Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the brain caused by JC virus (JCV) for which there is no cure. PML patients who have JCV-specific CD8+ cytotoxic T lymphocytes (CTL) in their blood have a better clinical outcome. We compared JCV-specific CTL responses in vitro elicited either by JCV peptide-loaded dendritic cells (DC) or by direct peptide stimulation of lymphocytes from 20 HLA A*0201+ healthy controls, HIV+ and PML patients. JCV peptide-loaded DC elicited a stronger CTL expansion in 13/15 responders. DC can induce potent JCV-specific CTL response in vitro, and may constitute a promising approach for PML immunotherapy.
Keywords: JC virus, Progressive Multifocal Leukoencephalopathy, Dendritic cells
Introduction
JC virus is the agent of progressive multifocal leukoencephalopathy (PML) (Koralnik, 2006) which occurs in up to 5% of people with AIDS (Antinori et al., 2003), as well as in patients with lymphoproliferative disorders, organ transplant recipients and individuals treated with natalizumab (Berger and Koralnik, 2005). After asymptomatic primary infection, JCV remains quiescent in the kidney, and can be found in urine of 30% of healthy and immunosuppressed individuals alike (Koralnik et al., 1999) and in lymphoid organs (Monaco et al., 1998).
In immunocompromised individuals, JCV may reactivate from sites of latency and causes a lytic infection of oligodendrocytes leading to multiple areas of demyelination in the central nervous system, and ultimately death within a few months. There is no effective treatment for this disease and those patients who survive are often left with devastating neurological sequellae (De Luca et al., 1998).
Therefore, a therapeutic vaccine may contain JCV replication, especially in the early stage of the disease, and may reduce the morbidity and mortality in patients with PML. The antibody response to JCV is unable to prevent development of PML or prevent disease progression (Weber et al, 1997). Conversely, the cellular immune response mediated by JCV-specific CD8+ cytotoxic T-lympocytes (CTL) plays a crucial role in the containment of PML (Du Pasquier et al., 2003; Du Pasquier et al., 2004; Koralnik et al., 2002). Hence, a treatment that improves the CTL response against JCV may be of benefit in patients with PML. Such therapies may consist of adoptive transfer of T cells after ex vivo stimulation with JCV antigens or dendritic cells (DC)-based immunotherapy (June, 2007; O'Neill and Bhardwaj, 2005). DC are the professional antigen-presenting cells in the body. A small number of DC and low level of antigens are able to induce a potent CD8+ T-cell response in vitro and in vivo (Adams et al., 2005). In addition, the safety of DC immunization has been established in numerous clinical trials (Dhodapkar and Bhardwaj, 2000). Furthermore, promising trials of DC vaccination have already been reported in patients with HIV infection (Lu et al., 2004).
The frequency of JCV-specific CTL in fresh blood is very low, and their function cannot usually be measured ex vivo by conventional assays without prior stimulation with JCV antigens (Lima et al., 2007). In our quest for a treatment for PML, we first developed a DC-based stimulation of JCV-specific T cells. Then, to determine the respective potential of autologous transfer of T cells and DC-based immunotherapy for PML, we compared the CTL response elicited in vitro from peripheral blood lymphocytes against JCV CTL epitopes by direct peptide stimulation, as performed in adoptive transfer of T cells, and by JCV-peptide loaded mature monocyte-derived DC.
Results
Peripheral blood samples were obtained from 20 JCV seropositive HLA-A*0201+ subjects, including 6 with PML(5 HIV+ and 1 HIV-) aged 40 to 69 years(47.8 ± 12.1 years [mean ± SD]), 4 HIV+ individuals aged 44 to 50 years(45.5 ± 3 years) and 10 healthy controls(HC) aged 24 to 44(34 ± 8 years). HIV+/PML and HIV+ patients had a mean CD4+ T cell count of 521 /μL ± 289 and 496 /μL ± 320 respectively, and a plasma HIV RNA level of 1.25 log10 copies/ml ± 0.5 and 1.98 log10 copies/ml ± 1.9, respectively. All HIV+ and HIV+PML patients were on highly active antiretroviral therapy (HAART). All but one PML patients were still alive an average of 9.2 years (3.4 to 15.2 years) after disease onset.
