Abstract
This study examined individual differences in male and female C57BL/6J (C57) mice responding for intravenous cocaine reinforcement. The experiment used 4 groups of mice, distinguished by sex and cocaine unit dose (0.3 or 1 mg/kg/infusion). Mice trained to lever respond for IV cocaine were given the drug initially on an FR2 schedule and then on a Progressive Ratio 2(PR2) schedule. Hierarchical linear modeling (HLM) techniques were used to examine data generated across four FR2 and four PR2 sessions, as well as within session data when cocaine was delivered on the PR2 schedule. HLM techniques, although uncommon in the animal literature, characterize individual differences in human studies and are likely to be useful in more complex preclinical studies. Analysis established distinct patterns of self-administration both across and within sessions. Responses for cocaine delivered on the FR2 schedule was dose-dependent, but did not differ according to sex. Response output was greater when either dose of cocaine was delivered on the PR2 than the FR2 schedule. Although response output for the more rewarding 1 mg/kg unit dose was similar for the two sexes, males responded more and had greater cocaine intake than females when the less reinforcing 0.3 mg/kg dose was delivered at the more behaviorally challenging PR2 schedule. HLM analysis of response patterns and cocaine intake within the PR2 sessions corroborated this sex difference and also indicated that trajectories differed for individual mice after accounting for the sex and dose factors. The reduced response output by females for cocaine in the present experiment is consistent with previous reports that sex differences in the rewarding effects of either alcohol or food reinforcement were revealed for C57 mice only when delivered on more behaviorally demanding schedules (e.g. PR2 or FR100).
Keywords: Drug Abuse, Cocaine Addiction, Cocaine Self-Administration, Animal Models, Progressive Ratio, Mouse, Hiearchical Linear Modeling
Introduction
Cocaine self-administration varies across individuals for both humans and laboratory animals. A number of factors contributing to individual differences can be controlled experimentally. However, even under carefully controlled experimental conditions, rodents continue to differ substantially in the amount of cocaine self-administered. The etiology of individual differences in cocaine self-administration has typically been approached by describing the relationship of self-administration behavior to a seemingly unrelated pre-existing trait or behavioral response. For example, the positive correlations beween cocaine self-administration and behavioral reactivity to novelty (Mantsch et al., 2001) and to grooming behavior (Homberg et al., 2002) in rats suggests that genetic background and/or developmental history might contribute to individual differences in cocaine-related behavior.
The importance of genetic background in cocaine self-administration experiments is also evident in the mouse. For example, several inbred mouse strains differ in their propensity to self-administer intravenous cocaine (Deroche et al., 1997; Kuzmin and Johansson, 2000; Rocha et al., 1998a) and deleting a single gene can alter cocaine self-administration (Colby et al., 2003; Rocha et al., 2002; Rocha et al., 1998b). Further, cross breeding of cocaine-preferring and non-preferring mouse strains produces hybrid strains with cocaine self-administration propensity that is directly related to percent of genetic make-up related to the preferring strain (Ruiz-Durantez et al., 2006). Thus, individual differences in cocaine self-administration are, at least in part, heritable.
Several, well characterized experimental factors such as cocaine unit dose, schedule of reinforcement, and sex of the subjects also contribute to the amount of cocaine self-administered. When cocaine is delivered on a low fixed ratio schedule of reinforcement, lever response output is typically inversely related to cocaine unit dose for both rats (Roberts et al., 1989b; Sizemore et al., 1997) and mice (Caine et al., 2002; Griffin and Middaugh, 2003; Thomsen and Caine, 2006). Further, increasing the behavioral effort required to obtain cocaine with progressive ratio schedules of reinforcement typically increases response output to a point at which the subject discontinues responding for the drug (i.e., breakpoint) (Depoortere et al., 1993; Spealman and Goldberg, 1978). In addition to the influence of unit dose and behavioral effort requirement, prior studies also indicate sex differences in cocaine self-administration. For example, female rats are reported to acquire cocaine self-administration more readily than males (Lynch and Carroll, 1999), an effect not diminished by gonadectomy (Hu et al., 2004). In addition, breakpoints for cocaine delivered on progressive ratio schedules of reinforcement were also reported to be higher for female than male rats (Carroll et al., 2002; Roberts et al., 1989a). Therefore, cocaine unit dose, schedule of reinforcement and sex are critical determinants of cocaine self-administration behaviors in rodents.
Although cocaine self-administration data generated across and within sessions is recognized to vary extensively across individuals, these data are typically analyzed with mixed within-between factorial analyses with emphasis on the means generated by the different groups of the design. Hierarchical linear modeling (HLM) techniques which provide a means for characterizing these individual differences are well suited for analyzing data from these experiments. HLM techniques are commonly used for analysis of data collected from longitudinal studies on humans; however, their use for evaluating data from animal models of drug abuse is limited to two recent reports on nicotine self-administration in rats (Donny et al., 2004; Lanza et al., 2004). HLM techniques, in addition to assessing the influence of time invariant factors (e.g., sex and cocaine unit dose) and continuously distributed variables (e.g., reaction to novelty or grooming) which can accomplished by factorial analyses, also directly assess whether individual animals have distinct patterns of lever pressing behavior not accounted for by these factors.
The present study served two purposes. The first purpose was to characterize cocaine self-administration in male and female C57 mice under conditions when the behavioral cost was low or high. The second purpose of the experiment was focus on the behavioral trajectories generated by individual mice under the different conditions using HLM techniques to help establish the extent to which individual variation in these parameters could be accounted for by the sex, dose, and reinforcement schedule factors.
Characterization of cocaine self-administration by male and female C57 mice was accomplished by delivering the drug at either low or high unit doses (0.3 or 1mg/kg) and according to reinforcement schedules that require either little effort, Fixed Ratio 2 (FR2) or substantial effort, Progressive Ratio 2 (PR2). The C57 strain was chosen because this strain self-administers cocaine (Fuchs et al., 2003; Grahame et al., 1995; Griffin and Middaugh, 2003; Rocha et al., 1998a) and is extensively used for backcrossing during the generation of many genetically-modified strains of mice (Crawley et al., 1997; Miner, 1997; Ruiz-Durantez et al., 2006). The experimental parameters were based on previous studies from our laboratory. First, male mice of this strain acquire lever-responding for cocaine without use of an alternative reinforcer, and maintain responding for these doses in a dose-dependent manner (Griffin and Middaugh, 2003). Second, although information about the influence of sex on intravenous cocaine self-administration in mice is not available, we previously reported that female C57 mice responded more than males for ethanol delivered on fixed ratio 2 (FR2) schedule of reinforcement, but responded less than males when ethanol was delivered on the more behaviorally demanding progressive ratio 2 (PR2) schedule (Middaugh and Kelley, 1999). This sex difference in responding for ethanol in mice is opposite that reported for rats self-administering cocaine (Carroll et al., 2002; Roberts et al., 1989a). Whether the apparent conflict is due to species or reinforcing agent remains to be determined.
HLM analytical techniques were used to assess the influence of sex, cocaine dose, and work effort requirement on individual differences in cocaine self-administration. These analytical techniques model the trajectories of lever pressing behavior over time (across and within sessions) for individual mice and it was hypothesized that behavioral trajectories would differ significantly amongst individuals. Further, we predicted that individual differences in behavioral trajectories could be partially explained by the influence of Sex and cocaine Dose. Specifically, we hypothesized that responding for cocaine reinforcement would be dose-dependent and, because of our previous study using ethanol (Middaugh and Kelley, 1999) that cocaine would maintain greater responding in the male mice on the PR2 schedule.
