Abstract
In Drosophila the fruit fly, coincident exposure to an odor and an aversive electric shock can produce robust behavioral memory. This behavioral memory is thought to be regulated by cellular memory traces within the central nervous system of the fly. These molecular, physiological or structural changes in neurons, induced by pairing odor and shock, regulate behavior by altering the neurons’ response to the learned environment. Recently, novel in vivo functional imaging techniques have allowed researchers to observe cellular memory traces in intact animals. These investigations have revealed interesting temporal and spatial dynamics of cellular memory traces. First, a short-term cellular memory trace was discovered that exists in the antennal lobe, an early site of olfactory processing. This trace represents the recruitment of new synaptic activity into the odor representation and forms for only a short period of time just after training. Second, an intermediate-term cellular memory trace was found in the dorsal paired medial neuron, a neuron thought to play a role in stabilizing olfactory memories. Finally, a long-term protein synthesis-dependent cellular memory trace was discovered in the mushroom bodies, a structure long implicated in olfactory learning and memory. Therefore, it appears that aversive olfactory associations are encoded by multiple cellular memory traces that occur in different regions of the brain with different temporal domains.
Keywords: Drosophila, mushroom body, olfactory learning, memory trace
Introduction
Two central goals of neuroscience are to understand how and where memories are stored. Over the last century, a variety of experimental approaches have been used to probe these questions in the brains of various mammalian model organisms. The resolution of these studies has improved from early behavioral experiments in which portions of the brain were removed to the development of electrophysiological experiments, which allowed the activity of individual cells to be analyzed. More recently, insects have become appealing model organisms for the study of memory formation. Quinn et al. (1974) showed that Drosophila melanogaster, the fruit fly, could form associative memories using an aversive conditioning paradigm involving odor and electrical shock. Fruit flies have the added benefit of being amenable to a growing number of genetic manipulations, and, though the scale of their central nervous system (CNS) is much smaller than mammalian organisms, the anatomy of their olfactory system closely mirrors that of vertebrates (Davis, 2004). Since the initial demonstration of olfactory classical conditioning (Tully and Quinn, 1985), the experimental approaches to studying olfactory memory formation in Drosophila have followed a similar trajectory as those used in mammalian studies — from ablation of different brain regions using pharmacological or genetic tools to the use of optical reporters that allow the activity of subsets of cells to be analyzed in response to stimuli in naive and conditioned animals.
The elemental unit of memory formation is the cellular memory trace. It can be defined as the molecular, physiological, or structural changes that occur in neurons to alter their response to the learned environment. The sum of all relevant cellular memory traces is the engram. In Drosophila a variety of experimental approaches have been used in the past to determine which neurons are involved in memory formation. In general, these studies involved mutating a particular gene expressed in a region of the fly brain or using a pharmacological or genetic tool to ablate or inactivate a region of the brain; meanwhile, the effect on memory is observed through behavioral performance. This basic approach utilized in numerous, clever ways has revealed many important features about Drosophila memory. For example, the mushroom bodies (MBs), prominent neural structures in the fly, preferentially express a number of proteins that have been found to be important in memory formation (Nighorn et al., 1991; Han et al., 1992; Skoulakis et al., 1993; Skoulakis and Davis, 1996; Grotewiel et al., 1998; Cheng et al., 2001; Folkers et al., 2006); the integrity of the MBs has been found to be critical for memory formation (Heisenberg et al., 1985; de Belle and Heisenberg 1994); and MB synaptic output is required during acquisition, storage, and retrieval of memories (Dubnau et al., 2001; McGuire et al., 2001; Krashes et al., 2007). While these studies have helped to localize the site of memory formation and established a role for at least some of the proteins necessary to form the engram, they lack the spatial and temporal resolution to determine which specific neurons form cellular memory traces, how the activity of these neurons changes to reflect the learned information, and how long these changes persist.
