Abstract
We have developed a tetracycline-repressible female-specific lethal genetic system in the vinegar fly Drosophila melanogaster. One component of the system is the tetracycline-controlled transactivator gene under the control of the fat body and female-specific transcription enhancer from the yolk protein 1 gene. The other component consists of the proapoptotic gene hid under the control of a tetracycline-responsive element. Males and females of a strain carrying both components are viable on medium supplemented with tetracycline, but only males survive on normal medium. A strain with such properties would be ideal for a sterile-insect release program, which is most effective when only males are released in the field.
There is increasing interest in biological methods for control of insect pests, in part because of increasing resistance to chemical insecticides and other factors such as environmental effects of insecticides. A biological method that has proven to be effective in the field for the area-wide control of some insects is called the sterile insect technique (SIT; ref. 1). SIT involves raising large numbers of insects that are then sterilized before field release. If sufficient numbers of competitive insects are released, most of the wild females in the field mate with the released sterile males and thus produce no viable offspring (1, 2). SIT can result in suppression or eradication of the target insect (1, 2). Successful past SIT programs include the eradication of screwworm from North America (2), tsetse fly from Zanzibar (3), Queensland fruit fly from Western Australia (4), and melon fly from the Okinawa islands (5). SIT has also been used for eradication or suppression of the Mediterranean fruit fly (medfly) in various parts of the world (1, 6).
For medfly, SIT has been shown to be most effective when only sterile males are released in the field (7). Current medfly SIT programs use so-called “genetic sexing strains” that facilitate the large-scale separation of males from females (8). The strains are made by classical genetic methods involving the isolation of Y:autosome translocations, where the translocation carries a dominant wild-type allele for a selectable gene (9). For example, genetic sexing strains have been made that are homozygous for a recessive temperature-sensitive lethal allele on chromosome 5, and males carry a Y:5 translocation that includes a wild-type allele of the temperature-sensitive lethal gene (9). In these genetic sexing strains, only male embryos survive incubation at the nonpermissive temperature. However, the strains can breakdown under mass rearing conditions because of male recombination (8, 9). An alternative method of making a genetic sexing strain is to use genetic engineering (10). Transgenic insects are made by using transposable elements that seem to have a broad host range (10). For example, transgenic medflies have been made by using the Minos (11) and piggyBac (12) transposable elements. Similarly, piggyBac has been used to make transgenic silk moth (13). Further, transgenic mosquitoes (Aedes aegypti) have been made by using the Hermes (14) and mariner (15) transposable elements.
Our aim was to construct a “terminator” gene that, under certain conditions, is lethal to transgenic female flies but otherwise has no effect on either male or female viability. Herein, we report the development of such a system with the vinegar fly Drosophila melanogaster. The terminator gene we choose was the proapoptotic gene head involution defective (hid; ref. 16), because ectopic expression of hid can lead to organismal death caused by induction of apoptosis (16). hid expression was regulated by the tetracycline-controlled transactivator (tTA), which is inactive in the presence of tetracycline (17). Expression of tTA was controlled with the female-specific enhancer from the Drosophila yolk protein 1 (yp1) gene (18). Because the components of the system are either conserved (yolk protein genes; ref. 19) or known to function in both Drosophila and mammalian cells (hid, ref. 20; tTA, refs. 17 and 21), we believe the system could be used to make genetic-sexing strains for a variety of insect pests that can be genetically engineered.
Methods
Construction of yp1-tTA and tetO-hid.
To construct yp1-tTA, a 158-bp DNA fragment containing the female-specific transcription enhancer of the yp1 gene (18) was obtained by PCR with D. melanogaster DNA as template. The forward primer was 5′-ATC TAT ATT TTA TGC ATT TAT TTG ATC-3′, and the reverse primer was 5′-AAT AGA CAC GGG GCC TAC CTA T-3′. The 50-μl reactions contained 200 ng of genomic DNA, 200 nM forward and reverse primers, 200 μM dNTPs, 1.6 mM MgCl2, and 1 unit of eLONGase (Life Technologies, Grand Island, NY) in buffer supplied by the manufacturer. Reactions were heated to 94°C for 3 min then cycled 35 times (30 s at 94°C; 30 s at 47°C; 30 s at 68°C) in a Perkin–Elmer 9600 thermocycler. A product of the correct size was purified by agarose gel electrophoresis, digested with Eco0109I then incubated at 75°C for 10 min to inactivate the enzyme. The DNA was then treated for 15 min at 25°C with the Klenow fragment of DNA polymerase I (New England Biolabs) in buffer supplied by the manufacturer supplemented with 33 mM dNTPs. After incubation at 75°C for 10 min to inactivate the enzyme, the DNA was digested with BclI. The resulting 124-bp fragment was inserted into the BamHI and EcoRV sites of the pBluescript II KS (−)vector (Stratagene). Cloning of the correct fragment was confirmed by DNA sequencing. The fragment containing the yp1 enhancer was excised with NotI and Asp718 and inserted into the NotI and Asp718 sites of the tTA transformation vector W.H.T. (21). W.H.T. is a CaspeR-derived vector with the NotI and Asp718 sites immediately upstream of the hsp70 minimal promoter that is used to drive expression of the tTA coding sequence.
