Abstract
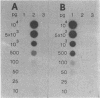
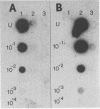
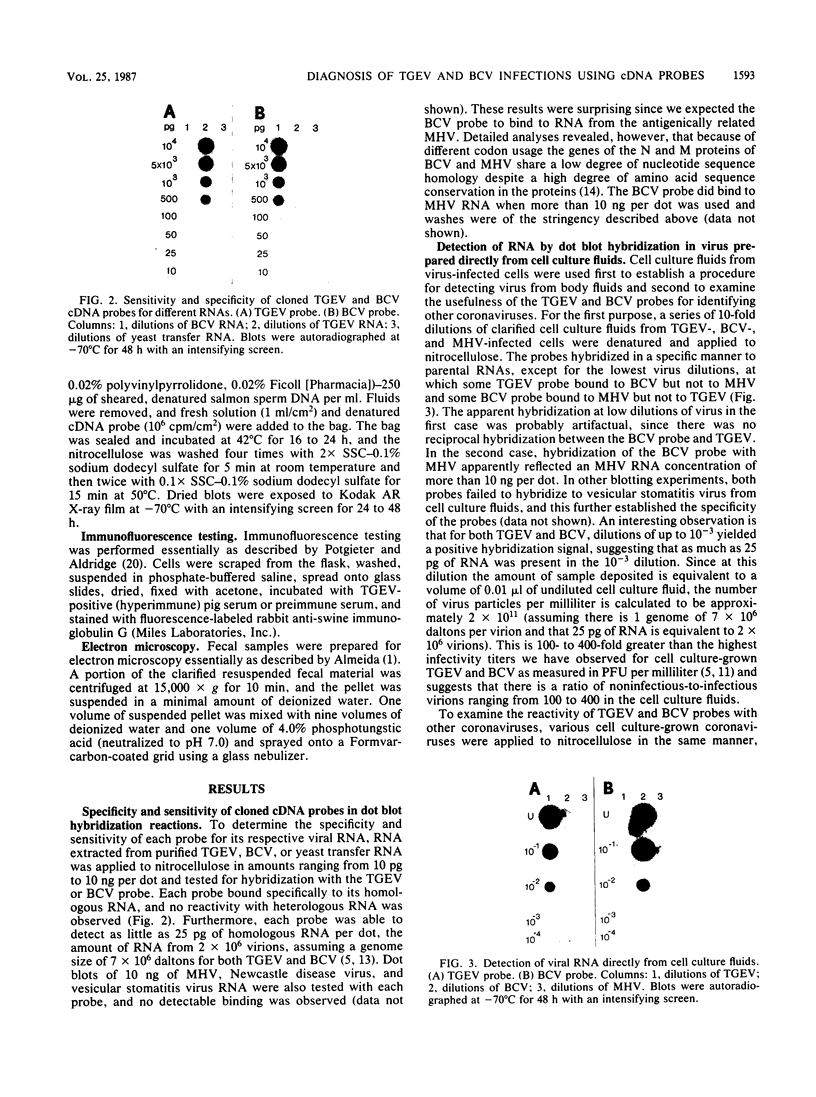
Molecular clones representing the first 2,000 bases from the 3' end of the porcine transmissible gastroenteritis coronavirus genome and the first 2,160 bases from the 3' end of the bovine enteric coronavirus genome were used in dot blot hybridization assays to detect viral RNA from cell culture and from fecal specimens. In each case, the cloned DNA represents approximately 10% of the genome. The cloned sequence for each virus encompasses the 3' noncoding region, the nucleocapsid protein gene, and a large portion of the matrix protein gene. 32P-labeled cDNA probes prepared from these clones detected as little as 25 pg of RNA from the parental virus but did not detect RNA from the nonparental virus even when amounts of up to 10 ng per dot were used. This specificity reflects the antigenic diversity between these two coronaviruses. The hybridization assay could also detect coronaviruses antigenically closely related to the parental virus but not coronaviruses belonging to an antigenically unrelated subgroup. Dot blot hybridization for transmissible gastroenteritis coronavirus diagnosis was compared with the routine procedures of virus isolation and electron microscopy as a diagnostic test.