Recently, a new class of tools has been developed that allow experimenters to look at memory trace formation at the level of individual neurons, and even within distinct regions of a neuron. Genetically encoded fluorescent reporters have been developed to monitor a variety of neural physiological processes, such as changes in intra-cellular calcium concentration, release of synaptic vesicles, changes in cAMP levels, or changes in voltage-sensitive ion channels. In Drosophila, the expression of these powerful tools can be controlled using the Gal4–UAS system. In this system, the yeast Gal4 transcriptional activator is expressed in particular cells within the brain using a tissue-specific promoter, while all cells contain a transgene composed of the Gal4 response element, UAS, coupled to the optical reporter. Once expressed, Gal4 seeks out the UAS element and drives expression of the optical reporter in the same tissue defined by the Gal4 promoter. Using this system, optical reporters can be expressed specifically and reproducibly in a particular neuron or set of neurons. Two reporters commonly used for functional imaging are synapto-pHluorin (spH) (Ng et al., 2002) and G-CaMP (Nakai et al., 2001). spH is a pH-sensitive green fluorescent protein (GFP) reporter that is fused to synaptobrevin, which targets it to synaptic vesicles. When these vesicles fuse with the plasma membrane, the reporter is exposed to the more pH-neutral environment of the synaptic cleft and its fluorescence increases. G-CaMP is one of the calcium-sensitive GFP reporters. When the calcium concentration inside the cell increases, the ions are bound by G-CaMP. This binding causes a conformational change that increases the GFP fluorescence. These tools have been utilized in innovative whole animal preparations, where the fly is completely intact except for a tiny window cut out of the head exoskeleton; in this way, flies can be exposed to a variety of stimuli, while the experimenter monitors the reporter activity. This functional in vivo imaging technique has been used to show how various stimuli are represented in the brains of naive flies and how those representations change after conditioning.
The optically recorded cellular memory traces that have been discovered thus far are consistent with the ideas about memory formation that stem from behavioral, genetic, and brain lesioning studies in Drosophila and other experimental organisms. Functional imaging has shown that memory traces are dynamic; they form and erode over different time courses. This observation correlates with behavioral conditioning which can generate distinct temporal memory phases often classified as short, intermediate, or long term. These different memory phases can have different molecular requirements, such as the necessity for protein synthesis during formation of the most durable forms of long-term memory; functional imaging experiments have additionally shown that some of these proteins and molecular pathways are necessary to form some cellular memory traces. Finally, the use of optical reporters has revealed that memory traces are distributed across different brain regions.
Olfactory-processing circuit in Drosophila
Aversive olfactory conditioning of an animal requires coincident exposure to an odor and an aversive electric shock in order to produce a robust behavioral memory. Therefore, it is critical to have an anatomical understanding of how these two forms of environmental information are received by the CNS. It is important to know which neurons carry this information from the periphery, where they distribute this information in the CNS, and, in particular, in which neurons or neuropil does the odor and shock information intersect. This information provides insights into how associative memory is acquired, stored, and retrieved. Due to the genetic tools available, and the existence of a simple, fairly discrete, and easily identifiable neural architecture, the odor-processing circuitry in the fruit fly has been extensively studied.
The odor information is first received via olfactory receptor neurons (ORNs), which are distributed across the antennae and maxillary palps on the front of the head (for review, see Davis, 2004). For the most part, each of these neurons expresses one and only one olfactory receptor along with a common odorant co-receptor. These receptors bind to volatile odorants in the environment and can lead to excitation or inhibition of the ORN. The ORN’s axonal projections bundle together in the antennal nerve (AN), and transfer the odorant information, via excitatory cholinergic output, to the first odor relay station in the fly brain, the antennal lobe (AL) (Fig. 1). The AL is a large structure which is characterized by ~50 discrete neuropil regions called glomeruli, where ORNs synapse onto either local interneurons or projection neurons (PNs). Even though there is no significant stereotyped arrangement of ORNs on the exterior structures of the head, ORNs with the same olfactory receptor project into the same glomeruli. A particular odor will bind to a particular olfactory receptor, activating its respective ORN, and lead to activation of a discrete set of glomeruli; thus, there is an odor mapping in the AL. The local interneurons are thought to participate in relaying or modulating information between glomeruli. This suggests that the AL may be an early site for modulating or transforming olfactory information before sending it further inside the brain. This processed olfactory information exits the AL via PNs, which have dendritic processes innervating specific glomeruli, and transmits olfactory information into two deeper regions in the brain. PNs send their axons through either of the two antennal cerebral tracts (ACTs). Both tracts have a final destination in a region called the lateral horn, LH, a still uncharacterized region of the brain with respect to olfactory learning. However, one PN tract also innervates a region called the calyx on its way to the lateral horn. The calyx is the dendritic neuropil belonging to the Kenyon cells, the cell bodies of prominent structures called mushroom bodies (MBs). Therefore, odor information leaves the AL and is deposited into the MBs and the lateral horn.
Fig. 1.
