Abstract
A simple and efficient method for the preparation of pyrimidine 2′-O-hydroxyethoxymethylribonucleosides and 2′-O-hydmxypropoxymethylribonucleosides has been developed. These modified nucleosides were incorporated into oligoribonucleotides, which were shown to form stable RNA/RNA duplexes. The effect of 2′-O-modification in the antisense and sense strands of small interference RNA was evaluated in multi-drug resistant NIH 3T3 cells.
Keywords: Oligoribonucleotides, ONs, RNA/RNA duplexes
Modified oligonucleotides (ONs) are widely used in molecular biology and medicinal chemistry. Antisense agents are valuable tools to inhibit the expression of a target gene in a sequence specific manner, and may be used for functional genomics, target validation and therapeutic purposes.[1] Research in this field increased in impact by the discovery of RNA interference.[2,3]
Numerous attempts have been made to improve the properties of natural ONs. It is believed that the 2′-carbohydrate modifications are the most universal and promising.[4–6] A typical strategy for the preparation of such ONs is the synthesis of modified nucleoside followed by conversion to the corresponding phosphoramidite suitable for automated ON synthesis. Most of these modifications were achieved via alkylation reactions of partially protected ribonucleosides. The heterocyclic bases should be protected in order to avoid their alkylation. For each nucleoside the specific blocking groups are used,[7] which in most cases are not compatible with standard automated ON synthesis. Very recently for the preparation of 2′-O-modified nucleosides we have developed another type of chemistry based on O-glycosylation reactions, which have several important advantages, such as increased yields and simplification.[8,9] A simple and effective method for the preparation of 2′-O-(β-d-ribofuranosyl)nucleosides starting from readily available 3′,5′-O-blocked N-acylribonucleosides and 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d-ribofuranose preactivated with tin tetrachloride in 1,2-dichloroethane at 0°C, has been recently developed.[10–12]
RESULTS AND DISCUSSION
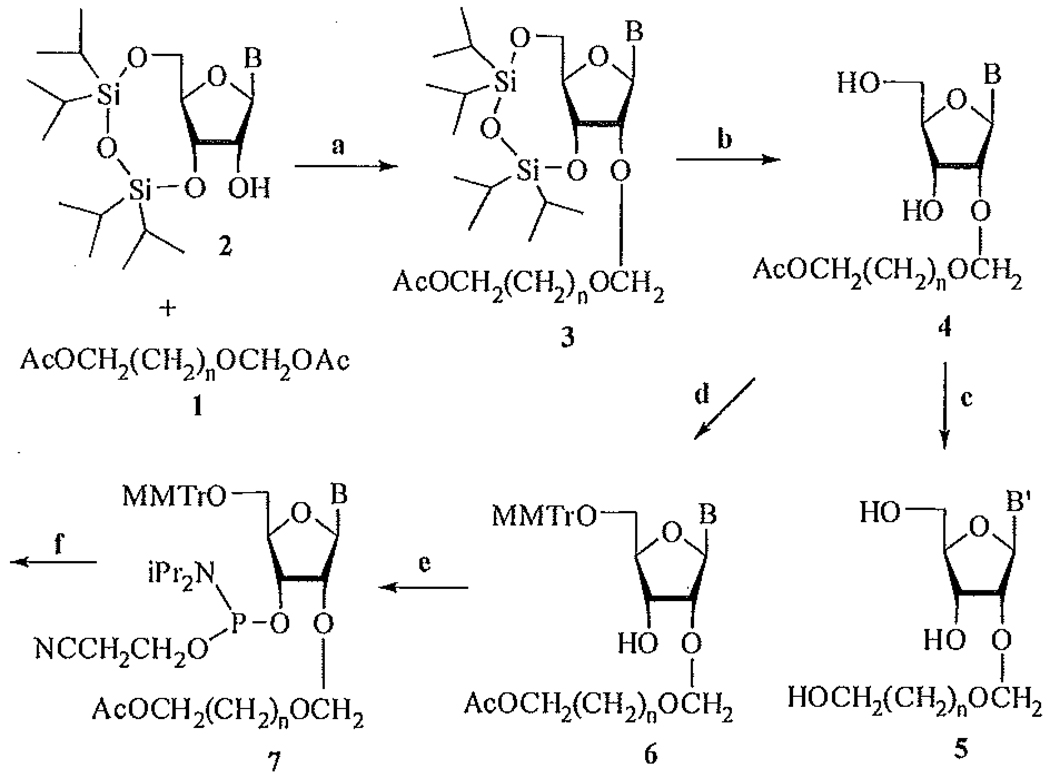