Methods
Subjects
Male and female C57 mice (7 weeks) obtained from Jackson Laboratories (Bar Harbor, ME) were individually housed in an AALAC accredited animal facility and were maintained on a 12 hour light cycle (lights on at 0600hr). Behavioral testing occurred during the light phase of their circadian cycle. The mice had free access to water, and food was restricted to maintain bodyweights at 90% of ad libitum weight after the jugular catheterization surgery. All experimental protocols were approved by the Institutional Animal Care and Use Committee and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Self-administration Apparatus
The self-administration chambers (model ENV-307A) were enclosed in sound-attenuating cabinets, both manufactured by MedAssociates, Inc. (Georgia, VT). Two response levers were located on the same wall of the chamber and were elevated 6.5 cm above the grid floor (lever model ENV-310). Two grams of force on a lever defined a response. A reinforced response on the active lever: 1) terminated the house light, 2) initiated a 2 second infusion of cocaine (19 μL) from an infusion pump (model PHM-100) outside the chamber, and 3) initiated a 2 second compound conditioned stimulus consisting of a tone (2,900 Hz, ENV-323A), a yellow LED stimulus light (ENV-321M) located directly above the active lever, and the infusion pump noise. Responses on the alternative lever (Inactive) were recorded but had no programmed consequences. A single channel fluid swivel (Instech Laboratories, Inc, 375 series) connected the jugular catheter to the infusion pump. Finally, the presentation of stimuli and the recording of experimental events was controlled by MedPC software.
Catheter Construction
The catheters were designed for insertion into the right jugular vein with a skull-mounted access port as previously reported (Griffin and Middaugh, 2003; Kelley et al., 1997). The bodies of the catheters were constructed of silastic tubing (0.30mm i.d., 0.64mm o.d.; Dow Corning, Inc). A small bead of silicon adhesive placed 1.2 cm from the end of the tubing served as an anchor point for the sutures. The access port of the catheter was constructed of 22-gauge stainless steel tubing cut and polished to a final length of 1.5 cm (Small Parts, Inc). The stainless steel tubing was smoothly bent at 90 degrees near the middle of its length. Additionally, the portion of the steel tubing that would lie against the skull was smoothly bent into a U-shape so that the end of the stainless steel tubing connected to the silastic catheter body faced 180 degrees from its original position and was parallel to the skull. This shaping procedure provided a flat, stable base for mounting the access port to the skull. The exposed end of the access port extended vertically from the skull and when not in use, was occluded by a short piece of tubing sealed at one end.
Catheterization Surgery
Catheter placement was conducted according to a previously described procedure (Griffin and Middaugh, 2003; Kelley et al., 1997). Mice were anesthetized with a mixture of xylazine (6mg/kg) and ketamine (120mg/kg). The right jugular vein was exposed by blunt dissection and phlebotomized. The catheter was inserted 1.2 cm and secured to the vein using 5-0 silk sutures. Internal adipose and connective tissue on either side of the catheter were sutured together over the catheter to secure it in the subcutaneous space and the incision was sutured. The catheter passed subcutaneously to an incision on top of the skull. The catheter port was secured to the skull using a light-cured resin as previously described (Groseclose et al., 1998) and the skin sutured together above the mound of cured resin. On the day of surgery, the mice were given a single intravenous dose of cefazolin (50 μL, 100mg/ml), followed by heparinized saline (20 μL, 90 U/ml).
Catheter Maintenance and Patency
Following surgery, the catheters were flushed twice daily with 20 μL of heparinized saline (30 U/ml first flush and 90 U/ml second flush). Catheter patency was assessed every 6 days, or as changes in behavior indicated, using a bolus infusion of methohexital HCl (20 μL, 6.25 mg/ml). Rapid sedation was regarded as a positive test. All mice with delayed or negative tests were excluded from the study and the analysis.
Experimental Procedure
Upon arrival, mice were handled for at least 5 to 7 days prior to the catheterization surgery to acclimate them to the process of catheter maintenance. They were assigned to one of four groups defined by a 2 (Sex) × 2 (Cocaine Dose) design. Forty-eight hours after surgery, mice were introduced to the operant chambers for daily, 2 hour sessions. Responding on the active lever delivered cocaine on a fixed ratio (FR) 1 reinforcement schedule (i.e. 1 lever press per cocaine infusion). After the first session, food was restricted to a single daily ration in order to maintain bodyweights at 90-95% of their ad libitum feeding weights, determined the day of surgery, for the duration of the study.
Mice were maintained on the FR1 schedule at least 4 days. During this acquisition period, food pellets and/or experimenter-initiated lever presses (priming infusions) were used to promote lever-pressing behavior in a few non-responding mice. These actions were limited to 1-2 infusions and/or pellets at the beginning of the 2 hour session. After at least two days of self-administering >10 reinforcers on the FR1 schedule, an FR2 schedule was initiated and the use of food pellets and priming infusions discontinued. Mice were required to maintain the rate of >10 reinforcers per 2-hr session and demonstrate >75% of total responding on the active lever during 2 consecutive FR2 sessions before advancing to a progressive ratio 2 (PR2) schedule with 3-hr sessions. The PR2 schedule was based on previously published work examining ethanol reward in male and female C57 mice (Middaugh and Kelley, 1999). According to this schedule, the initial response requirement for reinforcement was 2 lever presses and the ratio requirement increased by 2 lever presses thereafter (4,6,8,10,12, etc) to earn subsequent cocaine infusions. Mice were maintained on the PR2 schedule a minimum of 4 days.
Estrous Tracking
To determine if estrus state might influence cocaine self-administration in the female mice, the estrous cycle was tracked in a subset of females (n=14). Vaginal smears were taken at the same time each day beginning 2 weeks prior to the catheterization surgery and continuing for 2 weeks after the surgery. During self-administration phase, the smears were obtained in the colony room 1 to 2 hours prior to the daily self-administration session. The smears were stained using Geimsa stain and examined for changes in vaginal cytology (Zarrow et al., 1964).
Data Analysis
Responses made on the active lever (cocaine-contingent) were the primary data for the experiment. These data were analyzed across the last 4 sessions of access to cocaine on the FR2 schedule and the first 4 sessions on the PR2 schedule. Additionally, within-session lever presses and cocaine intake were analyzed during the PR2 sessions. The infusions earned were used to calculate cocaine intake (mg/kg). Finally, the ratio of active lever presses to total lever presses (active + inactive lever presses) was calculated for each group to assess the accuracy of responding during the PR2 sessions.
Hierarchical Linear Modeling
Active lever presses and cocaine intake (mg/kg) were analyzed using Hierarchical Linear Modeling (HLM) version 5 software (Bryk and Raudenbush, 1992; Raudenbush and Bryk, 2002), a method to examine the hierarchical structure of nested data. The multi-level approach to the analysis allows fitting individual trajectories for individual mice and also provides assessment of hypothesized group differences. Factors such as sex, cocaine unit dose and session are entered into the statistical model to determine whether they systematically influence individual trajectories. In this study a two component (linear and quadratic or “accelerated”) curve was used to describe responding and cocaine intake across days and a 4 component orthogonal polynomial was used to describe the more complex pattern of responding that occurred within sessions.
Two Level Hierarchical Model
To evaluate data from the experiment, a 2 level model similar to that of Lanza et. al. (Lanza et al., 2004) was employed with separate analyses for the FR2 and PR2 phases of the experiment. Behavior (active lever presses) was described by three level 1 parameters: intercept, linear slope, and accelerated slope. Level 1 parameters of the model reflect changes in the lever response by individual mice across the four test sessions while maintained on either FR2 or PR2. The level 1 parameters, in turn, are modeled as functions of level 2 parameters which are the between subject variables, Sex and Dose. Thus, the level 1 parameters are nested within the level 2 units.