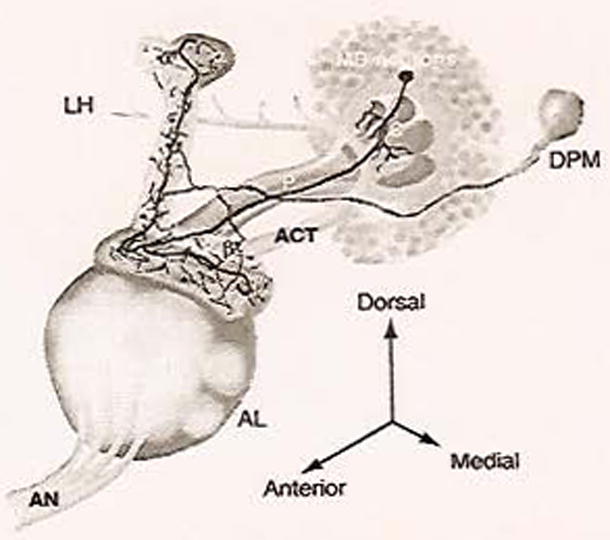
Olfactory processing circuit in Drosophila. A diagram of the important structures in olfactory learning and memory in the fruit fly (one hemisphere of the brain). AN, antennal nerve; ACT, antennal cerebral tract; LH, lateral horn; C, calyx; MB, mushroom body; P, peduncle; DPM, dorsal paired medial neuron. Adapted with permission from Yu et al. (2005). (Sec Color Plate 18.1 in color plate section.)
The MBs are the most extensively studied structures in the fruit fly and are critical for olfactory learning in insects. Approximately 2500 Kenyon cells in the very posterior of each hemisphere of the brain send out their dendrites into the calyx, where they receive olfactory information from cholinergic PNs. Their axons are bundled into the peduncle that extends anteriorly and bifurcates into vertical and horizontal lobes. These 2500 Kenyon cells are grouped into one of the three classes, γ, α′/β′, or α/β, based on the morphology of their bundled axons making up the MB lobes. The MB lobes thus consist of the vertical lobes made of α′ and α fibers, and the horizontal lobes consisting of β and β′ wrapped diffusely with γ (Fig. 1). Thus, odor information flows into the MB neurons via the calyx; presumably it is processed, and then transmitted to MB extrinsic neurons at axonal synapses in the lobes. Studies have identified various neurons that seem to innervate or surround the MB axonal lobes (Ito et al., 1998). However, the majority of these extrinsic neurons have not been genetically or physiologically characterized and a major unanswered question is where and how the odor information is transmitted from the MB neurons.
Conceptually, aversive olfactory learning would require that information about the negatively reinforcing electric shock must make its way to the learning and memory centers of the fruit fly CNS. How shock information is received at the periphery is less well understood than that of odor information. However, significant work has been performed to understand how shock information is conveyed inside the CNS. It was found that synaptic transmission from dopaminergic (DA) neurons that manufacture the neurotransmitter, dopamine, in the fly is required for aversive olfactory conditioning but it is dispensable for reward learning (Schwaerzel et al., 2003). The fact that these neurons have a shock-specific role suggests that shock information is conveyed from DA neurons. Early studies have shown that there are more than a hundred TH neurons scattered in clusters throughout the fly brain (Nassel and Elekes, 1992). Importantly, some of the DA neurons seem to be MB extrinsic neurons and thus have extensive innervation of the MB lobes (Riemensperger et al., 2005). Therefore, the MB lobe neuropil appears to represent a nexus in which odor and shock information coincide, and further supports their role in aversive olfactory learning.
One of the few MB extrinsic neurons that have been characterized anatomically and functionally is the dorsal paired medial (DPM) neuron (Fig. 1; Waddell et al., 2000). There is only one very large DPM neuron per hemisphere of the brain. Each of these seemingly sends only one large process out which moves anteriorly until it reaches the MB lobes, where it branches several times to fully innervate all of the MB lobes. In particular, one branch enters the MB lobe neuropil at the tip of the horizontal lobes, one branch enters toward a region near the branch point of the lobes, and the final branch enters at the tip of the vertical lobes. This architecture suggests that there might be functional significance between these branches and the various regions of the MBs they innervate. Based on membrane staining, there are no obvious dendritic processes for the DPM neuron. This type of anatomical evidence suggests the DPM neuron is a unipolar neuron, and thus may play a role in modulating activity in a MB-DPM local circuit.
Short-term memory traces
Experiments in the honeybee that attempted to localize the sites of memory formation found that the AL was involved in the formation of short-term memories. Specifically, it was demonstrated that inactivation of the ALs, using a probe to cool that region of the brain, blocked memory formation (Erber et al., 1980). Fruit flies, like bees, are able to form short-term memories. Prior to the development of functional imaging tools, much of the research on short-term memories in flies was focused on the MBs, where mutations in a number of proteins highly expressed in this structure inhibited memory when it was tested behaviorally 3 min after conditioning (Davis, 2005). The development of optical imaging provided an important tool to investigate the response properties of neurons, including those outside of the MBs. In the AL, anatomical data combined with receptor mapping studies suggested that odors could be encoded by patterns of activated glomeruli (Vosshall et al., 2000; Jefferis et al., 2001). Thus, functional imaging was used in the AL to determine how various stimuli are represented within this brain structure, and to determine whether a cellular memory trace forms within the AL neurons.