Here we present our recent results on the preparation of 2′-O-hydroxyalkoxymethylribonucleosides and their incorporation into oligoribonucleotides.[13] This scheme is nearly the same as for the preparation of disaccharide nucleosides and their incorporation into ONs.[14] The starting compounds 1(n = 1, 2)[15] were easily prepared from 1,3-dioxolane and 1,3-dioxalane, respectively, and were condensed with 3′,5′-O-blocked ribonucleosides 2 (B = Ura, CytBz) in the presence of tin tetrachloride in 1,2-dichloroethane at −12°C for 20 minutes (Figure 1). The yields (85–87%) of products 3 were even higher than in the case of above preparation of above mentioned disaccharide nucleosides.[10] The silyl group was cleaved to yield partially protected 4 and subsequent deblocking with ammonia in methanol gave free 2′-O-substituted nucleosides 5 in high overall yield. Nucleosides 4 were converted using standard procedures to the corresponding monomethoxytrityl derivatives 6 and their phosphoramidites 7. With these phosphoramidites, several oligoribonucleotides with one or more modifications were assembled. It was shown that modified ONs form stable RNA/RNA duplexes, with about 0.5°C destabilization per modification.
SCHEME 1.
B = Ura, CytBz; B′ = Ura, Cyt; n = 1,2. a) SnCl4/ClCH2CH2Cl,−12°C; b). Bu4NF/THF; c) NH3/MeOH, d) MMTrCl/Py;e) ClP(Nlpr2)(OCH2CH2CN);f) oligoribonucleotide syethesis.
In addition several siRNAs comprising one or more of the new modifications have been prepared and are currently under evaluation. The effects of 2′-O-modification in the antisense and sense strands of small interfering RNA targeting the MDR1 gene was evaluated in multi-drug resistant NIH 3T3 cells using previously described techniques.[16] Standard siRNA or siRNAs with 2′-O-hydroxyalkoxymethylribonucleosides in the sense strand effectively inhibited P-glycoprotein expression, whereas modifications in the antisense strand were not tolerated.
CONCLUSIONS
The general method for the preparation of 2′-O-β-d-ribofuranosylnucleosides was found to be applicable for the synthesis of pyrimidine 2′-O-hydroxyalkoxymethylribonucleosides. The 2′-O-substitutent was found to be stable during oligonucleotide synthesis. Additional work on the preparation of other 2′-O-functionalized nucleosides and oligonucleotides bearing amino groups is in progress and will be published shortly.
Acknowledgments
Financial support of the Russian Foundation for Basic Research, Programme "Molecular and Cellar Biology," INTAS and KUL Research Council is acknowledged.