To construct tetO-hid, a 3.9-kilobase EcoRI fragment containing the complete hid ORF (16) was inserted into the EcoRI site of the tetO vector W.T.P.2 (21). W.T.P.2 is also a CaspeR-derived vector that contains seven copies of tetO, a minimal promoter, and a unique EcoRI site between the hsp70 leader and hsp70 poly(A) region.
Drosophila Stocks.
Flies were usually raised on medium that had a high yeast content but contained no added corn meal (100 g of active dry yeast, 100 g of sugar, and 16 g of agar per liter). Alternative medium compositions that were used are described in the text. Crosses were performed at 25°C. All stocks not specifically mentioned have been described by Lindsley and Zimm (22). For germ-line transformation, constructs were coinjected into y w embryos with the Δ2,3 helper plasmid (23) by using the standard procedure (24). Single F1 progeny displaying a nonwhite eye color were backcrossed to y w then bred to homozygosity. Linkage of P [w+] was determined by following w+ segregation in the appropriate crosses.
Recombinant lines carrying both yp1-tTA and tetO-hid constructs were selected by first crossing homozygous yp1-tTA and tetO-hid lines where both lines had insertions on the third chromosome. The virgin female offspring were collected then mated with w; Tb/TM3, Sb males, and 100 male offspring from this cross were mated singly with w; Tb/TM3, Sb females on normal medium and also on medium supplemented with tetracycline (10 μg/ml). Crosses raised on normal medium that lacked w+ females were identified as probable recombinants. Homozygous lines were established by crossing w+ non-Sb males and females. Dissected larvae, pupae, and adults were stained for β-galactosidase activity by using the method of Simon and Lis (25).
Results
The Tetracycline-Controlled Female-Killing System.
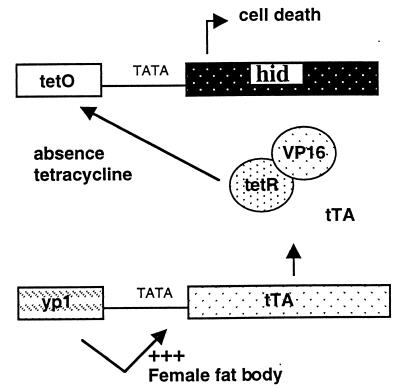
The system was designed such that female flies would die in the absence of tetracycline because of widespread cell death in the fat body. The system is shown schematically in Fig. 1. Expression of tTA is controlled by the female- and fat-body-specific enhancer from the yp1 gene (18). Binding of tTA to tetO results in activation of expression of the proapoptotic gene hid. Induction of apoptosis in fat body results in female-specific lethality, because the fat body is an important tissue for metabolism and food storage in insects. Females are viable when raised on culture medium supplemented with tetracycline, because the antibiotic inhibits the binding of tTA to tetO.
Figure 1.
The tetracycline-regulated female-killing system. Expression of tTA is controlled with the female- and fat-body-specific transcription enhancer from the yp1 gene (18). In the absence of tetracycline, tTA binds to tetO and induces expression of the proapoptotic gene hid. The loss of fat body results in female-specific lethality. In the presence of tetracycline, females are fully viable, because the binding of tTA to tetO is inhibited, switching off hid expression.