Expression of the calcium-sensitive optical reporter, G-CaMP, or the synaptic release reporter, spH, in the PNs of the AL was used initially, in isolated brain preparations, to determine how these neurons responded to odors. It was found that odors, as predicted, elicited stereotypic responses in overlapping subsets of PNs (Ng et al., 2002; Wang et al., 2003). To expand on these findings, spH was used for functional in vivo imaging of the AL neurons to determine how activity was altered by associative olfactory conditioning (Yu et al., 2004).
Using three different Gal4 drivers, spH expression was driven in each of the three neuron types that make up the AL: ORNs, local interneurons, and PNs. The responses of each neuron class were visualized in the synaptically dense glomeruli of the AL; however, because the flies were essentially intact, with only a small window cut in the cuticle covering the brain, only eight of the glomeruli were visible and identifiable. When flies were stimulated with an olfactory cue, all neuron types were found to respond; importantly, these responses were similar to earlier experiments in that each odor tested elicited a distinct, reproducible pattern of response among the visible glomeruli. Because cellular memory traces are thought to form in the neurons where odor and electric shock intersect, each of the AL neuron types was also tested for responses to shock. Unlike the responses to odor, only the PNs were found to respond to electrical shock. Because PNs responded to odor and shock, it was hypothesized that the PNs could encode a memory trace. Accordingly, the response in the PNs to the conditioned stimuli was recorded before and after training. It was found that, after training, the PNs from some of the glomeruli that were inactive in the naive flies were recruited into the representation of the learned odor. The glomeruli containing the newly recruited PNs were odor-specific and reproducible across flies. It was found that this cellular memory trace formed rapidly with recruitment of new PNs observed as quickly as 3 min after conditioning; the memory trace also faded quickly, as the conditioned response was lost by 7 min. Importantly, the cellular memory trace formed only with a delay conditioning protocol in which the odor was paired temporally with the shock, just as for behavioral conditioning (Fig. 2). Other conditioning protocols, such as odor only, shock only, and trace conditioning, in which the shock follows the odor presentation by a significant delay, were ineffective at producing the cellular memory trace and behavioral conditioning.
Fig. 2.
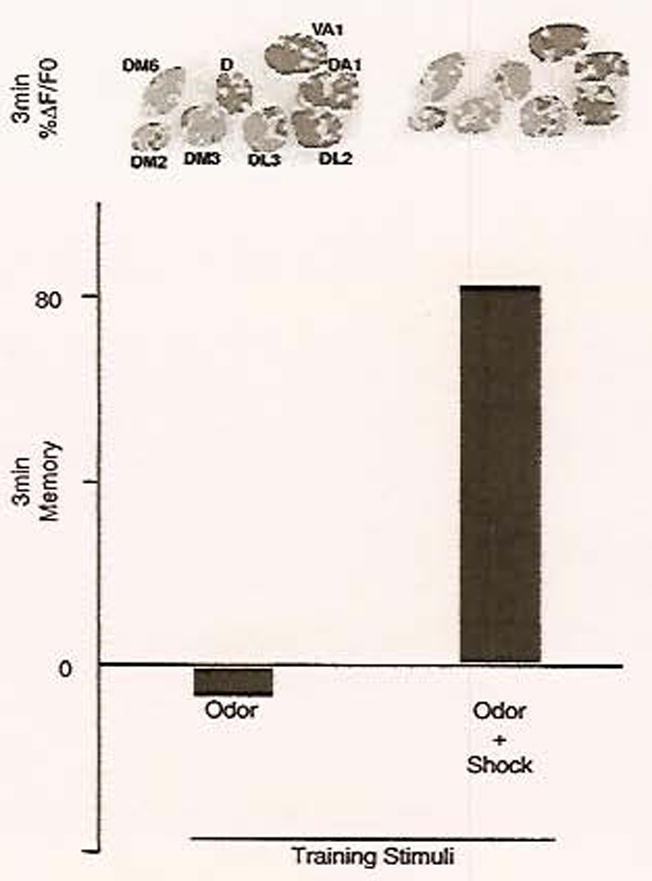
The AL memory trace involves recruitment of new neurons into the representation of the learned odor and is correlated with behavioral memory. Flies were trained in an olfactory classical conditioning paradigm capable of generating short-term memory. Flies were trained with stimuli consisting of either odor alone, odor paired with shock, or other protocols (not shown). After training, flies were tested, either using a behavioral memory task or using functional imaging, for their response to the trained odor. Three-minute memory scores revealed that paired odor and shock, but not odor alone, was sufficient to form memory and behavioral avoidance of the trained odor (scores indicate the percentage of flies that demonstrated learned behavior). In the flies that were tested using functional imaging, changes in the pattern of optical reporter activity were also observed in response to training with odor and shock; specifically, activity in the PNs of glomerulus D was only elicited when these stimuli were delivered simultaneously. Pseudocolor images indicate the percentage change in fluorescence of the optical reporter during odor stimulation. In this preparation, eight glomeruli were visible and identifiable. These results correlate the cellular memory trace with learned behavior. Adapted with permission from Yu et al. (2004). (See Color Plate 18.2 in color plate section.)