JCV-specific CTL responses against VP1p36 and/or VP1p100 epitopes, were detected in 14/20 study subjects, including 5/6 PML patients, 3/4 HIV+ individuals and 6/10 HC after direct peptide stimulation of lymphocytes. These responses were enhanced using peptide-loaded DC stimulation in 14/16 comparisons performed in these 14 individuals (Table 1). The average amplification factor of JCV-specific CD8+ T-cell response using peptide-loaded DC compared to peptide stimulation alone was 6.8x for PML patients, 3.9x for HIV+ patients, and 31.0x for HC. Furthermore, we measured a JCV epitope-specific CTL response after DC stimulation in 1 HIV+ individual (#1, VP1p100) and 2 HC (#3, VP1p100 and #9, VP1p36) who had no detectable CTL after stimulation of lymphocytes with peptide alone The median percentage of JCV-specific CD8+ T-cells in peptide alone and peptide-loaded-DC stimulation of lymphocytes was 0.7% (0.1-40.1%) vs 7.3% (0.2-48.8%) in the whole study population including both JCV VP1p36 and VP1p100 epitopes. There was a significant increase in the JCV-specific CTL response for both epitopes in peptide-loaded DC versus peptide alone stimulation of lymphocytes in all 15 responders (p=0.004, Wilcoxon matched-paired test)
Table 1.
Detection of JCV VP1p36- and JCV VP1p100-specific CD8+T cells in lymphocyte cultures stimulated with peptide-loaded autologous DC or peptide alone
| Subjects | % JCV VP1p36- specific CD8+T cells |
Amplification Factor | % JCV VP1p100- specific CD8+T cells |
Amplification Factor | ||
|---|---|---|---|---|---|---|
| Peptide alone | Peptide-loaded DC | Peptide alone | Peptide-loaded DC | |||
| PML patient # | ||||||
| 1 | 0.1 | 0.4 | 4.0 X | NA a | NA | |
| 2 | 0.4 | 1.2 | 3.0 X | 2.2 | 16.5 | 7.5 X |
| 3 | b | ~ | O̸ | 0.6 | 1.0 | 1.7 X |
| 4 | 1.0 | 24.5 | 24.5 X | NA | NA | |
| 5 | ~ | ~ | O̸ | ~ | ~ | O̸ |
| 6 | 40.1 | 3.6 | 0.1 X | NA | NA | |
| HIV+ patient # | ||||||
| 1 | NA | NA | ~ | 0.2 | c | |
| 2 | NA | NA | 0.4 | 3.2 | 8.0 X | |
| 3 | NA | NA | 3.6 | 8.0 | 2.2 X | |
| 4 | NA | NA | 4.2 | 7.3 | 1.7 X | |
| Healthy control # | ||||||
| 1 | 0.7 | 48.8 | 69.7 X | NA | NA | |
| 2 | 0.2 | 15.0 | 75.0 X | 4.9 | 7.4 | 1.5 X |
| 3 | 0.8 | 22.1 | 27.6 X | ~ | 0.3 | c |
| 4 | 0.5 | 12.3 | 24.6 X | NA | NA | |
| 5 | ~ | ~ | O̸ | NA | NA | |
| 6 | ~ | ~ | O̸ | NA | NA | |
| 7 | ~ | ~ | O̸ | 8.6 | 6.6 | 0.7 X |
| 8 | ~ | ~ | O̸ | NA | NA | |
| 9 | ~ | 2.6 | c | 2.6 | 47.1 | 18.1 X |
| 10 | ~ | ~ | O̸ | NA | NA | |
Notes:
No amplification
NA, Not available
-, undetectable
JCV VP1p36 and VP1p100-specific CD8+T cells detectable after peptide-loaded DC stimulation only.