To test the system, homozygous tetO-hid and yp1-tTA lines were crossed, and the offspring were raised on either normal medium or medium supplemented with tetracycline (10 μg/ml; Table 1). Thus, the offspring of the crosses carry one copy of each construct. We found that, for most of the crosses raised on normal medium, there was a highly significant decrease in female viability. In particular, for the cross yp1-tTA line 19 with tetO-hid line 53, 99.7% of the offspring were male. Female lethality occurred during the pupal stage. From the crosses where most of the females died (e.g., yp1-tTA line 19 crossed with tetO-hid line 27), the females that emerged either died shortly after eclosion or were sterile and showed a variety of defects such as wing bubbles. We attribute the variable level of female killing to the position of integration affecting the level of expression of either the tTA or hid genes. In contrast, for crosses raised on medium supplemented with tetracycline, we found that males and females were equally viable.
Table 1.
Viability of males and females carrying one copy each of the tetO-hid and yp1-tTA constructs
| yp1-tTA line | tetO-hid line | Tetracycline, 10 μg/ml | No. female | No. male | Percentage male |
|---|---|---|---|---|---|
| 19 | 53 | − | 1 | 330 | 99.7 |
| 19 | 53 | + | 376 | 362 | 49.1 |
| 19 | 27 | − | 18 | 195 | 91.5 |
| 19 | 27 | + | 175 | 138 | 44.1 |
| 19 | 8 | − | 61 | 99 | 61.9 |
| 19 | 8 | + | 46 | 33 | 41.8 |
| 6 | 53 | − | 2 | 89 | 97.8 |
| 6 | 53 | + | 181 | 162 | 47.2 |
| 22 | 53 | − | 216 | 194 | 47.3 |
| 22 | 53 | + | 209 | 189 | 47.5 |
| 30 | 53 | − | 47 | 120 | 71.8 |
| 30 | 53 | + | 165 | 112 | 40.4 |
For an SIT program that typically involves raising millions of flies, it would not be practical to mate separate lines each carrying one of the components of the female-killing system. Therefore, we wanted to determine whether a line could be maintained that carried both components of the system. yp1-tTA line 19 was mated with tetO-hid line 53; recombinant offspring were identified and either bred to homozygosity or maintained with a balancer chromosome. Only males survived when the homozygous line (which carries two copies of each construct) was raised on normal medium, but both males and females survive equally when raised on medium supplemented with tetracycline (Table 2). Further, the homozygous males from the culture raised without tetracycline are fertile. Thus, we conclude that it is possible to maintain a line that contains both components of the female-killing system.
Table 2.
Tetracycline-repressible female-specific lethality of a recombinant line with two copies of the tetO-hid and yp1-tTA constructs
| Tetracycline, 10 μg/ml | Male | Female |
|---|---|---|
| − | 222 | 0 |
| + | 139 | 186 |
The recombinant line was obtained by mating tetO-hid line 53 with yp1-tTA line 19 and selecting for recombinant offspring.
Female- and Fat-Body-Specific Expression of tTA.
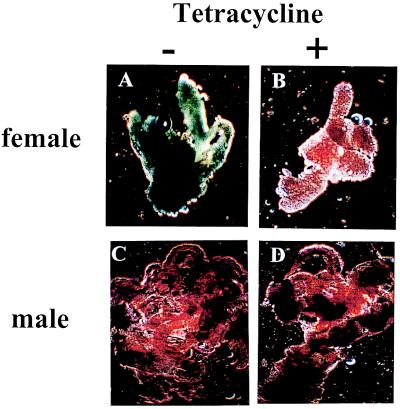
To confirm that in yp1-tTA lines tTA was expressed in the female fat body, yp1-tTA line 19 was crossed with a line carrying a tetO-lacZ reporter gene (21). The offspring of the cross were dissected and stained for β-galactosidase activity. We found strong β-galactosidase expression in the fat body of female larvae (Fig. 2), pupae, and adults (data not shown) raised on normal medium but not in females raised on medium that contained tetracycline (Fig. 2). There was little staining in the fat body of male larvae (Fig. 2), pupae, or adults (not shown) raised on either normal medium or medium supplemented with tetracycline (Fig. 2).
Figure 2.
Expression of tTA is confined to the female fat body and is inhibited by tetracycline. Climbing third instar larvae were sexed, dissected, and stained for β-galactosidase expression (25). Strong staining was seen in fat body of female larvae raised on normal medium (A) but not medium that contained tetracycline (10 μg/ml; B). Little staining above background level was seen in fat body from male larvae raised on either normal medium (C) or medium supplemented with tetracycline (D).
A Yeast-Rich Diet Is Essential for Induction of Female Lethality.