In summary, functional imaging revealed a short-term memory trace in the PNs of the AL, which correlated with short-term behavioral memory. This memory trace was formed by the recruitment of previously inactive neurons into the representation of the learned odorant. Furthermore, it revealed that, in the Drosophila brain, memory traces can be formed in integrative neurons outside of the MBs.
Intermediate-term memory traces
Behavioral experiments have suggested that aversive olfactory memory in fruit flies contains a distinct intermediate memory phase, often referred to as middle-term memory. This intermediate memory was first deduced using flies that had a mutation in the amnesiac (amn) gene, which is thought to encode a putative neuropeptide (Tully and Quinn, 1985; Moore et al., 1998). These flies had relatively normal memory immediately after training, but this memory decayed more rapidly than normal within the first 30 min to an hour after training. However, if assayed at later time points, the amn mutant’s memory reaches a level similar to that of wild-type animals. Therefore, amn is considered an intermediate memory gene due to its preferential role at times between short-term and long-term memories.
An important question is which neurons are involved in intermediate memory. The AMN protein was found to be preferentially expressed in the DPM neuron (Waddell et al., 2000). Importantly, transgenically expressing AMN specifically in the DPM neuron of an amn mutant fully rescues the intermediate memory defects. Therefore, this suggests that the DPM neuron plays an important role in intermediate memory. This was further supported by studies utilizing a technique that temporally restricts synaptic transmission in a neuron during defined time points in a behavioral memory assay. This is done by altering the temperature of an animal expressing, within a genetically specified neuron, a temperature-sensitive dominant-negative dynamin protein (shibirets) that is critical for synaptic vesicle recycling and thus synaptic transmission. With this, it has been shown that synaptic transmission from the DPM neuron is not required during training or retrieval of the memory, but is required during a window of time that closely correlates with the intermediate memory phase (Keene et al., 2004). Thus, it seems likely that the DPM neuron, via expression of AMN, functions in intermediate memory in Drosophila.
Due to its important role in intermediate memory, the DPM neuron is a likely site for an intermediate memory trace. This has been addressed using the optical imaging with the intact animal preparation used in the AL studies above. The DPM neuropil that innervates the MB lobes was found to respond to a large variety of odorants in two important ways (Yu et al., 2005). First, odor exposure evoked a temporary increase in intracellular Ca2+ levels, as reported by G-CaMP. This, along with the fact that the DPM neuron responds to many different odors and has no obvious dendritic tree, suggests that the DPM neuron is receiving odor information from the MB neurons and thus it is postsynaptic to the MB lobes. Second, using the spH reporter, odors also evoked synaptic transmission from the DPM neuron. It has been proposed then that this synaptic transmission is releasing neurotransmitters or neuropeptides, such as AMN, back onto the MB axonal fibers. Therefore, it seems likely that the DPM neuron represents a modulatory neuron that is both presynaptic and postsynaptic to the MB neurons.
In addition to odor information, the DPM neuron also responds to electric shock via both increase in Ca2+ levels and synaptic transmission. Therefore, since odor and shock information can intersect within the DPM neuron, aversive olfactory memory traces may form in this neuron. To probe this possibility, flies were trained with an odor and electric shock and then their response to this trained odor was assayed at different times after training. Remarkably, it was found that prior coincidence of electric shock with an odor significantly increased the DPM neuron’s post-response to this odor but not to an odor that was not paired with shock. Given that this altered response is specific only to the trained odor and to pairing an odor with shock, it is clear that a cellular memory trace forms within the DPM neuron.
The DPM memory trace has intriguing characteristics. First of all, this memory trace is delayed in its formation. The response to the trained odor is not altered at 3 or 15 min after training, but appears at 30 min. Thus, this delayed DPM memory trace interestingly coincides with the temporal requirements for synaptic transmission of this neuron for intermediate memory. A second characteristic is that the DPM memory trace is dependent upon AMN expression within the DPM neuron. All of these observations suggest the intriguing possibility that the DPM memory trace may, in part, regulate intermediate memory. Finally, the DPM memory trace is only observed in the DPM neuropil that innervates the vertical branch of the MB lobes (Fig. 3). The role that this branch specificity plays in aversive olfactory memory remains a fascinating unknown.
Fig. 3.