To accomplish the analysis, the HLM analysis was completed in several stages. Initially, a completely unconditional model was developed to define the overall between and within subject variance estimates across the 4 test periods without regard to the Sex and Dose variables. Next, the linear and accelerated “predictor” components were sequentially added, and the resultant improvement of fit evaluated with likelihood ratio tests. Preliminary analyses indicated that addition of a cubic component did not improve the model fit.
The equations describing the level 1 model were as follows: [Behaviorti= β0i + β1i(session) + β2i(session2) + eti] where subjects range from i=1 to N, and session from 1 to 4. β0i, β1i, β2i are, respectively, the intercept, linear, and accelerated growth parameters. eti is the level 1 error (i.e., the difference between the predicted and actual response data).
As described by Lanza et. al. (Lanza et al., 2004), we modeled accelerated change by adding the square of the Session to the linear component, so that the instantaneous slope increased or decreased systematically across sessions. It should be noted that, in contrast to an orthogonal polynomial representation, the linear component is altered by the inclusion of the quadratic and, in general, the linear and quadratic components covary. Nevertheless, this procedure was preferred since the use of independent (orthogonal) components have a less natural interpretation in this context. The analysis employed is not invalidated by this dependence between parameters.
After developing the unconditional hierarchical model, the level 2, between subjects (Sex and Dose), factor equations were included to produce the final model. These second level equations express each of the level 1 coefficients as functions of level 2 variables (i.e. Sex and Dose). In this case the 3 parameters describing the trajectory of responses generated by individual animals across session are modeled as functions of Dose and Sex which are coded as dummy variables (male=0, female=1, 0.3mg/kg dose=0, 1mg/kg dose=1). Most of the standard regression techniques may be used at level 1, level 2, or both, so that possible interactive effects of Dose and Sex on the behavior can be represented by the product of the two dummy coded variables. Thus, the level 2 equations are the following:
As indicated by the equations, a level 2 regression analysis is conducted for each of the three level 1 parameters. By substituting the dummy codes into the level 2 equations, coefficients can be calculated: γx0 is the average coefficient (x) of the level 1 regression for low dose males; γx0 + γx1 is the coefficient for high dose males; γx0 + γx1 + γx2 is the average coefficient for the low dose females, and, γx0 + γx1 + γx2 + γx3 is the average coefficient for the high dose females. Since the coefficients of the dummy coded regressions represent differences between predicted values for subjects with 0 vs 1 for the variable, tests of these coefficients against zero test particular aspects of the model. Specifically, a test of γ01 against zero would determine if low and high dose males differ and a test of γ02 against zero determines if low dose males and females differ. A test of γ03 against zero determines if sex has a different influence at the high vs the low dose. Tests of the level 2 intercepts, i.e. γ00, γ10, and γ20, reflect whether the average level 1 coefficient for low dose males is different from 0.
The rxi terms reflect the variation in the level 1 coefficient around its predicted value. By eliminating this term, we would have a “fixed” rather than “random” effect at level 1. With a fixed effect, all subjects in a group defined by the level 2 model (i.e. Dose and Sex) would have the same coefficient. For example, fixing the linear and acceleration terms forces all subjects to show the same pattern of results, but allow the overall response magnitude (indicated by the intercept) to vary between subjects. This corresponds roughly to a traditional ANOVA. On the other hand, keeping the rxi term fits a separate trajectory for each subject and hypotheses can be tested regarding populations of coefficients. For example, a test of residuals from the model with fixed vs random effects indicates whether fitting separate trajectories decreases the residual. In this instance, a reduced residual indicates more variation amongst individual trajectories; whereas, unchanged residuals indicate that the same coefficient could be used for all subjects.
Three Level Hierarchical Model
A three-level hierarchical linear model was used to analyze the within session time course data collected at 5-min intervals across the three hour period of the PR2 sessions. Time-interval was nested within session and session within subject. Sessions at level 2 were represented by 3 indicator-coded contrasts using the first session as a reference point. The lever response or cocaine intake data trajectories across time were modeled as sets of orthogonal polynomial contrasts. Preliminary analysis indicated that a fourth degree function (i.e. Quartic) captured the systematic trend, eliminating the need for higher order functions.
The level 1 hierarchical regression equations were
where π0, π1, π2, π3, and π4 are the regression coefficients, respectively, for the intercept, linear, quadratic, cubic and quartic components. εijk is the level 1 error.
The level 2 equations were
where π refers to the level 1 coefficient as a function of the three dummy coded contrasts and SCODE1, SCODE2 and SCODE3 for session 1 vs 2 and 1 vs 3 and 1 vs 4 respectively and the β are the regression coefficients.
The level 3 equations are given by
Omnibus tests of Sex, Dose, and the Sex × Dose interaction were conducted by performing joint multivariate tests including all of the contrasts other than the intercept (Raudenbush and Bryk, 2002). Since orthogonal polynomials are “centered” variables, a test of the intercept corresponds to a test of the overall mean across the session. Additional repeated contrasts at level 2 (e.g. session 1 vs session 2) were performed by changing the dummy coding of the sessions and rerunning the analysis.
Results
Subjects
The experiment included 4 groups of mice, distinguished by sex (19 males; 25 females) and cocaine unit dose (0.3 or 1 mg/kg/infusion). Four male mice were excluded from data analysis: two because of catheter failures, one died, and one failed to meet lever discrimination criteria. Attrition was greater for female mice, elimination due to catheter failure (7), sickness (4), and aberrantly high responding throughout the FR2 and PR2 sessions (1; >2 standard deviations from the mean). The higher catheter failure rate in the female mice may have been due to the use of shorter catheter insertion lengths (1 cm) in the initial surgeries but the combination of shorter catheters and extra handling (vaginal smears) cannot be ruled out. Thus, the final group compositions available for analysis were: Male, 0.3 mg/kg dose (n=8) and 1 mg/kg dose (n=7); Female, 0.3 mg/kg dose (n=7) and 1 mg/kg dose (n=6).
Estrous Cycle Tracking
Estrous cycle was tracked in a subset of females (n=14, 4 included in final data set; 10 excluded as described in the preceding paragraph). Evidence of estrus phase (cornified epithelial cells) was found in each female mouse at least once, usually twice, in the 2 weeks prior to surgery. However, none of these mice exhibited signs of estrus after surgery. Therefore, it appears that normal estrous cycling was interrupted by surgery, onset of food restriction (Nelson et al., 1985), onset of cocaine self-administration, or a combination of these factors, all of which occurred within a two day period. Because the estrus cycle was interrupted, we were unable to evaluate its impact on cocaine self-administration by female mice.
Fixed-Ratio 2 (FR2) Responding Across Sessions
Active lever responding across the last four sessions that cocaine was delivered on the FR2 schedule of reinforcement is summarized in Figure 1. Panel A of the figure provides mean ± S.E.M. values for groups of male and female mice responding for either 0.3 or for 1.0 mg/kg unit doses. Panel B provides similar values predicted from the 2-level hierarchical model for lever pressing across the 4 sessions. Both methods of data presentation indicated that mice responded more for the 0.3 mg/kg than for the 1 mg/kg unit dose of cocaine, that the sexes did not differ in response output, and that responding tended to increase across sessions.
Figure 1.