Female D. melanogaster that are starved from eclosion show a basal level of yolk protein synthesis that is rapidly induced by supplying a normal diet (26). The control element for this diet-dependent response was mapped to an 890-bp fragment upstream of the yp1 gene (26). Further studies showed that the nutritional response could be mediated by any of several smaller fragments of the 890-bp fragment, including the 124-bp enhancer used in this study (27). In the experiments described above, the flies were raised on a relatively rich medium that contained 100 g of active dried yeast per liter. Because the cost of the culture medium can be significant in a SIT program (1), we wanted to determine whether the female-killing system was affected by diet. yp1-tTA line 19 was mated with tetO-hid line 53 and raised on medium that contained a low, intermediate, or high amount of yeast. We found that the efficiency of the female-killing system increased with the level of yeast in the diet (Table 3). Addition of corn meal to low-yeast medium did not affect female viability. Thus, efficient induction of female lethality depends on diet, particularly the level of yeast in the culture medium.
Table 3.
Medium with a high yeast content is required for efficient induction of female-specific lethality
| Medium type* | Yeast, g/liter | Cornmeal, g/liter | No. male† | No. female† |
|---|---|---|---|---|
| High yeast | 100 | 0 | 330 | 1 |
| Intermediate yeast | 62 | 0 | 103 | 1 |
| Low yeast | 32 | 0 | 201 | 205 |
| Intermediate yeast + cornmeal | 62 | 107 | 235 | 5 |
| Low yeast + cornmeal | 32 | 107 | 170 | 226 |
All types of culture medium contained 100 g of sugar and 16 g of agar per liter.
Males and females are the offspring of a cross between yp1-tTA line 19 with tetO-hid line 53 and thus carry one copy of each construct.
Discussion
We have developed a repressible female-specific lethal system that under certain conditions results in complete female lethality. Further, we have maintained a strain homozygous for both components of the system for several generations on medium supplemented with tetracycline. When transferred to medium without tetracycline, the males that emerge are viable and fertile. Such properties are suitable for a strain that is to be used in a sterile release program. Ideally, it would be preferable if female-specific lethality occurred at the embryonic stage rather than pupal stage, because of the costs associated with raising large numbers of larvae. However, such a system would require a female-specific promoter or enhancer that is expressed earlier in development than the yolk protein genes. Although the system has been developed to make a strain suitable for a sterile release program, it may also be possible to release fertile males to control the target insect, because female viability depends on tetracycline in the diet. From the matings between the released males and females in the field, only male offspring will survive, and these males in turn will produce only male offspring. However, the results presented in this study suggest that the efficiency of this approach could depend on the quality of the diet of the insects in the field. A relatively poor diet may result in survival of some female offspring of the released males, unless the terminator gene is very effective.
The amount of induced ectopic cell death is very sensitive to the level of ectopic hid expression (28), which in the female-lethal system depends directly on the level of tTA expression. Transgene expression is influenced by the local chromatin environment, and tTA expression is controlled by the yp1 enhancer, which may explain why the efficiency of the system depends on the sites of integration of the constructs and the level of yeast in the diet. The position effects could be minimized by bracketing the yp1-tTA and tetO-hid constructs with insulator elements (29). The effect of diet on female lethality is consistent with previous studies that showed that the yp1 fat body enhancer is responsive to diet, particularly yeast (26, 27). It will be of interest to determine whether the diet response is mediated via either the sex-specific double-sex protein or the proteins that bind to the b-zip or w3 sites of the enhancer, because the binding sites for all three proteins are required for enhancer function in vivo (30). Genes involved in the diet response potentially could be identified by carrying out sensitive genetic screens (31) for mutations that either enhance female lethality on a low-yeast diet or suppress lethality on a high-yeast diet. Such screens potentially could also identify genes that act downstream of hid in the induction of apoptosis in fat body. The efficiency of the system could potentially be improved by including a second proapoptotic gene such as reaper or grim also controlled by a tetracycline-responsive element. In the central nervous system, midline cells reaper, grim, and hid seem to act cooperatively to induce apoptosis (32, 33). Further, reaper and grim but not hid seem to activate specifically the Drosophila caspase DCP-1 in vivo (34).
