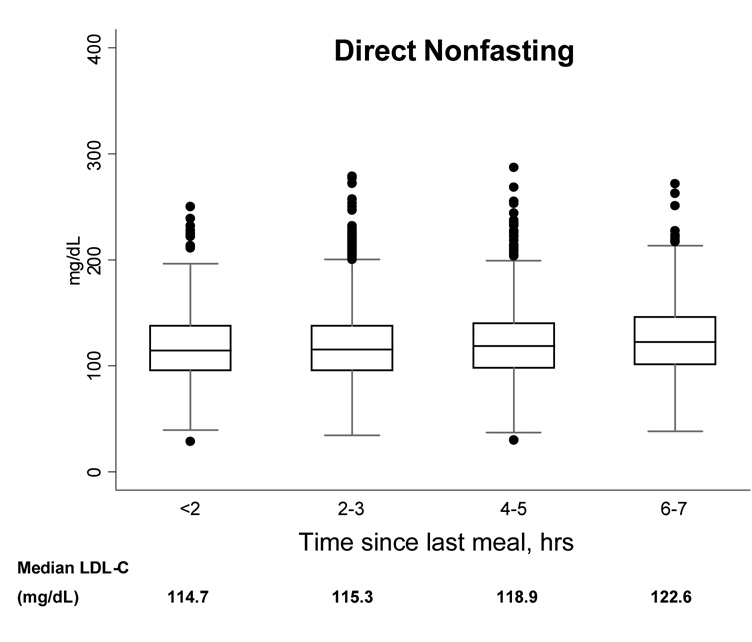
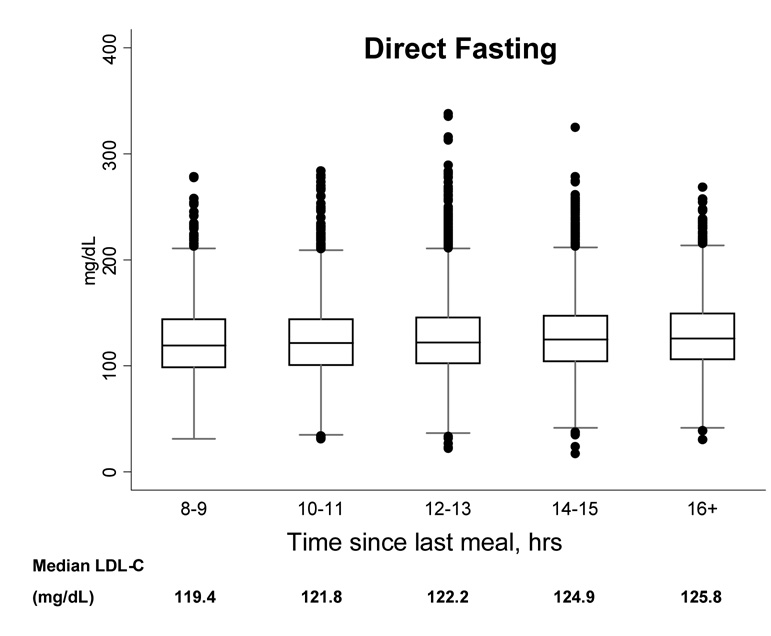
Next, to determine the effect of postprandial time on LDL-C concentrations, we plotted the 2.5, 25, 50, 75, and 97.5% cumulative percentile and extreme values by 2-hour intervals of time to last meal. With greater time elapsed since last meal, the median concentration of direct LDL-C was somewhat higher, but the overall distributions of LDL-C were not substantially different (Figure 2). Similar results were found for LDL-C by Friedewald calculation (results not shown).
Figure 2.
Boxplots showing the 2.5, 25, 50, 75, and 97.5% cumulative percentile values and extreme values, according to time since last meal, for direct nonfasting LDL-C (left) and direct fasting LDL-C (right).
Relationship of Baseline LDL-C with Incident CVD
During a median follow-up of 11.4 years, a total of 945 first CVD events occurred in the women with known fasting/nonfasting status. Associations of LDL-C by Friedewald calculation and direct method with incident CVD were examined by quintile cutpoints as well as by 1-SD and 10 mg/dL increments (Table 3). After adjusting for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index, fasting LDL-C by either method was significantly associated with incident CVD to a similar extent. The adjusted HR per 1-SD increment in fasting Friedewald LDL-C was 1.22 (95% CI 1.14–1.30), almost identical to the fasting direct LDL-C HR of 1.23 (95% CI 1.15–1.32). Similarly, when compared per 10 mg/dL increments, the fasting HRs were identical, with a 6% increase in CVD risk for each 10 mg/dL increase in LDL-C. Only fasting LDL-C was associated with CVD. Nonfasting direct LDL-C showed no association with CVD (P=0.72), nor did nonfasting Friedewald LDL-C (P=0.92).
Table 3.