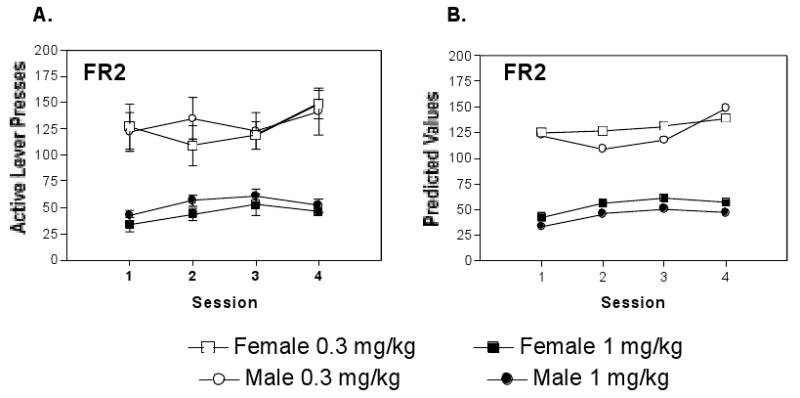
Responding for intravenous cocaine delivered on an FR 2 schedule of reinforcement. Open squares and open circles, respectively, represent females and males responding for the 0.3 mg/kg unit dose. Closed squares and closed circles, respectively, represent females and males responding for the 1mg/kg unit dose. (A) The mean ± S.E.M. across sessions for the 4 groups of mice. (B) The predicted values from the 2 level hierarchical model for lever pressing across the 4 sessions by the same mice summarized in Panel A. The hierachical analysis indicated that responding was dose-dependent but did not vary according to sex.
FR2 Responding: Two Level Hierarchical Model
The sequential hierarchical modeling for active lever presses across days during the FR2 phase of the experiment are shown in middle column of Table 1. The upper portion of the column summarizes the level 1 model without the Sex and Dose factors (i.e. the Unconditional Model). The lower portion of the table summarizes the final, level 2, model containing the Sex and Dose factors. The sequential estimates of the level 1 model characterize the basic structure of the within subject data. As noted in the table, active lever presses varied across the four sessions with the within subject variance representing 30.0% of the total overall variance. Mean responses per session averaged across the four sessions were 92.0 ± 9.0 (See Intercept Estimate). Addition of a linear component accounted for a large portion of the total overall within subject variance (See Linear Component Estimate, 44% p< 0.001). As noted, these slopes were generally positive and approached significance (X2 =6.22, p= 0.084). More importantly, the highly significant Residual Test associated with the Linear Component Estimate indicates that individual mice differed in the trajectories they generated across the four sessions differed as indicated by a significant reduction in residual variance by inclusion of the random effects term (i.e., the rxi term derived from generating an individual slope for each subject). The significant difference in trajectories generated by the different mice questions the appropriateness of the fixed effects term used in ANOVA or typical regression analyses, in which all subjects are fitted to a common slope (Bryk and Raudenbush, 1992; Raudenbush and Bryk, 2002).
Table 1. Hierarchical Linear Models for Active Lever Presses.
| Unconditional Model | Fixed Ratio 2 | Progressive Ratio 2 | ||
|---|---|---|---|---|
| Percent Within Variation | 30.0% | 47.4% | ||
| Intercept Est. (S.E.) | 92.0 (9.0) | 680.8 (74.5) | ||
| Linear Component Est. (S.E.) | 6.22 (3.55) | 97.8 (38.1) | ||
| Residual Test | χ2 = 16.8, 3 df, p = 0.001 | χ2 = 58.1, 3 df, p = 0.000 | ||
| % Variation | 44.0 % | 57.0 % | ||
| Individual Variation | χ2 = 4.28, 1 df, p = 0.036 | χ2 =46.2, 2 df, p = 0.000 | ||
| Different from Zero | t=1.79, 27 df, p= 0.084 | t = 2.57, 27 df, p = 0.016 | ||
| Accelerated Component Est. (S.E.) | 1.12 (2.61) | -24.9 (19.9) | ||
| Residual Test | χ2 = 8.37, 4 df, p = 0.078 | χ2 = 9.06, 4 df, p = 0.059 | ||
| % Variation | 26.0 % | 11.0 % | ||
| Individual Variation | χ2 = 8.34, 3 df, p = 0.039 | χ2 = 8.12, 3 df, p = 0.043 | ||
| Different from Zero | t= 0.428, 27 df p= 0.67 | t =-1.26, 27 df, p = 0.221 | ||
| Final Mode1 Including Sex and Dose factors | Fixed Ratio 2 | Progressive Ratio 2 | ||
| Predicted Values (S.E.) | P-value | Predicted Values (S.E.) | P-Value | |
| Intercept (β00) | 125.1 (17.8) | 0.000 | 540.5 (54.6) | 0.000 |
| Dose (γ01) | -82.5 (18.3) | 0.000 | 2.2 (80.7) | 0.978 |
| Sex (γ02) | -2.5 (26.4) | 0.924 | -16.5 (126.7) | 0.898 |
| Dose × Sex (γ03) | -6.9 (27.3) | 0.802 | -148.9 (151.7) | 0.337 |
| Linear Slope (β10) | 0.22 (11) | 0.984 | 460.1 (199.7) | 0.030 |
| Dose (γ11) | 18.2 (11.4) | 0.125 | -383.8 (211.6) | 0.082 |
| Sex (γ12) | -24.8 (32.9) | 0.458 | -484.6 (207.7) | 0.028 |
| Dose × Sex (γ13) | 23.5 (33.4) | 0.489 | 518.4 (250.8) | 0.049 |
| Accelerated Slope (β20) | 1.5 (3.6) | 0.410 | -70.5 (51.03) | 0.180 |
| Dose (γ21) | -6.0 (3.9) | 0.136 | 70.3 (54.2) | 0.207 |
| Sex (γ22) | 9.6 (9.7) | 0.332 | 59.8 (54.7) | 0.285 |
| Dose × Sex (γ23) | -9.3 (10) | 0.361 | -73.4 (72.6) | 0.322 |
As noted in the Accelerated Component section of the table, adding the accelerated slope component to the model accounted for an additional 26.0% of the overall within subject variance, a marginal significant increase (p< 0.078). Despite the marginal significance, the large percentage of the overall individual variation accounted for, and the significant subject to subject variability (See Individual Variation in the table) argue in favor of including the accelerated slope component in the final model. The low, nonsignificant average coefficient for the component (1.12 ± 2.61) indicates distinct trajectories for different subjects across the four sessions, with some exhibiting a concave and others a convex trajectory.
The level 1 analysis discussed above suggests that both the slopes and intercepts of response data generated under the FR2 schedule were sufficient to justify examination of the level 2 model. The coefficients for each group of mice generated by the level 2 model are tested against zero, and the actual slope and intercept for the aggregate curves for each of the four groups in Fig 1B are derived from the regression coefficients.
The final, level 2 model is summarized in the lower middle portion of Table 1 and suggests relatively simple between subject relationships in FR-2 responding over the 4 sessions with only a strong Dose (γ01) effect associated with the Intercept (β00). As noted for the mean values in Fig 1A, the predicted trajectories in Fig 1B indicate that mice of both sexes responded similarly for cocaine delivered on the FR2 schedule and had substantially higher response rates for the low than the high dose of cocaine. The addition of linear and accelerated trajectory components to the model made no significant contribution toward accounting for variance associated with Dose, Sex, or the Sex × Dose interaction.