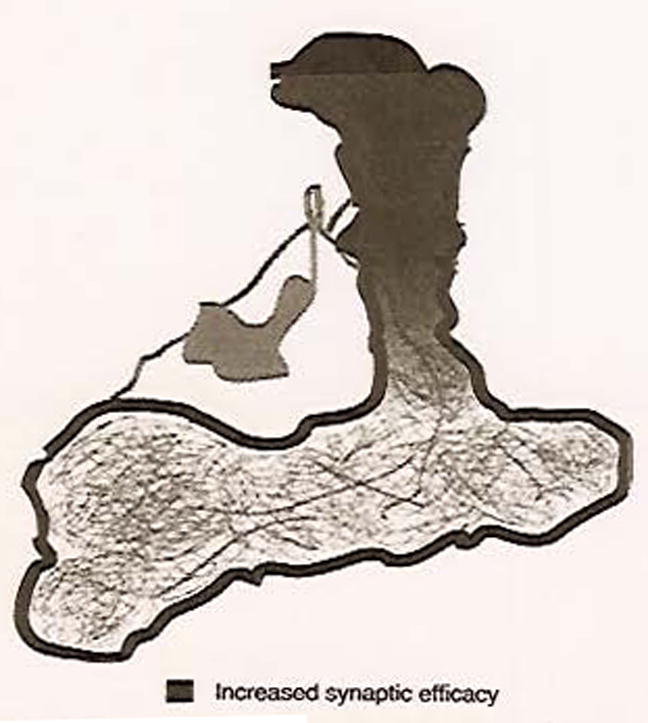
Branch-specific cellular memory trace in the DPM neuron. Diagram of DPM neuron and its innervation of the MB lobes with overlay of branch-specific enhancement of synaptic activity.
Another MB extrinsic memory trace
Riemensperger et al. (2005) have studied stimuli-evoked Ca2+ responses of neurons that are thought to mediate the transmission of shock information to the CNS. By using a Ca2+ fluorescent reporter, it was discovered that DA neurons that innervate the MB lobes respond to odor and electric shock. Therefore, tests were performed to determine whether a memory trace could form in the DA neurons themselves. Unlike in the DPM neuron, the amplitude of the Ca2+ responses to an odor did not change in the DA neurons after pairing the odor with shock. However, the calcium responses to the trained odor had a prolonged duration. This indicates that there is a training-induced net increase in Ca2+ within the DA neurons when exposed to a trained odor, thus suggesting the existence of a DA cellular memory trace. Unfortunately, the training protocol used in this study involved multiple presentations of short pairings and was very different from the more classical aversive olfactory paradigm used in the studies of the AL and the DPM neurons. Therefore, the kinetics of the DA neuron cellular memory trace are more obscure. However, the memory trace appears to form by 15 min after the first pairing of odor and shock.
Long-term memory traces
The MBs have long been a focus of cellular and molecular memory research in Drosophila. An initial role for this anatomically distinctive collection of neurons came from the realization that a number of proteins involved in cAMP regulation are preferentially expressed in the MB; cAMP signaling has been shown in Drosophila and other organisms to be necessary for memory formation (Mayford and Kandel, 1999). Consequently, it was found that ablation of the MBs was sufficient to block memory, while rescue of mutant proteins involved in cAMP regulation by expression of wild-type forms only in the MBs was sufficient to restore memory (Zars et al., 2000; McGuire et al., 2003; Mao et al., 2004). The use of a temperature-sensitive shibire to block synaptic output showed that synaptic activity in the MB neurons was required during acquisition, storage, and retrieval of learned information (Dubnau et al., 2001; McGuire et al., 2001; Schwaerzel et al., 2002; Isabel et al., 2004; Krashes et al., 2007). Olfactory conditioning using a spaced training schedule has been shown to form long-term memories in Drosophila; these memories specifically require protein synthesis (Tully et al., 1994). Subsequently, genetic studies have suggested that this long-term memory is dependent upon the vertical lobes of the MBs (Pascual and Preat, 2001). The development of optical reporters and their use in functional imaging have allowed researchers to examine the function of MB neurons in olfactory perception and in the formation of cellular memory traces.
As with the AL and DPM neurons, the initial application of functional imaging in the Drosophila MBs was focused on determining how these neurons respond to olfactory information. Expression of the G-CaMP reporter was driven in the MBs using the Gal4 system, and the reporter activity was monitored in the calyx and the cell bodies of the MB neurons while olfactory stimulation was delivered (Wang et al., 2004). This technique revealed odor-specific patterns of increasing intracellular calcium concentration in both regions of the MBs. This finding suggested that the spatial code of odor information established in the AL is preserved as that information is transmitted to the MBs.