The amplification factor, defined as the ratio between frequency of JCV specific CTL frequency after stimulation with peptide-loaded DC versus peptide alone, and varies from 0.1 to 75 X in patients with detectable JCV response.
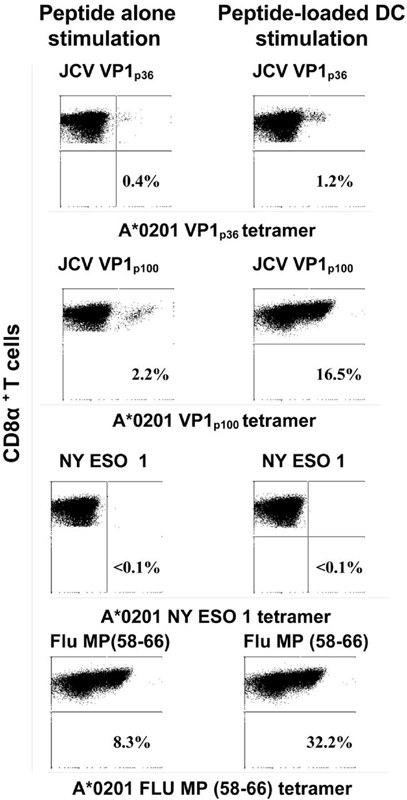
In 1 PML patient and 4 HC, we did not observe any JCV-specific CTL response using either method. Strong CTL responses to positive control Flu matrix protein (MP) peptide were elicited using peptide-loaded DC stimulation in all tested cases, while no responses were elicited against negative control NY ESO cancer peptide, as expected (data not shown). A representative example of CTL response elicited by direct peptide or peptide-loaded DC stimulation of lymphocytes is shown in Fig 1.
Figure 1. Comparison of JCV VP1p36, JCV VP1p100, NY ESO 1 and Flu matrix protein-specific CTL responses in PML patient # 2 after stimulation of lymphocytes with peptide alone or peptide-loaded autologous DC.
Staining of peripheral blood lymphocytes from PML patient #2 with tetrameric HLA-A*0201 JCV VP1p36, JCV VP1p100, NY ESO 1, and Flu matrix protein complexes is shown after in vitro stimulation with either the peptide alone or with autologous peptide-loaded DC for 10 to 14 days. The percentage of CD8α+ T cells that bind the tetramer (right upper quadrant of each panel) is indicated. An increase in JCV epitope-specific CTL and Flu matrix protein positive control could be seen using peptide-loaded DC compared to peptide stimulation alone, while no CTL response could be elicited against negative control cancer epitope NY ESO 1 using either method
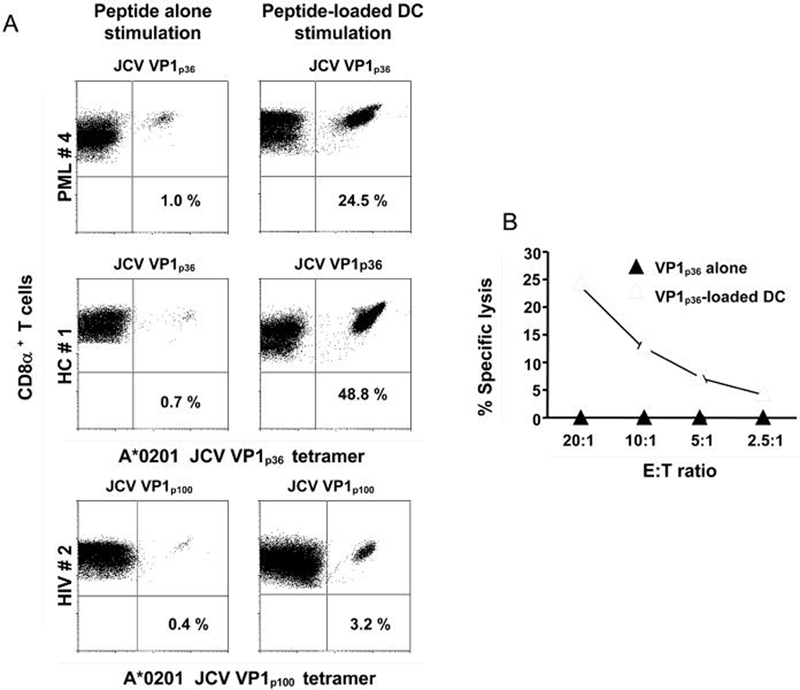
Interestingly, among the PML group, we detected the strongest JCV-specific CTL response using DC, in PML patient #4 diagnosed only two months before testing. In contrast, PML #5 who developed PML 15.2 years before testing had no detectable JCV-specific CTL response with either peptide or peptide-loaded DC stimulation. Only two study subjects, PML patient #6 and HC #7 had a stronger CTL response against JCV epitopes using peptide alone compared to peptide-pulsed DC stimulation. A representative example of the increase in the JCV VP1p36 or JCV VP1p100- specific CTL response obtained using peptide-loaded DC compared to peptide alone stimulation of lymphocytes is shown in Fig 2A, for