FR Responding: Performance Criteria for Transition to the Progressive Ratio Sessions
The number (X ± SE) of sessions required to meet the criteria for advancing to the PR2 schedule for males and females, respectively, were 10.3 ± 0.5 and 9.5 ± 0.3 sessions for the 0.3 mg/kg unit dose and 9.8 ± 0.2 and 9.5 ± 0.3 sessions for the 1 mg/kg unit dose.
Progressive Ratio-2 (PR2) Responding: Accuracy of responding
To assure that the lever response number was appetitive for cocaine rather than reflecting a motor activation, the percent of Total responses made on the cocaine Active Lever was determined when the drug was delivered on the PR2 schedule of reinforcement. Mean percentage of responses on the cocaine appropriate lever for the 0.3 mg/kg and 1 mg/kg cocaine doses were, respectively, 87% and 95% for males and 88% and 95% for females. The high percentages of responses on the cocaine-producing lever indicates mice discriminated the active and inactive lever when responding for cocaine delivered on the PR2 schedule of reinforcement regardless of sex or cocaine dose.
PR2 Responding Across Sessions
Active lever responses for the four groups of mice across the first four sessions when cocaine was delivered on the PR2 schedule are summarized in Fig 2. As described for the FR2 data in Fig 1, mean responses on the drug lever by the four groups for cocaine are summarized in Panel A, and the predicted values from the level 2 hierarchical model are summarized in Panel B. Both methods of data presentation indicate that responses for 1 mg/kg cocaine (filled symbols) delivered on the PR2 schedule increased across sessions at a similar modest rate for both sexes, with response output slightly (but not significantly) higher for males than females. In contrast, when responding for the 0.3 mg/kg unit dose of cocaine, response trajectories across the four test days were remarkably dissimilar for the two sexes with responses slightly declining for females and increasing substantially for males, apparently approaching an upper asymptote at the later sessions.
Figure 2.
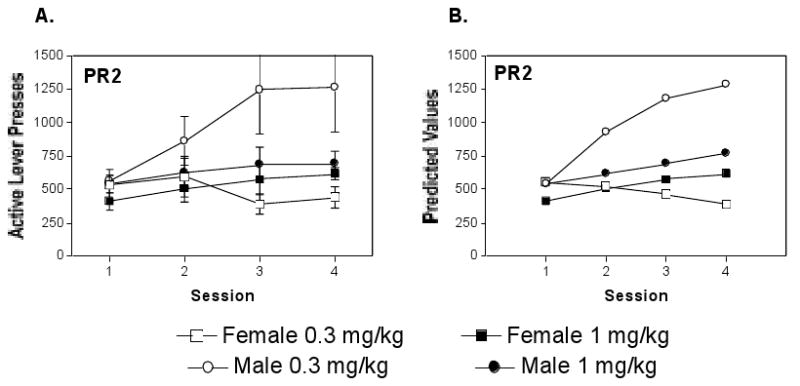
Responding for cocaine delivered on a behaviorally demanding PR2 schedule for the same 4 groups of mice shown in Figure 1. Symbols are as described for Fig 1. (A) The mean ± S.E.M. across 4 sessions on PR2 was elevated in comparison to response output on the FR2 schedule. (B) The 2 level hierarchical model predicted values for lever pressing across sessions for the same mice. The analysis indicated that responses for the 0.3 mg/kg unit dose of cocaine were greater for male than for female mice and responses where similar between the sexes for the 1 mg/kg cocaine unit dose.
PR2 Responding Across Sessions: Two Level Model
The hierarchical analysis of active lever responses during the PR2 phase of the experiment associated with the aggregate trajectories of Figure 2B are summarized in the right panels of Table 1 as described for the FR2 phase of the experiment. The within subject variation was substantially greater when cocaine was delivered on the PR2 schedule (47%) than when delivered on the FR2 schedule (30%) reflecting the greater session to session variation. In addition, the overall Intercept Estimate was considerably larger for the PR than the FR2 phase indicating that the mice responded rapidly in the first session to the contingency change from FR2 to PR2. The Linear Component accounted for 57% of the total within subject variance, was highly positive (b = 97.8 ± 38.1; t = 2.57, df = 13 p = 0.016), and reflects substantial individual variation (p = 0.001). Addition of an Accelerated Component accounted for an additional 11% of the within subject variance and approached the 0.05 level of significance (p = 0.059). Although Individual variation was significant (p = 0.043), the negative sign for the accelerated component coupled with the marginal overall significance (p = 0.059) suggests some mice approached asymptotic responding and appears to be important for males responding for the low cocaine dose as discussed below in the Final Conditional Model (Table 1, bottom) and individual trajectories (Fig 3).
Figure 3.
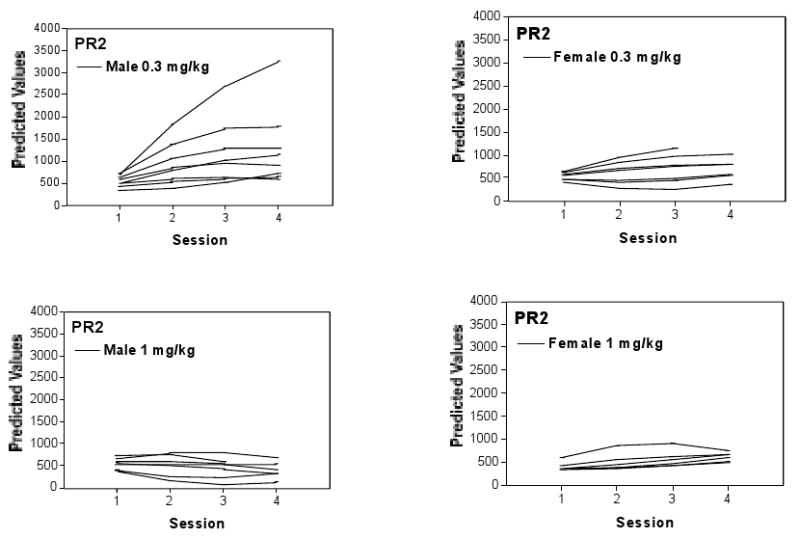
Behavioral trajectories across the four sessions for indiviudal mice during the PR2 reinforcement sessions. Curves represent trajectories of individual subjects, grouped according to Sex and Dose. Individual mice had distinct trajectories of lever pressing behavior across sessions while maintained on the PR2 schedule of reinforcement, even after accounting for Sex and Dose.
The estimates from the level 2 model in Figure 2B are consistent with the group means summarized in Figure 2A. As shown at the bottom of Table 1, neither of the level 2 effects (Sex or Dose) associated with the intercept were significant. The linear slope, however, depended upon an interaction of Sex and Dose. Male mice responding for the low dose of cocaine were distinguishable from all other groups, exhibiting a very rapid (b=460.1 ± 199) increase in responses across sessions in comparison to shallow slopes other groups (female low = -20.3 ± 54, male high = 76.2 ± 69 and female high =110.3 ± 122). The animal's sex impacted the linear slope of responding for the low cocaine dose (t = 2.35, df = 13, p = 0.036) but not the high dose. Interestingly, level 2 variables did not affect the accelerated component of the trajectory, likely because of the low statistical power associated with the relative few animals in the study. As noted above, a subset of mice (Low dose Males) appeared to account for accelerated component included in the unconditional model. Although not statistically supported, analysis of individual groups suggested a more substantial accelerated coefficient for males responding for the low dose (-70.5 ± 51) than for other groups, though group differences did not reach significance (t = 1.38, df = 25, p = 0.18).