To determine whether a cellular memory trace was formed in the MB neurons, fluorescence of the G-CaMP reporter was monitored in the horizontal and vertical lobes of the α/β MB neurons (Yu et al., 2006). Changes in the observed G-CaMP fluorescence indicated that both lobes responded to stimulation with odors and electric shock, as would be expected if the neurons were to integrate these stimuli and form a memory trace. To determine whether a memory trace formed, changes in reporter activity were assessed 3, 9, and 24 h after an olfactory conditioning protocol designed to generate either short-term or long-term memory. Only the long-term memory conditioning, consisting of multiple spaced training cycles, was capable of forming a MB cellular memory trace that appeared between 3 and 9 h after training and persisted at 24 h after training. This memory trace took the form of increased amplitude in intracellular calcium levels in response to the learned odor and was, intriguingly mirroring the DPM memory trace, only observed in the α tip of the MB vertical lobe (Fig. 4). This conditioning protocol also generated behavioral memory in trained flies when they were tested at 24 h, which suggests that, under these conditions, the observed MB cellular memory trace underlies the formation of long-term memory. In many organisms, long-term memory has been shown to require protein synthesis as well as a transcription factor, the cyclic AMP response element-binding protein (CREB) (Kandel, 2001). Treatments that interfered with either of these requirements blocked the formation of both the cellular memory trace and the learned behavior when tested at 24 h.
Fig. 4.
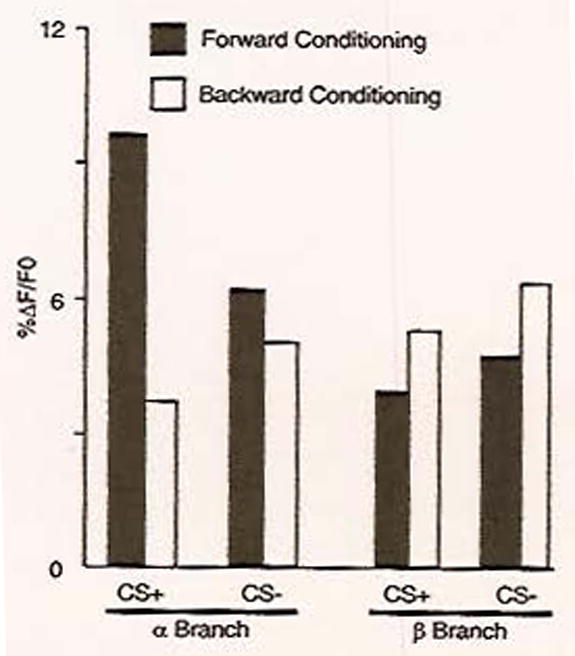
A cellular memory trace in the MB neurons is localized to the vertical branch. The optical reporter G-CaMP was expressed in both the α (vertical) and the β (horizontal) branches of the MB neurons. Flies were conditioned in a behavioral apparatus such that they received either an odor paired with shock (CS+) or an odor not paired with shock (CS−). Forward conditioning, in which the CS + and the shock were presented simultaneously, was spread over multiple, spaced trials to generate long-term memory. As a control, backward conditioning, in which the CS+ loses its predictive value because it is delivered at the end of the shock presentation, was also tested. Twenty-four hours post-conditioning, the reporter’s response to both the CS+ and the CS− odors was measured and quantified as the percentage change in fluorescence. A significant increase in reporter activity was observed only in response to forward conditioning with the CS+ odor and only in the α branch of the MB neurons. Adapted with permission from Yu et al. (2006).
Functional imaging in the Drosophila MBs supports their established role in memory formation. Furthermore, it has demonstrated that a memory trace forms in the vertical lobe of a subset of MB neurons, and only in response to a conditioning schedule that generates long-term behavioral memory. These observations are consistent with an earlier genetic experiment that was performed to focus on the role of specific MB neurons in memory formation (Pascual and Preat, 2001). That study took advantage of a mutant protein, alpha-lobes-absent, which can prevent proper development of the MB vertical lobe. In those flies where the mutation ablated only the vertical lobe, no long-term memory was observed 24 h after training; however, other forms of memory were unaffected.
Concluding thoughts and future considerations
As discussed above, associating an odor with an aversive electric shock can create robust behavioral memories in fruit flies that have characteristic temporal phases. In addition, in vivo imaging techniques have revealed that these temporal phases of behavioral memory might be regulated by discrete cellular memory traces, registered as changes in neuronal activity after training. These functional in vivo data offer important insights. First, they strongly suggest that the overall behavioral memory of an animal is regulated by a combination of memory traces that are located in different regions of the brain. So, the memory of an associative event, like a negatively reinforced odor, is encoded in several different regions of the brain. This is evidenced by a short-term memory trace in the AL, an intermediate memory trace in the DPM neurons, and a long-term memory trace in the MBs. Second, the imaging data imply that the spatially segregated memory traces regulate behavioral memory at different times after the association is made. Therefore, in Drosophila, it appears that different brain structures form cellular memory traces that regulate the behavior of the animal during dedicated temporal domains (Fig. 5). Since the currently observed memory traces do not cover all temporal domains of behavioral memory, such as times between the AL and DPM memory trace (between 7 and 30 min after training), there are presumably unknown cellular memory traces that exist in undefined locations which would regulate the animal’s behavior during these gaps.