one subject of each study group. To verify the function of the tetramer-staining cells, a 51Cr release assay was performed after both types of stimulation in sample from HC#1 (Fig 2B). Only the peptide-loaded DC stimulation was potent enough to generate a sufficient number of functionally active effector CD8+ T-cells that could be detected by the 51Cr assay. While peptide-specific CTL are usually undetectable in fresh blood samples in most individuals (Du Pasquier et al., 2003) our findings suggest that stimulation of peripheral blood lymphocytes in vitro with peptide-loaded autologous DC elicit in most cases a stronger CTL response than stimulation with peptide alone in individuals of the 3 study groups. The lack of any detectable JCV CTL expansion in lymphocytes of 4 HC and 1 PML patient could be explained by an immune response directed toward other JCV epitopes or by a very low frequency of JCV epitope-specific CTL response in fresh blood. Our results confirm and expand previous reports showing that phenotypic and functional maturation of monocyte-derived DC from HIV-1 infected individuals is similar to healthy volunteers (Hsieh et al., 2003).
Figure 2. JCV peptide-specific CTL response are enhanced in subjects from 3 study groups by stimulating peripheral blood lymphocytes with JCV peptide-loaded autologous DC compared to peptide alone.
Panel A
Staining of peripheral blood lymphocytes from PML #4, HIV #2, HC #1 with tetrameric HLA-A*0201/JCV VP1p36 or VP1p100 complexes is shown after in vitro stimulation with either the JCV VP1p36 or VP1p100 peptide alone or with JCV VP1p36 or VP1p100-loaded autologous DC for 10 to 14 days. The percentage of CD8α+ T cells that bind the tetramer (right upper quadrant of each panel) are indicated.
Panel B
Stimulated lymphocytes from HC #1 were then assessed for the presence of functionally active effectors cells in a 51Cr release assay. Lysis of JCV VP1p36-pulsed autologous B lymphoblastoid cell line (B-LCL) could be detected only using lymphocytes from HC# 1 stimulated with JCV VP1p36 -loaded DC but not by those stimulated with peptide alone. The percentage of specific lysis indicates the difference in specific 51Cr release between target cells pulsed with JCV VP1p36 peptide and those pulsed with an irrelevant control peptide18). Effector target (E: T) ratios are shown.