Evidence that the aggregate group values presented in Fig 2B represent the individual trajectories generated by individual mice is provided in Fig 3, which summarizes the trajectories for individual mice for each sex and cocaine dose. Comparison of the two figures suggests that the group trajectories of Fig 2B do indeed capture the trends for the individual trajectories generated by mice within each of the groups. Further, inspection of the trajectories for the male mice responding for the low cocaine dose in Fig 3 indicates a pattern of response increases across the session with some appearing to asymptote with little change between Sessions 2 and 3 or between Sessions 3 and 4.
PR2 Responding Within Sessions: Three Level Model
The within session time course of responding across the four test sessions was evaluated by extending the 2 level model in two ways. The first major extension was that data collected during 5-min time bins within each of the four sessions provided level 1 dependent variables for a 3- rather than a 2- level model (i.e. time is nested within session and sessions are nested within animals). Thus, the regression coefficients describing the fluctuation in responses across each three hour test are modeled as the dependent variable for a level 2 regression, and our analysis modeled the level 1 coefficients as a function of session number. The coefficients of the level 2 model (sessions) are described by the level 3 variables, Sex and Dose. The indicator for “sessions” was coded with the first session serving as the reference session. The second major extension for establishing a level 3 model used to describe the within session response patterns is that a 4th degree polynomial was required to model the more complex trajectories of individual mice over the 36, 5-min bins of a three hour test session across the 4 sessions, rather than trajectories for only total responses generated during each of the 4 sessions.
Initial analyses indicated that the within session complex response pattern trajectories could be adequately described by four polynomial coefficients (linear, quadratic, cubic, and quartic). Figure 4 describes the predicted trajectories calculated from the empirical Bayes residuals for two individual mice with quite different response patterns. The calculated trajectories are superimposed on the response output per 5-min bin during a three hour test session. Comparison of the two figures indicates that in spite of the considerable difference in response pattern for the two mice, the polynomial equation captures the overall trajectory for each mouse.
Figure 4.
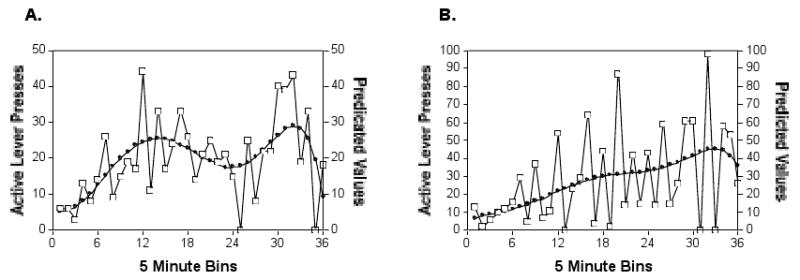
Within-session lever presses compared to the predicted values derived from the 3 level hierarchical model for 2 representative mice to illustrate the relationship of predicted to actual responses. Open squares are active lever presses and the smoothed dark lines are the responses predicted by the model. (A) A female mouse responding for the 0.3 mg/kg unit dose of cocaine during session 1. (B) A male mouse responding for the 1 mg/kg unit dose of cocaine during session 4. The response estimates accurately represent the behavioral trajectory of each mouse.
Figure 5 summarizes the within session response trajectories (Fig 5A,B,C,D) and associated cocaine intake (Fig 5E,F,G,H) generated by the different Sex and Dose groups for each of the four test days. In general, the within session model supported and extended conclusions drawn from the two level model constructed from the session totals. Differences at level 2 (intercept, slope) and level 3 (Dose and Sex) were assessed with a joint test of the set of polynomial coefficients against zero (Bryk and Raudenbush, 1992). The within session response pattern depended upon an interaction of Sex and Dose (χ2=25.7, df=12, p=0.012). Follow up analyses indicated that Sex influenced the response trajectory generated during the test sessions at the low cocaine dose (See Fig A,C; χ2=34.5, df=20, p=0.023), but not the high dose (See Fig E,G; χ2=3.5, df=20, p>0.500).
Figure 5.
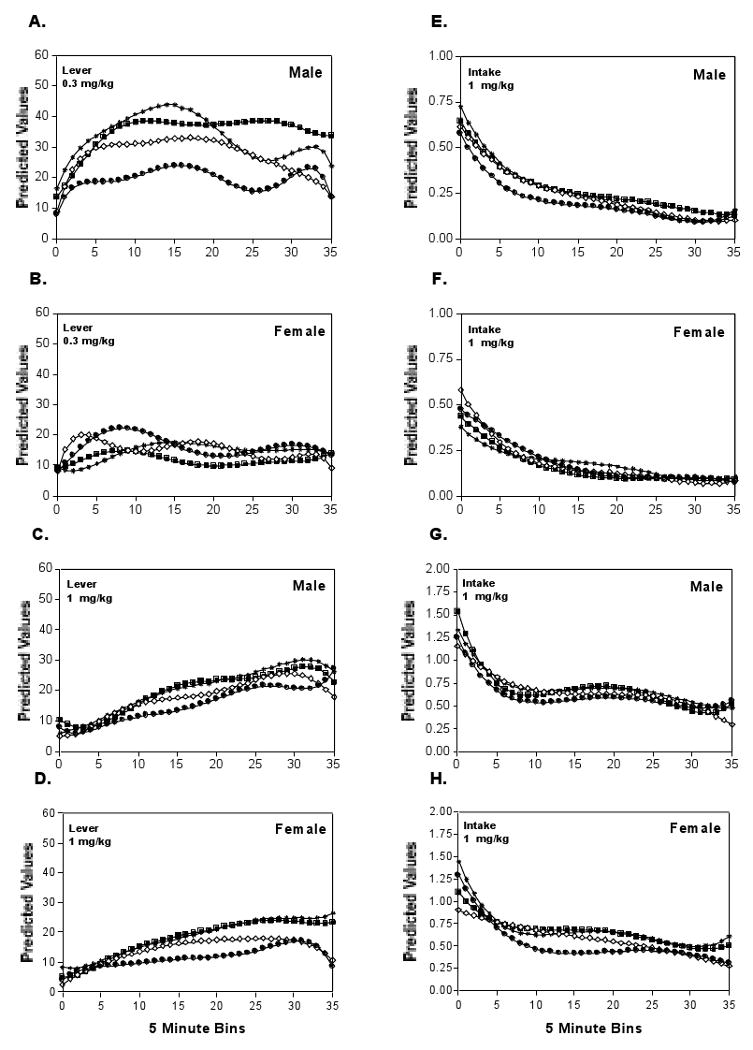
Within and across session predicated values (Right panels, A-D) and corresponding cocaine intake estimates (Left panels, E-H) derived from the 3 level hierarchical model for the first four PR2 sessions. (A,E) Males responding for the 0.3 mg/kg unit dose show dynamic changes both within and across sessions, but despite high local rates of responding during the early and middle portions of the sessions, cocaine intake declined because high response rates were not maintained. (B,F) Females responding for the 0.3 mg/kg unit dose generally showed stable responding across and within sessions, but because of the increasing ratio requirements of the PR2 schedule, steady cocaine intake was not maintained. (C,G) Responding for the 1mg/kg unit dose increased steadily within session for males and females, suggesting this dose was more reinforcing than the lower dose. (D,H) In contrast to the the low dose, the steady increases in responding for the high dose within sessions lead to relatively stable periods of intake during the middle of the session.