Although we have demonstrated that the system is effective in Drosophila, we think it is likely that the system will be applicable to other insects. The tTA is functional in both Drosophila (21) and in mammalian cells (17) and is thus likely to be functional in other insects. Similarly, the Drosophila hid gene has been shown to induce apoptosis in mammalian cells (20). However, it is possible that the Drosophila yp1 enhancer may not retain the correct tissue and sex specificity in other insects. Indeed, the regulatory regions from the housefly yolk protein genes show the correct tissue specificity but not sex specificity in Drosophila (35), suggesting that it might be necessary to isolate the yolk protein genes from the insect species of interest. Yolk protein genes have been isolated from a number of insect species including the medfly (36). The availability of these genes, methods for germ-line transformation (11, 12), and the current use of SIT to control the medfly make this species attractive for testing the repressible female-lethal genetic system. Our results suggest that culture medium will be an important consideration in developing this system in other insects.
After submission of this article, a similar system for controlling female viability was reported by Thomas et al. (37). In their system, the female- and fat-body-specific enhancer from the yolk protein 3 (yp3) gene (38) was used to drive expression of tTA. The terminator gene regulated by tTA is Ras64Bval12, which encodes a constitutively active Ras, a key component of the receptor tyrosine kinase signaling pathway (39). Thomas et al. (37) report 100% lethality for females carrying one copy of each of the yp3-tTA and tetO-Ras64Bval12 constructs when raised on normal food that lacks tetracycline (37). It is difficult to compare the efficiency of the two female-killing systems directly. Both yp3-tTA lines tested by Thomas et al. (37) were equally effective, which may indicate that the Ras64Bval12 gene is a more effective terminator than the hid gene. Additionally, the yp3 enhancer may be stronger or less sensitive to position effects than the yp1 enhancer used in this study. However, the two yp3-tTA lines tested by Thomas et al. (37) were chosen on the basis of strong expression of the white+ marker gene (D. D. Thomas and L. S. Alphey, personal communication), and thus, the yp3-tTA construct may have integrated into sites that were favorable for high levels of tTA expression. Further, the medium used contained high levels of yeast (D. D. Thomas and L. S. Alphey, personal communication). Like the yp1 enhancer, the yp3 enhancer is also responsive to diet (40). It will be desirable to compare the two female-killing systems directly by crossing a yp1-tTA line with a tetO-Ras64Bval12 terminator line and also crossing a yp3-tTA line with a tetO-hid line on normal medium that contains either low or high yeast.
Acknowledgments
We express great appreciation to Steve Dobson for use of his computer in preparing a draft of the manuscript and for comments on the manuscript. We also thank Catherine Day, John Tweedie, and Rebecca Henry for comments on the manuscript. We are grateful to Dean Thomas, Luke Alphey, and Mary Bownes for communicating information that was important for completing the revised version of the manuscript. We are grateful to Hermann Steller and Bruno Bello for gifts of plasmid DNA. This work was supported by a grant from Wool Pro to M.J.S.
Abbreviations
- tTA
tetracycline-controlled transactivator
- SIT
sterile insect technique
- medfly
Mediterranean fruit fly
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140142697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140142697
References
- 1.Gilmore J E. In: Fruit Flies: Their Biology, Natural Enemies and Control. Robinson A S, Hooper G, editors. 3B. Amsterdam: Elsevier; 1989. pp. 353–363. [Google Scholar]
- 2.Knipling E F. Sci Am. 1960;203(4):54–61. doi: 10.1038/scientificamerican1060-54. [DOI] [PubMed] [Google Scholar]
- 3.Vreysen M J B, Salch K M, Ali M Y, Abdulla A M, Zhu Z-R, Juma K G, Dyck A, Msangi A R, Mkonyi P A, Feldman H U. J Econ Entomol. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Fisher K T. In: Fruit Flies and the Sterile Insect Technique. Calkins C O, Klassen W, Liedo P, editors. Boca Raton, FL: CRC; 1994. pp. 237–246. [Google Scholar]
- 5.Kakinohana H, Kuba H, Kohama T, Kinjo K, Taniguchi M, Nakamori H, Tanahara A, Sokei Y. Jpn Agric Res Q. 1997;31:91–100. [Google Scholar]
- 6.Hendrichs J, Franz G, Rendon P. J Appl Entomol. 1995;119:371–377. [Google Scholar]
- 7.McGinnis D O, Tam S, Grace C, Miyashita D. Ann Entomol Soc Am. 1994;87:231–240. [Google Scholar]
- 8.Robinson, A. S., Franz, G. & Fisher, K. (2000) Trends Entomol., in press.