Full text
PDF

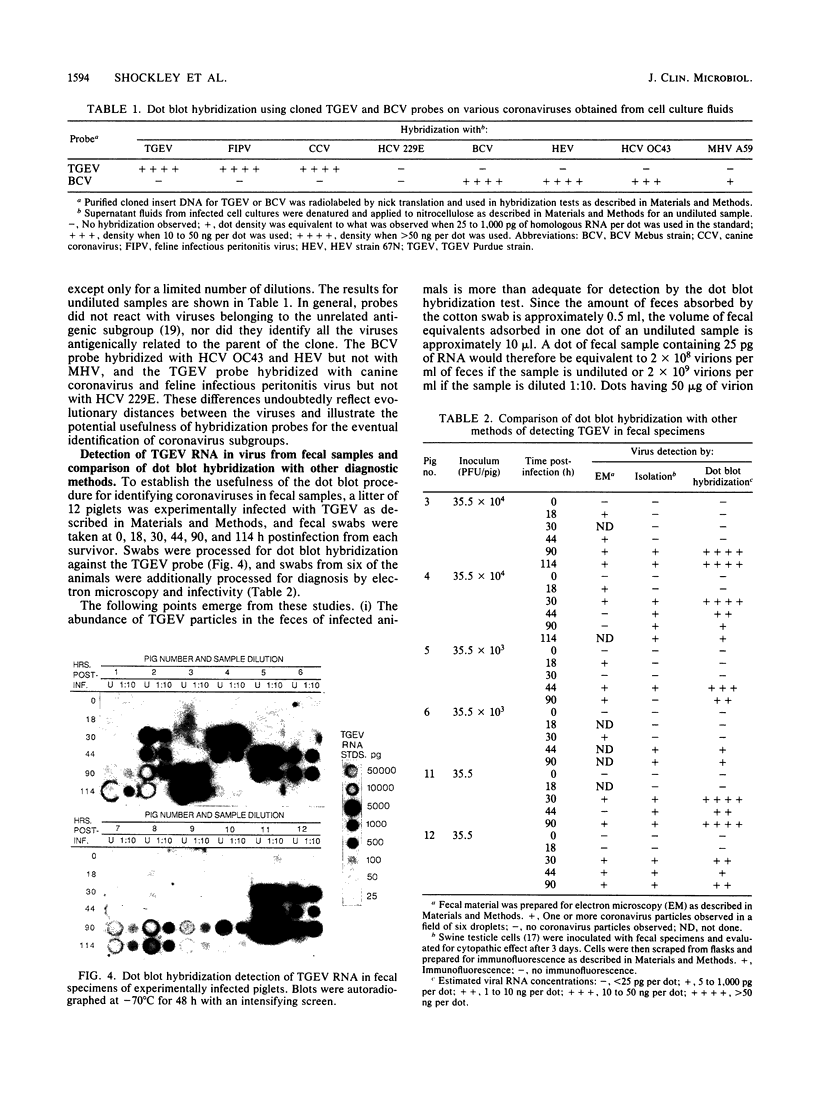


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D. Practical aspects of diagnostic electron microscopy. Yale J Biol Med. 1980 Jan-Feb;53(1):5–18. [PMC free article] [PubMed] [Google Scholar]
- Almeida J. D. Uses and abuses of diagnostic electron microscopy. Curr Top Microbiol Immunol. 1983;104:147–158. doi: 10.1007/978-3-642-68949-9_9. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Spasic P. Evidence of a viral aetiology in endemic (Balkan) nephropathy. Lancet. 1975 Dec 27;2(7948):1271–1273. doi: 10.1016/S0140-6736(75)90609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Mathan M., Mathan V. I., Jesudoss S., Swaminathan S. P. Chronic enterocyte infection with coronavirus. One possible cause of the syndrome of tropical sprue? Dig Dis Sci. 1982 Nov;27(11):1039–1043. doi: 10.1007/BF01391753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian D. A., Dennis D. E., Guy J. S. Genome of porcine transmissible gastroenteritis virus. J Virol. 1980 May;34(2):410–415. doi: 10.1128/jvi.34.2.410-415.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks J. S., DeVald B. L., Jankovsky L. D., Gerdes J. C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980 Aug 22;209(4459):933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- Dennis D. E., Brian D. A. RNA-dependent RNA polymerase activity in coronavirus- infected cells. J Virol. 1982 Apr;42(1):153–164. doi: 10.1128/jvi.42.1.153-164.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H., Cross R. F. Pathogenicity of an attenuated strain of transmissible gastroenteritis virus for newborn pigs. Am J Vet Res. 1976 Feb;37(2):165–169. [PubMed] [Google Scholar]
- Hyypiä T., Stålhandske P., Vainionpä R., Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984 Mar;19(3):436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapke P. A., Brian D. A. Sequence analysis of the porcine transmissible gastroenteritis coronavirus nucleocapsid protein gene. Virology. 1986 May;151(1):41–49. doi: 10.1016/0042-6822(86)90102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Brian D. A. Bovine coronavirus structural proteins. J Virol. 1982 May;42(2):700–707. doi: 10.1128/jvi.42.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapps W., Brian D. A. Oligonucleotide fingerprints of antigenically related bovine coronavirus and human coronavirus OC43. Arch Virol. 1985;86(1-2):101–108. doi: 10.1007/BF01314116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapps W., Hogue B. G., Brian D. A. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein genes. Virology. 1987 Mar;157(1):47–57. doi: 10.1016/0042-6822(87)90312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Imai M., Bellamy A. R., Ikegami N., Furuichi Y., Summers D., Nuss D. L., Deibel R. Diagnosis of rotavirus infection with cloned cDNA copies of viral genome segments. J Virol. 1985 Aug;55(2):509–512. doi: 10.1128/jvi.55.2.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin A. W., Norman J. O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Med Vet Sci. 1966 Jul;30(7):190–198. [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C., Ward J., Mengeling W. L. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol. 1978;58(1):45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgieter L. N., Aldridge P. L. Use of the indirect fluorescent antibody test in the detection of bovine respiratory syncytial virus antibodies in bovine serum. Am J Vet Res. 1977 Sep;38(9):1341–1343. [PubMed] [Google Scholar]
- Richman D. D., Cleveland P. H., Redfield D. C., Oxman M. N., Wahl G. M. Rapid viral diagnosis. J Infect Dis. 1984 Mar;149(3):298–310. doi: 10.1093/infdis/149.3.298. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Welling G. W., Welling-Wester S., Niesters H. G., Lenstra J. A., Van der Zeijst B. A. Predicted membrane topology of the coronavirus protein E1. Biochemistry. 1986 Mar 25;25(6):1335–1339. doi: 10.1021/bi00354a022. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]