Session symbols are: 
When responding for the low cocaine dose (Fig 5A), male mice increased their response output during the first half of each session. Response output by this group plateaued near the middle of the first three sessions at rate which did not maintain constant cocaine (See Fig 5A vs E). During the fourth sessesion, response output by this group of mice peaked about 60 minutes into the test period, and then rapidly declined suggesting the likelihood of response extinction. Female responses for this cocaine dose (Fig5B) increased only modestly during the early part of most sessions and cocaine intake fell continuously throughout each session
Response trajectories associated with the high cocaine dose were similar for male and female mice, with responses increasing almost throughout the session (See Fig 5 C & D). Cocaine intake plateaued at 30 min (bin 6) to 100 min (bin 20) into the session. During this 70 min time period, responding by both sexes was sufficient to maintain constant drug intake. At approximately one hour and forty min into the session the increased response output required by the demanding PR2 schedule for constant cocaine intake delivered on was not maintained and intake declined during the final minutes of each session. Responses increased modestly across the four sessions for both sexes. Although the change across the first two sessions appeared to be greater for females than for males, the sex difference was not supported statistically, and was likely due to a greater increase for males during the first two sessions.
Discussion
In the present study C57 mice pressed levers to obtain either 0.3 mg/kg (Low dose Group) or 1.0 mg/Kg (High dose Group) unit doses of cocaine, first on an FR2 schedule and then on a PR2 schedule of reinforcement. Response trajectories across the four sessions under each reinforcement schedule of cocaine delivery differed substantially for individual mice. Hierarchical analyses of data gathered when cocaine was delivered on either schedule established that in spite of strict experimental control, and the use of genetically identical subjects, individual differences in the response trajectories generated by generated by these mice accounted for large proportions of the total experimental variance. Not surprisingly, HLM established that responding for cocaine was influenced by the unit dose as well as the schedule of reinforcement. A sex difference in cocaine self-administration emerged when the behavioral cost was great (PR schedule) and the reward small (0.3 mg/kg dose), with males responding more than females as previously observed in C57 mice responding for food (Gentry and Middaugh, 1988) or ethanol (Middaugh and Kelley, 1999).
Use of hierarchical modeling techniques to describe lever pressing behaviors
A particular advantage of the hierarchical analysis of data is that in addition to group differences (i.e., Sex and Dose) assessed by the traditional ANOVA, HLM provides important information about individual differences in a behavioral response across time which can be important in drug studies. The model used in our experiment was similar to that described for nicotine self-administration (Donny et al., 2004; Lanza et al., 2004) and provided information about how the behavioral trajectories across time differed for individual mice. In addition, the procedure modeled how these individual trajectories, as well as the group trajectories, were influenced by Sex or Dose. It is important to note that other factors with continuous distributions could be added to the 2 level model in addition to, or instead of, Sex and Dose to explain the behavioral variance amongst individual mice. For example, activity levels for each mouse in response to novelty could have been directly entered into the model as covariates. Moreover, as demonstrated by Donny et. al. (Donny et al., 2004), neurobiological measurements such as nicotinic receptor binding (e.g. Bmax) in specific brain regions can be added to hierarchical models to determine their impact on behavioral trajectories. Thus, hierarchical analysis begins by modeling individual trajectories and subsequently incorporates between groups factors, like the time invariant factors of Sex and Dose or variables with continuous distributions such nicotinic receptor binding, to explain individual variance.
The hierarchical analysis extended information provided by ANOVA by quantifying individual variation in lever pressing and cocaine intake both within and across the 4 daily sessions. Importantly, individual variation is addressed in a systematic manner thus reducing problems of large error variances associated with relatively small group sizes. Expanding the model to 3 levels allowed examination of individual response patterns within each session as well as across the 4 daily sessions to provide accurate models of how these complex response patterns change across time.
The 3 level HLM accurately represented within session lever pressing and cocaine intake for individual mice of the four groups of the Sex × Dose design during the PR2 schedule phase of the experiment, and the aggregate group trajectories were useful for examining cocaine intake patterns. For example, the steady increase in lever pressing for the 1.0 g/kg unit dose of cocaine within the sessions produced relatively stable cocaine intake during the middle portion of the sessions. In addition, the steady increase in responses for the 1 mg/kg, but not 0.3 mg/kg, unit dose of cocaine across the session indicates that the former was a more efficacious reinforcer as defined by its maintenance of responding with increasing behavioral demand (Stafford et al., 1998). In contrast, for the 0.3mg/kg unit dose, neither consistent lever pressing by the females nor rapid increases in lever presses early in the session by the males maintained stable periods of cocaine intake which declined within the session. The within session plots noted in Fig 6 suggest that mice were approaching the traditional “breakpoint” defined as a prolonged period of behavioral extinction after the last earned infusion (Richardson and Roberts, 1996), albeit the downward deflections in the predicted values could be artifacts related to fitting orthogonal polynomial functions to the data. Nevertheless, the trajectories do suggest that within session behavioral extinction would occur if the session were lengthened and future experiments will address this issue.
Although the orthogonal polynomials provided an accurate description of the behavioral trajectory in the present experiment, they do not correspond to any obvious physiological construct beyond the accelerated growth component established by the 2 level model. Inclusion of a physiologically relevant covariate which changes across time (e.g. blood levels of cocaine, extracellular dopamine, etc) in future experiments would contribute to the biological relevance of the 3 level model. Modeling individual active lever presses associated with changes in cocaine blood concentration could be achieved by calculating moment to moment lever press probability using within subject hierarchical logistic regression (Bryk and Raudenbush, 1992).
Influence of Sex and Dose on cocaine self-administration in C57 mice
The lever pressing for cocaine delivered on the FR2 schedule observed in this experiment is consistent with a previous experiment using C57 mice (Griffin and Middaugh, 2003) and other reports for mice (Caine et al., 2002; Thomsen and Caine, 2006). The absence of sex-related differences when cocaine was delivered on the FR2 schedule in our experiment is consistent studies using rats maintained on low response requirement fixed ratio schedules (Caine et al., 2004; Dalton et al., 1986; Roberts et al., 1989b). The lower response rate for the high than the low cocaine unit does delivered on the FR2 schedule suggests that the doses were on the descending limb of the cocaine dose-response function for C57 mice.
Although responses for cocaine did not differ according to sex when delivered on the low cost FR2 schedule, when delivered on the behaviorally more demanding PR2 schedule, response output for cocaine increased, and importantly, depended upon the interaction of the dose and sex factors. Response output for cocaine delivered on the PR2 schedule was similar for the two sexes when the unit does was relatively high (1.0 mg/kg); however, at the lower dose (0.3 mg/kg) male mice responded more than females. HLM analysis indicated that the higher response output by males for the low dose of cocaine was due to initial high response rates in the early part of test session that peaked at approximately one hour and then declined. In contrast, female mice responded steadily at low rates across the two hour sessions. These findings suggest that increasing the behavioral cost for obtaining cocaine revealed a sex difference in its reward value, with males expending more effort than females for smaller amounts of cocaine. This sex difference is consistent with previous reports on C57 mice responding for food (Gentry and Middaugh, 1988) or ethanol reinforcement (Middaugh and Kelley, 1999). Together, these results suggest that male C57 mice will exert more effort than females to obtain both drug and natural reinforcers when challenged by a behaviorally demanding reinforcement schedule.
The higher response output by males than females when cocaine was delivered on a PR2 schedule, however, is opposite to two reports of studies using rats (Carroll et al., 2002; Roberts et al., 1989a). Methodological differences such as cocaine doses, PR reinforcement schedule, in addition to species, likely contribute to the contradictory findings. Although the direction of the sex difference in responding for cocaine reinforcement remain unclear, the differences were observed only under behaviorally demanding conditions.