REFERENCES
- 1.Kurrech J. Antisence technologies, Improvements through novel chemical modifications. Eur. J. Biochem. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- 2.Sioud M, Iversen PO. Ribozymes, DNAzymes and small interfering RNAs as therapeutics. Curr. Drug Targets. 2005;6:647–647. doi: 10.2174/1389450054863653. [DOI] [PubMed] [Google Scholar]
- 3.Coppelti FM, Grandis JR. Oligonucleatides as anticancer agents: From the benchside to the clinical and beyond. Cure Pharm. Des. 2005;11:2825–2840. doi: 10.2174/1381612054546752. [DOI] [PubMed] [Google Scholar]
- 4.Manoharan M. 2′-Carbohadrate modification in antisense oligonucleotide therapy: Importance of conformation, configuration and conjugation. Biochim. Biophys. Acta. 1999;1489:117–130. doi: 10.1016/s0167-4781(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 5.Freier SM, Altmann K-H. The ups and downs of nucleic acid duplex stability: Structure-stability studies on chemically-modified DNA:RNA duplexes. Nucleic Acids Res. 1997;25:4429–4443. doi: 10.1093/nar/25.22.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin S, Miduturu CV, McKinney DC, Silverman SK. Synthesis of amine- and thiole-modified nucleoside phosphoramidites for site-specific introduction of biophysical probes into RNA. J. Org. Chem. 2005;70:4284–4299. doi: 10.1021/jo050061l. [DOI] [PubMed] [Google Scholar]
- 7.Zatsepin TS, Romanova EA, Oretskaya TS. Synthesis of 2′-O-alkylnucleosides. Russ. Chem. Rev. 2002;71:513–534. [Google Scholar]
- 8.Efimtseva EV, Mikhailov SN. Disaccharide nucleosides. Russ. Chem. Rev. 2004;73:401–414. [Google Scholar]
- 9.Nauwelaerts K, Efimtseva EV, Mikhailov SN, Herdewijn P. Disaccharide nucleosides, an important group of natural compounds. In: Schinazi RF, Liotta DC, editors. Frontiers in Nucleosides and Nucleic Acids. Tucker, GA: IHL Press; 2004. pp. 187–220. [Google Scholar]
- 10.Mikhailov SN, Efimtseva EV, Gurskaya GV, Zavodnik VE, De Bruyn A, Janssen G, Rozenski j, Herdewijn P. An efficient synthesis and physico-chemical properties of 2′-Oβ-D-ribofuranosyl-nucleosides, minor tRNA components. J Carbohydr. Chem. 1997;16:75–92. [Google Scholar]
- 11.Mikhailov SN, Rodionov AA, Efimtseva EV, Ermolinsky BS, Fomitcheva MV, Padyukova NSh, Rothenbacher K, Lescrinier E, Herdewijn P. Formation of Trisaccharide Nucleoside during disaccharide nucleoside synthesis. Eur. J. Org. Chem. 1998:2193–2199. doi: 10.1080/15257779908041543. [DOI] [PubMed] [Google Scholar]
- 12.Mikhailov SN, Efimtseva EV. Disaccharide nucleosides and oligonucleotides on their basis. Collect. Czech. Chem. Commun. Symp. Ser. 2002;5:181–194. doi: 10.1023/a:1020963207320. [DOI] [PubMed] [Google Scholar]
- 13.Bobkov GV, Brilliantov KV, Mikhailov SN, Rozenski J, Van Aerschot A, Herdewijn P. Synthesis of oligoribonucleotides containing pyrimidine 2′-O-[(hydroxyalkoxy)methyl] ribonucleosides. Collection Czech. Chem. Comm. 2006;71:804–819. [Google Scholar]
- 14.Efimtseva EV, Bobkov GV, Mikhailov SN, Van Aerschot A, Schepers G, Busson R, Rozenski J, Herdewijn P. Oligonucleotides containing disaccharide nucleosides. Helv. Chim. Acta. 2001;84:2387–2397. [Google Scholar]
- 15.Senkus M. Reaction of some cyclic acetals with acetic anhydride. J. Am. Chem. Soc. 1946;68:734–736. [Google Scholar]
- 16.Xu D, Kang H, Fisher M, Juliano RL. strategies for the inhibition of MDRl gene expression. Mol. Pharmacol. 2004;66:268–275. doi: 10.1124/mol.66.2.268. [DOI] [PubMed] [Google Scholar]