- 9.Franz G, Gencheva E, Kerremans P. Genome. 1994;37:72–82. doi: 10.1139/g94-009. [DOI] [PubMed] [Google Scholar]
- 10.O'Brochta D A, Atkinson P W. Sci Am. 1998;279(6):60–65. doi: 10.1038/scientificamerican1298-90. [DOI] [PubMed] [Google Scholar]
- 11.Loukeris T G, Livadaras I, Arcà B, Zabalou S, Savakis C. Science. 1995;270:2002–2005. doi: 10.1126/science.270.5244.2002. [DOI] [PubMed] [Google Scholar]
- 12.Handler A M, McCombs S D, Fraser M J, Saul S H. Proc Natl Acad Sci USA. 1998;95:7520–7525. doi: 10.1073/pnas.95.13.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toshiki T, Chantal T, Corinne R, Toshio K, Eappen A, Mari K, Natuo K, Jean-Luc T, Bernard M, Gerard C. Nat Biotechnol. 2000;18:81–84. [Google Scholar]
- 14.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates C J, Jasinskiene N, Miyashiro L, James A A. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grether M E, Abrams J M, Agapite J, White K, Steller H. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garabedian M J, Shepherd B M, Wensink P C. Cell. 1986;45:859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- 19.Sappington T W, Raikhel A S. Insect Biochem Mol Biol. 1998;28:277–300. doi: 10.1016/s0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 20.Haining W N, Carboy-Newcomb C, Wei C L, Steller H. Proc Natl Acad Sci USA. 1999;96:4936–4941. doi: 10.1073/pnas.96.9.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bello B, Resendez-Perez D, Gehring W J. Development (Cambridge, UK) 1998;125:2193–2202. doi: 10.1242/dev.125.12.2193. [DOI] [PubMed] [Google Scholar]
- 22.Lindsley D L, Zimm G G. The Genome of Drosophila melanogaster. New York: Academic; 1992. [Google Scholar]
- 23.Laski F A, Rio D C, Rubin G M. Cell. 1986;44:7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- 24.Rubin G M, Spradling A C. Science. 1982;218:348–352. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 25.Simon J A, Lis J T. Nucleic Acids Res. 1987;15:2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bownes M, Scott A, Shirras A. Development (Cambridge, UK) 1988;103:119–128. doi: 10.1242/dev.103.1.119. [DOI] [PubMed] [Google Scholar]
- 27.Søndergaard L, Mauchline D, Egetoft P, White N, Wulff P, Bownes M. Mol Gen Genet. 1995;248:25–32. doi: 10.1007/BF02456610. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann A, Agapite J, McCall K, Steller H. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 29.Geyer P K. Curr Opin Genet Dev. 1997;7:242–248. doi: 10.1016/s0959-437x(97)80134-7. [DOI] [PubMed] [Google Scholar]
- 30.An W, Wensink P C. EMBO J. 1995;14:1221–1230. doi: 10.1002/j.1460-2075.1995.tb07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon M A, Bowtell D D, Dodson G S, Laverty T R, Rubin G M. Cell. 1991;15:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Schnitzler A, Agapite J, Schwartz L M, Steller H, Nambu J R. Proc Natl Acad Sci USA. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing J P, Zhou L, Schwartz L M, Nambu J R. Cell Death Differ. 1998;5:930–939. doi: 10.1038/sj.cdd.4400423. [DOI] [PubMed] [Google Scholar]
- 34.Song Z, Guan B, Bergman A, Nicholson D W, Thornberry N A, Peterson E P, Steller H. Mol Cell Biol. 2000;20:2907–2914. doi: 10.1128/mcb.20.8.2907-2914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortiglione C, Bownes M. Dev Genes Evol. 1997;207:264–281. doi: 10.1007/s004270050114. [DOI] [PubMed] [Google Scholar]
- 36.Rina M, Savakis C. Genetics. 1991;127:769–780. doi: 10.1093/genetics/127.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas D D, Donnelly C A, Wood R J, Alphey L S. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 38.Ronaldson E, Bownes M. Genet Res. 1995;66:9–17. doi: 10.1017/s0016672300034340. [DOI] [PubMed] [Google Scholar]
- 39.Fortini M E, Simon M A, Rubin G M. Nature (London) 1992;355:559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- 40.Bownes M, Ronaldson E, Mauchline D. Dev Biol. 1996;173:475–489. doi: 10.1006/dbio.1996.0041. [DOI] [PubMed] [Google Scholar]