The noted sex difference in cocaine reward suggests a possible difference in motivation to obtain cocaine reinforcement. Dopaminergic systems have been strongly implicated in the motivation to seek cocaine (De Wit and Wise, 1977; Ranaldi and Wise, 2001; Ritz et al., 1987; Roberts et al., 1977; Roberts and Koob, 1982) and sex differences in the dopaminergic systems of mice have been documented. For example, potassium stimulated release of extracellular dopamine was reported to be greater in male than female CD1 mice (McDermott et al., 1994). Relevant to this study, we recently reported greater cocaine-induced increases in striatal extracellular DA for male than female C57 mice (Griffin and Middaugh, 2006). Other reports indicate that in comparison to males, female mice have more efficient DA uptake in striatal preparations (Bhatt and Dluzen, 2005) as well as reduced D2 receptor density (Boggan et al., 1996). Given the importance of the dopaminergic system as a neurobiological substrate for motivation (Berridge and Robinson, 1998; Salamone, 1996) and the noted sex differences in DA systems, it is reasonable to postulate that differences in the dopaminergic systems of male and female mice may contribute to the difference in cocaine seeking behavior observed in the present study.
Although females reportedly metabolize cocaine more rapidly than male mice (Leibman et al., 1990; Morishima and Whittington, 1995), another report indicates an intraperitoneal injection of cocaine produced equivalent brain levels of cocaine in male and female C57 mice (Azar et al., 1998). The latter report as well as the similar response for the two sexes when cocaine was delivered on the FR2 schedule makes it unlikely that differences in cocaine metabolism or brain concentrations can account for the lower response output by female than male mice responding for cocaine delivered on the PR2 schedule.
Conclusion
Male and female C57 mice self-administered intravenous cocaine under two schedules of reinforcement (FR2 & PR2) for two unit doses of cocaine (1.0 and 0.3 mg/kg). HLM analysis demonstrated that individual mice exhibited distinct behavioral trajectories across and within sessions even after eliminating the contribution of the fixed factors, Cocaine dose and Sex. When cocaine was delivered on an FR2 schedule, responding was dose dependent and response rates were similar for male and female mice at each dose. When cocaine was delivered on a PR2 schedule, responses were equivalent for the two sexes at the high unit dose; however, at the lower unit dose, male mice responded more than females for cocaine by completing higher final ratios. Within session analysis of the PR sessions corroborated the sex difference and further indicated that cocaine was more reinforcing at the 1.0 mg/kg than the 0.3 mg/kg unit dose. Thus, sex differences in cocaine reinforcement were evident when behavioral demand increased for low unit doses of cocaine.
Acknowledgments
WCG was supported by NIH T32 DA07288 and the research was supported by NIDA DA14185 & DA16511 to LDM. The authors thank Kimber L. Price for valuable technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azar MR, Acar N, Erwin VG, Barbato GF, Morse AC, Heist CL, Jones BC. Distribution and clearance of cocaine in brain is influenced by genetics. Pharmacol Biochem Behav. 1998;59:637–640. doi: 10.1016/s0091-3057(97)00471-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;1035:188–195. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Boggan WO, Xu W, Shepherd CL, Middaugh LD. Effects of prenatal ethanol exposure on dopamine systems in C57BL/6J mice. Neurotoxicol Teratol. 1996;18:41–48. doi: 10.1016/0892-0362(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models. Sage; Newbury Park, CA: 1992. [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacol. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacol. 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dalton JC, Vickers GJ, Roberts DC. Increased self-administration of cocaine following haloperidol: sex-dependent effects of the antiestrogen tamoxifen. Pharmacol Biochem Behav. 1986;25:497–501. doi: 10.1016/0091-3057(86)90130-9. [DOI] [PubMed] [Google Scholar]
- De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45(3):539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6 × SJL and BALB/cByJ mice. Pharmacol Biochem Behav. 1997;57:429–440. doi: 10.1016/s0091-3057(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Lanza ST, Balster RL, Collins LM, Caggiula A, Rowell PP. Using growth models to relate acquisition of nicotine self-administration to break point and nicotinic receptor binding. Drug Alcohol Depend. 2004;75:23–35. doi: 10.1016/j.drugalcdep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Middaugh L, See RE. Conditioned-stimulus induced reinstatement of extinguished cocaine seeking in C57BL/6 mice: A mouse model of drug relapse. Brain Res. 2003;973:99–106. doi: 10.1016/s0006-8993(03)02560-5. [DOI] [PubMed] [Google Scholar]
- Gentry GD, Middaugh LD. Prenatal ethanol weakens the efficacy of reinforcers for adult mice. Teratology. 1988;37:135–144. doi: 10.1002/tera.1420370206. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Phillips TJ, Burkhart-Kasch S, Cunningham CL. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol Biochem Behav. 1995;51:827–834. doi: 10.1016/0091-3057(95)00047-z. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. Acquisition of lever pressing for cocaine in C57BL/6J mice: effects of prior Pavlovian conditioning. Pharmacol Biochem Behav. 2003;76:543–549. doi: 10.1016/j.pbb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- Groseclose CH, Draughn RA, Tyor WR, Sallee FR, Middaugh LD. Long-term intracranial cannula stabilization in mice with light cured resin composites. J Neurosci Meth. 1998;79:31–36. doi: 10.1016/s0165-0270(97)00158-1. [DOI] [PubMed] [Google Scholar]
- Homberg JR, van den Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, de Vries TJ. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological Basis of Sex Differences in the Propensity to Self-administer Cocaine. Neuropsychopharmacol. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Bandy AL, Middaugh LD. A novel chronic and detachable indwelling jugular catheterization procedure for mice. Physiol Behav. 1997;62:163–167. doi: 10.1016/s0031-9384(97)00029-2. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Donny EC, Collins LM, Balster RL. Analyzing the acquisition of drug self-administration using growth curve models. Drug Alcohol Depend. 2004;75:11–21. doi: 10.1016/j.drugalcdep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Leibman D, Smolen A, Smolen TN. Strain, sex and developmental profiles of cocaine metabolizing enzymes in mice. Pharmacol Biochem Behav. 1990;37:161–165. doi: 10.1016/0091-3057(90)90057-o. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacol. 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- McDermott JL, Liu B, Dluzen DE. Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol. 1994;125:306–311. doi: 10.1006/exnr.1994.1034. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Morishima HO, Whittington RA. Species-, gender-, and pregnancy-related differences in the pharmacokinetics and pharmacodynamics of cocaine. NIDA Res Monogr. 1995;158:2–21. [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32(3):515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci. 2001;21:5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. Second. Sage; Newbury Park, CA: 2002. [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacol. 1989a;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacol. 1989b;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ. Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacol. 1998a;138:82–88. doi: 10.1007/s002130050648. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998b;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci. 2002;22:10039–10045. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Durantez E, Hall SK, Steffen C, Self DW. Enhanced acquisition of cocaine self-administration by increasing percentages of C57BL/6J genes in mice with a nonpreferring outbred background. Psychopharmacol. 2006;186:553–560. doi: 10.1007/s00213-006-0379-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods. 1996;64:137–149. doi: 10.1016/0165-0270(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Gaspard TM, Kim SA, Walker LE, Vrana SL, Dworkin SI. Dose-effect functions for cocaine self-administration: effects of schedule and dosing procedure. Pharmacol Biochem Behav. 1997;57:523–531. doi: 10.1016/s0091-3057(96)00437-6. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18 doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacol. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacol. 2006;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Yochim JM, McCarthy JL. Experimental endocrinology, a sourcebook of basic techniques. Academic Press; New York: 1964. p. 519. [Google Scholar]