Discussion
Since there is no specific antiviral treatment against JCV, enhancement of the cellular immune response against the virus may represent an important therapeutic option for PML. Indeed, we have demonstrated that an early detection of JCV-specific CTL has an 87% predictive value for subsequent control of PML (Du Pasquier et al., 2004). In addition, these cells have an effector memory phenotype early after disease onset (Lima et al., 2007) and are present in the cerebrospinal fluid of PML survivors (Du Pasquier et al., 2005). Furthermore, CD8+ T-cells tend to aggregate around JCV infected glial cells in PML lesions (Wuthrich et al., 2006). Our current results suggest that it may be possible to significantly expand the JCV-specific CTL response in PML patients even during the early stage of the disease. In turn, these CTL may penetrate into the brain where they may destroy virus-infected glial cells and help contain PML. This is to our knowledge the first demonstration that JCV-specific CTL can be elicited by DC stimulation of lymphocytes. This is remarkable since the frequency of JCV-specific memory T cells is very low in the peripheral blood, in some cases less than 1/100,000 PBMC (Du Pasquier et al., 2003). Furthermore, these results also demonstrate that CTL specific for a pathogen different than HIV can be elicited in immunosuppressed HIV-infected patients. It could be argued that those patients who are able to mount a CTL response against JCV will not need an additional immunotherapy for PML. However we have observed that this response may not be constant and that relapses of PML do occur (Du Pasquier et al., 2004). In addition, PML survivors are often left with devastating neurological sequellae. Therefore, our goal would be to administer the DC-based immunotherapy to PML patients soon after diagnosis to prevent the occurrence of irreversible neurological dysfunction. While the most dramatic effect of peptide-loaded DC were observed in healthy controls, a new category of patients at risk for PML include individuals with MS and Crohn's disease treated with natalizumab (Berger and Koralnik, 2005). We have previously shown that the baseline response to JCV in MS patients is at least as good as healthy controls (Du Pasquier et al., 2006.) Therefore, such patients may also benefit from DC-based immunotherapy.
Our study of in vitro expansion of JCV-specific CTL by peptide-loaded autologous DC is novel and constitutes the proof of concept necessary to bring DC-based immunotherapy for PML to the clinical stage. The potential benefits of such immunotherapy in patients with PML are substantial since a persistent JCV-specific CTL response may limit the extent of the neurological damage caused by JCV and eventually improve their survival.
Materials and methods
JCV serology was determined by HIA or ELISA as previously described (Miller et al., 1983; Viscidi et al.,2003).
For direct peptide stimulation, peripheral blood lymphocytes were isolated by centrifugation on Ficoll-diatrizoate gradient and cultured in RPMI-12% fetal calf serum with A*0201-restricted JCV VP1p36 (Du Pasquier et al., 2003)and VP1p100 (Koralnik et al., 2002)or control epitope peptides NY ESO 1(Velazquez et al., 2007) and Flu MP(58-66) (Dhodapkar and Bhardwaj, 2002) at a concentration of 1 to 2.5 μg/ml, and after 3 days, with 25 U of rhIL-2/ml.
For peptide-loaded DC stimulation, DC were derived from peripheral blood mononuclear cells (PBMC) using two different methods. For PML patients #2 to #5 and all HC, PBMC were plated and allowed to adhere to plastic for 1-2 hours at 37°C in 5% pooled human serum (HPS). For PML patient #1 and all HIV+ patients monocytes were isolated from PBMC using CD14 Human Microbeads (MACS, Milteny Biotech). Plastic-adherent or CD14+ cells (monocyte-enriched fraction) were cultured as previously described (O'Neill and Bhardwaj, 2005). On day 7, DC had a mature phenotype assessed based on three parameters:
Morphology: Mature DC were loosely attached to the wells, veiled and clustered,
Immunophenotyping: Phenotypic analysis of immature and mature DC was performed by flow cytometry. Briefly, DC were considered mature when they were CD83+, CD80+, CD86+, MHC class I+, MHC class II+, CCR7 +.
Mixed Leukocyte Reaction: In addition, the functionality of mature DC was tested in each category of study subjects in a mixed lymphocyte reaction. (Waeckerle-Men et al., 2004).
Mature DC were incubated with 1 to 2.5 μg/ml of JCV or control peptide for 2 to 4 hours at 37°C.
After washing, DC were resuspended in RPMI-5% HPS and mixed with autologous non adherent cells or CD14- fraction (T cells enriched fraction) at a ratio 1:10 and supplemented with IL2 (50 U/ml) starting at day 3. After 10-14 days in culture, lymphocytes stimulated by peptide alone of peptide-loaded autologous DC were tested for the presence of peptide-specific CTL by tetramer staining and 51Cr release assay as previously described (Du Pasquier et al., 2004).