Fig. 5.
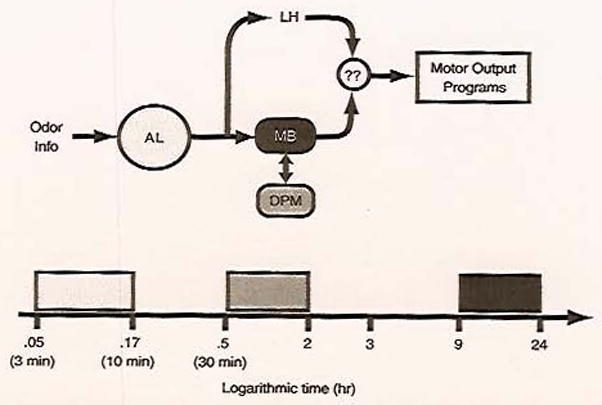
Spatial and temporal cellular memory traces regulate behavior. A diagram illustrating how odor information flowing through the olfactory circuit can be regulated by distinct brain regions to create a conditioned behavioral response. Behavior is posited to be regulated by multiple regions of the brain, with different regions participating during various time windows. AL, antennal lobe; LH, lateral horn; MB, mushroom bodies; DPM, dorsal paired medial; ??, unknown downstream integrator/effector neurons. The light-gray rectangle indicates the period of activity of the PN memory trace, the medium-gray rectangle the period of activity of the DPM neuron memory trace, and the black rectangle the period of the MB memory trace. Presumably, other memory traces not yet discovered participate during period not covered by these three memory traces.
There are still many unanswered questions that will be the focus of future research in the field of functional in vivo imaging in Drosophila. First, all of the optical memory traces observed have only been shown to exist in animals that display behavioral memory and are absent in animals that do not. This is a correlation that needs to be strengthened. This will require novel whole animal preparations that allow the measurement of conditioned behavior while imaging brain activity. In addition, we will need to understand the molecular mechanisms underlying each of the traces so that we can specifically disrupt the cellular memory trace without a non-specific disruption of all neuronal activity. This will allow us to show that a given memory trace is required for a behavioral output in an animal. One would also like to show sufficiency by artificially creating a memory trace and showing the appearance of a conditioned behavior in an untrained fly. For example, this may be possible by experimentally increasing Ca2+ influx or synaptic transmission in the DPM innervation of the vertical MB lobes. Finally, aversive olfactory conditioning may initiate the different cellular memory traces independently, but, due to different maturation rates, they have varying windows in which they effectively guide behavior. Alternatively, a subset of the memory traces may be initiated during training, and in addition to guiding early behavior, they are in some way utilized to copy the relevant learned information into a new more stable medium in a different neuronal structure. This hypothesis would suggest that later-occurring memory traces would be dependent on earlier-born memory traces. Consequently, further research is required to elucidate the nature and role of spatial and temporally distributed cellular memory traces in the context of the entire olfactory learning and memory circuit.
Acknowledgments
Research in the laboratory of the authors is supported by grants from the National Institutes of Health, the Mather’s Charitable Trust, and the R.P. Doherty-Welch Chair in Science to R.L.D.
Abbreviations
- ACT
antennal cerebral tract
- AL
antennal lobe
- AMN
amnesiac
- CNS
central nervous system
- CREB
cyclic AMP response element-binding protein
- CS
conditioned stimulus
- DA
dopaminergic
- DPM
dorsal paired medial
- MB
mushroom body
- ORN
olfactory receptor neuron
- PN
projection neuron
- spH
synapto-pHluorin
References
- Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclin II is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associate odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Erber J, Mashur T, Menzel R. Localization of short-term memory in the brain of the bee Apis mellifera. Physiol, Entomol. 1980;5:343–358. [Google Scholar]
- Folkers E, Waddell S, Quinn WG. The Drosophila radish gene encodes a protein required for anesthesia-resistant memory. Proc, Natl Acad Sci USA. 2006;103:17496–17500. doi: 10.1073/pnas.0608377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem. 1998;5:52–77. [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci USA. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;10:1126–1129. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Moreillo P, Miesenboeek G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Nighorn A, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Heisenberg H, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulakis EM, Davis RL. Olfactory learning in mutants for Leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron. 1996;17:931–944. doi: 10.1016/s0896-6273(00)80224-x. [DOI] [PubMed] [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Alkalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–676. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]