Associations of Friedewald and direct LDL cholesterol with cardiovascular disease events
| Per 1-quintile increase | P for linear trend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Range | ||||||
| Friedewald, fasting | <102.3 mg/dL | 102.3–120.6 | 120.7–137.9 | 138.0–159.6 | >159.6 | |
| Direct, fasting | <97.8 mg/dL | 97.8–115.5 | 115.6–132.2 | 132.3–153.9 | >153.9 | |
| Direct, nonfasting | <97.8 mg/dL | 97.8–115.5 | 115.6–132.2 | 132.3–153.9 | >153.9 | |
| HR (95% CI) | ||||||
| Friedewald, fasting | 1.00 | 1.21 (0.90–1.62) | 1.40 (1.06–1.85) | 1.65 (1.26–2.16) | 1.93 (1.49–2.51) | <0.001 |
| Direct, fasting | 1.00 | 1.31 (0.97–1.77) | 1.53 (1.15–2.05) | 1.99 (1.51–2.63) | 2.11 (1.60–2.78) | <0.001 |
| Direct, nonfasting | 1.00 | 0.96 (0.59–1.55) | 1.25 (0.79–1.98) | 1.16 (0.73–1.85) | 1.20 (0.74–1.93) | 0.33 |
| Per 1-SD increase | Per 10 mg/dL increase | P | ||||
| HR (95% CI) | ||||||
| Friedewald, fasting | 1.22 (1.14–1.30) | 1.06 (1.04–1.08) | <0.001 | |||
| Direct, fasting | 1.23 (1.15–1.32) | 1.06 (1.04–1.08) | <0.001 | |||
| Direct, nonfasting | 1.03 (0.89–1.18) | 1.01 (0.97–1.05) | 0.72 | |||
HRs and 95% CIs were adjusted for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index. P value for linear trend was obtained using the median value for each quintile. Fasting was defined as ≥ 8 hours from last meal.
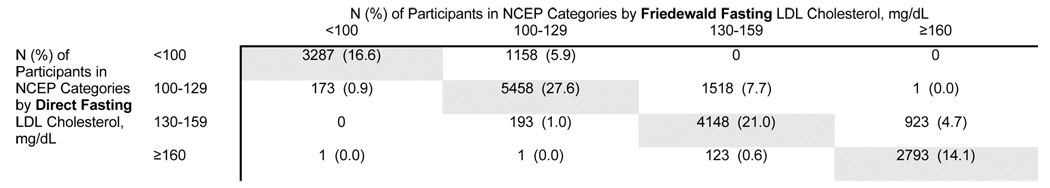
Finally, using clinical cut-points for LDL-C based upon NCEP categories of risk in the fasting participants (N=19,777), Friedewald and direct LDL-C concentrations classified participants into the same NCEP risk category in 79.3% (15,686/19,777) of participants (Table 4). A total of 4,088 participants (20.7%) differed by one NCEP category, with a large proportion (3,599/4,088 or 88.0%) of these participants being classified into a lower risk NCEP category by direct LDL-C compared with Friedewald calculation. Only 3 participants differed by 2 or more NCEP categories.
Table 4.
Concordance of Friedewald and direct fasting LDL cholesterol for classifying participants into NCEP categories of risk, N=19,777
 |
Discussion
In this prospective study of 27,331 initially healthy women, mean baseline concentrations of fasting and nonfasting direct LDL-C were lower by ~5 to 10 mg/dL, respectively, compared with fasting Friedewald LDL-C. The direct method used in this study correlated highly with Friedewald calculation. In fasting samples, the association of direct LDL-C with incident CVD was nearly identical to LDL-C by Friedewald calculation. However, the lower LDL-C concentrations measured by this direct assay may misclassify a substantial proportion of individuals into a lower NCEP risk category. Moreover, the lack of association of nonfasting direct LDL-C with CVD questions the clinical utility of a direct assay for LDL-C measurement on a nonfasting blood sample.
Several direct methods for measuring LDL-C are currently available,(10) but there is sparse data evaluating their predictive performance in relation to clinical events. It is believed that potential advantages of direct measurement of LDL-C may be better precision of the assay due to the single measurement, and its being relatively unaffected by the presence of increased triglyceride concentrations or a nonfasting blood draw.(5) Some studies have shown that direct assays are generally accurate when compared to the β-quantification reference method or the Friedewald calculation.(11–14) However, other studies have questioned the specificity of direct assays and their ability to meet the NCEP goal for a total error of <12%.(15) In addition, clinical trials demonstrating the benefit of LDL-C lowering with statin therapy have used Friedewald calculation for determining LDL-C concentration,(16) with the exception of the Heart Protection Study that used a direct assay.(17) Our study findings demonstrate no clear advantage for using a direct assay for LDL-C compared with Friedewald calculation. Moreover, LDL-C concentration with the direct assay used in this study was ~ 5 to 10 mg/dL lower than by Friedewald calculation. While small, this systematic difference in mean LDL-C concentrations may be clinically important when assessing the need for drug intervention in a particular individual based on NCEP risk categories.