Statistical analysis
The Wilcoxon matched-paired test was employed to calculate the difference between the median of JCV-specific CTL response for both epitopes in peptide-loaded DC versus peptide alone stimulation of lymphocytes in all 15 responders. This non parametric test was used since the data were sampled from a non Gaussian distribution. The test was two tailed and α of 0.05 was employed. Calculations were performed with GraphPad prism 5.0 for PC (GraphPad Software, San Diego, CA).
Acknowledgements
This work was supported in part by Public Health Service Grants R01 NS/AI 041198 NS 047029, and K24 NS 060950 to IJK.
References
- 1.Adams S, O'Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005;25(3):177–88. doi: 10.1007/s10875-005-4086-2. [DOI] [PubMed] [Google Scholar]
- 2.Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, Grisetti S, Moretti F, Vigo B, Bongiovanni M, Del Grosso B, Arcidiacono MI, Fibbia GC, Mena M, Finazzi MG, Guaraldi G, Ammassari A, d'Arminio Monforte A, Cinque P, De Luca A, Italian Registry Investigative Neuro AIDS Study Group Clinical epidemiology and survival of progressive multifocal leucoencepalophathy in the era of highly active antiretroviral therapy: Data from the Italian Registry Investigative Neuro AIDS (IRINA) J Neurovirol. 2003;9(Suppl 1):47–54. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353(4):414–6. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 4.De Luca A, Ammassari A, Cingolani A, Giancola ML, Antinori A. Disease progression and poor survival of AIDS-associated progressive multifocal leukoencepalophathy despite highly active antiretroviral therapy. AIDS. 1998;12:1937–1938. [PubMed] [Google Scholar]
- 5.Dhodapkar MV, Bhardwaj N. Active immunization of humans with dendritic cells. J Clin Immunol. 2000;20(3):167–74. doi: 10.1023/a:1006681312249. [DOI] [PubMed] [Google Scholar]
- 6.Dhodapkar MV, Young JW, Chapman PB, Cox WI, Fonteneau JF, Amigorena S, Houghton AN, Steinman RM, Bhardwaj N. Paucity of functional T-cell memory to melanoma antigens in healthy donors and melanoma patients. Clin Cancer Res. 2000;6(12):4831–8. [PubMed] [Google Scholar]
- 7.Du Pasquier RA, Autissier P, Zheng Y, Jean-Jacques J, Koralnik IJ. Presence of JC virus-specific CTL in the cerebrospinal fluid of PML patients: rationale for immune-based therapeutic strategies. AIDS. 2005;19(19):2069–76. doi: 10.1097/01.aids.0000194804.97164.86. [DOI] [PubMed] [Google Scholar]
- 8.Du Pasquier RA, Kuroda MJ, Schmitz JE, Zheng Y, Martin K, Peyerl FW, Lifton M, Gorgone D, Autissier P, Letvin NL, Koralnik IJ. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1 (p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J Virol. 2003;77(22):11918–26. doi: 10.1128/JVI.77.22.11918-11926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127(Pt 9):1970–8. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 10.Du Pasquier RA, Stein MC, Lima MA, Dang X, Jean-Jacques J, Zheng Y, Letvin NL, Koralnik IJ. JC virus induces a vigorous CD8+ cytotoxic T cell response in multiple sclerosis patients. J Neuroimmunol. 2006;176:181–6. doi: 10.1016/j.jneuroim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 12.Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol. 2001;7(4):353–7. doi: 10.1080/13550280152537238. [DOI] [PubMed] [Google Scholar]
- 13.Hall C, Dafni D, Simpson U, Clifford D, Wetherill D, Cohen B, McArthur J, Hollander H, Yainnoutsos C, Major E, Millar L, Timpone J, AIDS Clinical Trials Group 243 Team Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. N Engl J Med. 1998;339(19):1345–61. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh SM, Pan SC, Hung CC, Chen MY, Chang SC. Differential impact of late-stage HIV-1 infection on in vitro and in vivo maturation of myeloid dendritic cells. J Acquir Immune Defic Syndr. 2003;33(4):413–9. doi: 10.1097/00126334-200308010-00001. [DOI] [PubMed] [Google Scholar]