For nonfasting individuals, the direct assay has been suggested to be the preferred method for assessment of LDL-C.(1) The findings from this study question the clinical utility of performing a direct assay for LDL-C measurement in nonfasting samples, since nonfasting LDL-C by direct assay was not associated with CVD. By contrast, nonfasting levels of apolipoprotein B100 and the ratio of apolipoprotein B100/A-1 were associated with CVD in this group of women, although the association with CVD events was stronger in fasting samples.(18) Importantly, the ratio of total/HDL cholesterol was associated with CVD to a similar magnitude in both fasting and nonfasting samples, and can be obtained at no additional cost.(18) Direct assays add to healthcare costs and are more expensive than measuring triglycerides, total cholesterol, or HDL cholesterol. Future studies are needed to assess the association with CVD of direct LDL-C assays in other populations, examined according to fasting status.
There are several possible limitations of the present study. Lipid measurements were only available once at baseline and results could not be corrected for potential regression dilution bias. Although we assessed only the direct Roche method, this assay is commonly used and commercially available in the U.S. Our study included healthcare professionals who were women, mostly white, apparently healthy, and recruited from a variety of geographic locations across the US; thus, it is unclear if our results would be applicable to other ethnic populations or men. Time to last meal was self-reported, and we did not have paired samples of fasting and nonfasting measurements in the same individuals. Finally, this was a primary prevention population and further studies are needed before the data can be extended to secondary prevention populations that are frequently treated with lipid lowering medications.
Strengths of the present study include the large number of healthy women participants with simultaneous concentrations of direct and Friedewald LDL-C. Additionally, all lipid measurements were performed at a core laboratory facility that is certified for lipid testing by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program. Detailed information on cardiovascular risk factors was available, allowing for analysis by the presence or absence of these factors, such as fasting status.
In summary, the direct assay used in this study correlated highly with Friedewald calculation but was generally lower by ~5 to 10 mg/dL. The lower LDL-C concentrations by direct assay may misclassify a substantial proportion of individuals into a lower NCEP risk category. While the association of LDL-C with CVD by the two methods was nearly identical in fasting samples, the lack of association of nonfasting direct LDL-C with CVD questions the clinical use of a direct assay in nonfasting blood samples.
Acknowledgments
Funding Sources
The Women’s Health Study is supported by grants HL-43851 and CA-47988 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, and by grants from the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation, with additional support from an Investigator-Initiated Studies Program from Merck. Dr Mora is supported by grants from the American Heart Association (0670007N), Sandra Daugherty Foundation, and Lerner Research Young Investigator Award. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this manuscript.
Footnotes
Clinical trial registration URL http://clinicaltrials.gov/ct/show Unique identifier NCT00000479.
Conflict of Interest Disclosures
The authors report no conflicts.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 3.Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1414–1420. [PubMed] [Google Scholar]
- 4.Washington: US Governement Printing Office; Recommendations on lipoprotein measurement from the working group on lipoprotein measurement. 1995:1–194. National Institutes of Health Publication No. 95-3044.
- 5.Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- 6.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Hainline A, Karon J, Lippel K. Manual of Laboratory Operations: Lipid Research Clinics Program and Lipid and Lipoprotein Analysis. 2nd ed. Bethesda, Md: Dept of Health and Human Services; 1982. [Google Scholar]
- 10.McNamara JR, Warnick GR, Cooper GR. A brief history of lipid and lipoprotein measurements and their contribution to clinical chemistry. Clin Chim Acta. 2006;369:158–167. doi: 10.1016/j.cca.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Rifai N, Iannotti E, DeAngelis K, Law T. Analytical and clinical performance of a homogeneous enzymatic LDL-cholesterol assay compared with the ultracentrifugation-dextran sulfate-Mg2+ method. Clin Chem. 1998;44:1242–1250. [PubMed] [Google Scholar]
- 12.Sugiuchi H, Irie T, Uji Y, Ueno T, Chaen T, Uekama K, Okabe H. Homogeneous assay for measuring low-density lipoprotein cholesterol in serum with triblock copolymer and alpha-cyclodextrin sulfate. Clin Chem. 1998;44:522–531. [PubMed] [Google Scholar]
- 13.Nauck M, Graziani MS, Bruton D, Cobbaert C, Cole TG, Lefevre F, et al. Analytical and clinical performance of a detergent-based homogeneous LDL-cholesterol assay: a multicenter evaluation. Clin Chem. 2000;46:506–514. [PubMed] [Google Scholar]
- 14.Nauck M, Rifai N. Analytical performance and clinical efficacy of three routine procedures for LDL cholesterol measurement compared with the ultracentrifugation-dextran sulfate-Mg(2+) method. Clin Chim Acta. 2000;294:77–92. doi: 10.1016/s0009-8981(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 15.Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem. 2002;48:489–498. [PubMed] [Google Scholar]
- 16.Faas FH, Earleywine A, Smith G, Simmons DL. How should low-density lipoprotein cholesterol concentration be determined? J Fam Pract. 2002;51:972–975. [PubMed] [Google Scholar]
- 17.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]