- 15.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: Has the disease outgrown its name? Ann Neurol. 2006;60(2):162–73. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 17.Koralnik IJ, Boden D, Mai VX, Lord SCI, Letti NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52(2):253–60. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 18.Koralnik IJ, Du Pasquier RA, Kuroda M, Schmitz JE, Dang X, Zheng Y, Lifton M, Letvin NL. Association of prolonged survival in HLA-A2+ Progressive Multifocal Leukoencephalopathy patients with a cytotoxic T lymphocyte response specific for a dominant JC virus epitope. J Immunol. 2002;168:499–504. doi: 10.4049/jimmunol.168.1.499. [DOI] [PubMed] [Google Scholar]
- 19.Lima MA, Marzocchetti A, Autissier P, Tompkins T, Chen Y, Gordon J, Clifford DB, Gandhi RT, Venna N, Berger JR, Koralnik IJ. Frequency and phenotype of JC virus-specific CD8+ T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J Virol. 2007;81(7):3361–8. doi: 10.1128/JVI.01809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 21.Marra CM, Rajicic N, Barker DE, Cohen BA, Clifford D, Donovan Post MJ, Ruiz A, Bowen BC, Huang ML, Queen-Baker J, Andersen J, Kelly S, Shriver S, Adult AIDS Clinical Trials Group 363 Team A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS. 2002;16(13):1791–7. doi: 10.1097/00002030-200209060-00012. [DOI] [PubMed] [Google Scholar]
- 22.Miller N, Major EO, Wallen WC. Transfection of human fetal glial cells with molecularly cloned JCV DNA. Prog. Clin. Biol. Res. 1983;105:29–40. [PubMed] [Google Scholar]
- 23.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72(12):9918–23. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill D, Bhardwaj N. Generation of autologous peptide- and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods Mol Med. 2005;109:97–112. [PubMed] [Google Scholar]
- 25.Royal W, 3rd, Dupont, McGuire B, Chang D, Goodkin L, Ernst T, Post MJ, Fish D, Pailloux G, Poncelet H, Concha M, Apuzzo L, Singer E. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9(3):411–9. doi: 10.1080/13550280390201740. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O'Neill D, Zavilevich K, Albukh T, Christos P, Mazumdar M, Pavlick A, Polsky D, Shapiro R, Berman R, Spira J, Busam K, Osman I, Bhardwaj N. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;12:7–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Viscidi RP, Rollison DE, Viscidi E, Clayman B, Rubalcaba E, Daniel R, Major EO, Shah KV. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin Diagn Lab Immunol. 2003;10(2):278–85. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waeckerle-Men Y, Scandella E, Uetz-Von Allmen E, Ludewig B, Gillessen S, Gillessen S, Merkle HP, Gander B, Groettrup M. Phenotype and functional analysis of human monocyte-derived dendritic cells loaded with biodegradable poly(lactide-co-glycolide) microspheres for immunotherapy. J Immunol Methods. 2004;287(12):109–24. doi: 10.1016/j.jim.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Walsh SR, Bhardwaj N, Gandhil RT. Active immunization of humans with dendritic cells. J Clin Immunol. 2000;20(3):167–74. doi: 10.1023/a:1006681312249. [DOI] [PubMed] [Google Scholar]
- 30.Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic CJ, Schulz-Schaeffer WJ, Kretzschmar HA, Enzensberger W, Hunsmann G, Lüke W. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis. 1997;176(1):250–4. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 31.Wuthrich C, Kesari S, Kim W, Williams K, Gelman K, Elmeric D, De Girolami U, Joseph JT, Hedley-Whyte T, Koralnik IJ. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: co-localization of CD8(+) T cells with JCV-infected glial cells. J Neurovirol. 2006;12(2):116. doi: 10.1080/13550280600716604. [DOI] [PubMed] [Google Scholar]