Abstract
Refractive surgery is a popular method used to reduce or eliminate dependence on glasses and contact lenses. Corneal haze is one of the common complications observed after photorefractive keratectmomy (PRK). The objective of this study was to develop an in vivo mouse model that consistently produces moderate to severe corneal haze in the anterior stroma of the mouse cornea after excimer laser treatment to study myofibroblast biology and corneal wound healing in a genetically defined model. Regular- or irregular-phototherapeutic keratectomy (PTK) was performed on black C57BL/6 mice with the Summit Apex excimer laser (Alcon, Ft. Worth, TX). Different numbers of laser pulses (45; ablation depth ~10 μm) were fired on the central cornea, after scraping the epithelium prior to excimer laser ablation. Irregularity was generated by positioning a fine mesh screen in the path of laser after firing 50% of the pulses. Eyes were collected 1, 2, 3 or 4 weeks after the procedure. Haze formation was gauged with slit lamp biomicroscopy. Immunocytochemistry was used to determine number of myofibroblasts in the mouse cornea using antibodies specific for the myofibroblast marker alpha-smooth muscle actin (SMA). The numbers of SMA-positive cells/400X microscopic were determined by counting within the stroma. Statistical analysis was performed using analysis of variance (AVOVA) with the Bonferonni-Dunn adjustment for repeated measures. Regular-PTK with epithelial scrape (Group 3) and irregular-PTK with epithelial scrape (Group 4) in the mouse eyes were performed to produce corneal haze. Eyes collected 4 weeks after regular- or irregular-PTK after epithelial scrape showed 22 ± 6.6 (Group 3) or 34 ± 7.9 (Group 4) SMA-positive cells in the anterior cornea. The difference in the SMA-positive cells detected among the groups was statistically significant (p < 0.01). Less than 4 SMA-positive cells were detected in the tissue sections of the mouse eyes collected after 1, 2 or 3 weeks of regular (Group 3) or irregular PTK (Group 4) or controls (Group 1 and 2). The optimized PTK excimer laser conditions developed in this study produces haze selectively in anterior stroma of the mouse cornea immediately beneath the epithelial basement membrane. Irregular PTK performed after epithelial scrape by applying 45 laser pulses was found to be the most effective method to generate myofibroblasts. This PTK technique for inducing haze in mouse cornea in vivo provides a useful model for studying wound healing and myofibroblast biology in transgenic mice.
Keywords: Haze, cornea, phototheraputic keratectomy, stroma, myofibroblasts
1. Introduction
Development of corneal haze is a common complication of photorefractive keratectomy (PRK), especially in eyes undergoing correction for high myopia (Seiler and McDonnell, 1995; Lipshitz et al., 1997; Sakimoto et al., 2006). Mechanical and/or surgical injury to the cornea triggers a wound healing response causing changes in extracellular matrix organization and cellular phenotype and density (Wilson et al., 2001; Jester et al., 1999a; Fini and Stramer, 2005). Numerous studies have shown that injury to the cornea facilitates release of multiple cytokines and growth factors from both corneal cells and the lacrimal glands (Fini, 1999; Mohan et al., 2003; Zieske, 2001; Baldwin and Marshall, 2002). Cytokines that have been shown to play important roles in maintaining corneal transparency and consequently clear vision are platelet-derived growth factor, transforming growth factor beta, fibroblast growth factor, and the interleukins (Jester et al., 1995; Jester et al., 2002; Girard et al., 1991; Masur et al., 1996). Transforming growth factor beta has been shown to have a central role for inducing opacity (haze) in the cornea by promoting trans-differentiation of progenitor cells, including keratocytes and, possibly, bone marrow-derived cells into myofibroblasts (Jester et al., 1997; Masur et al., 1996, Dupps and Wilson, 2006). The precise mechanisms of corneal haze formation are still unclear. However, the basement membrane of the epithelium has been shown to have a central role in modulating myofibroblast generation, and, therefore haze (Netto et al., 2006).
In previous studies, rabbits (Mohan et al., 2003; Wilson et al., 2003; Netto et al., 2006), rats (Power et al., 1995), hens (Martinez-Garcia et al., 2006) or monkeys (Del Pero et al., 1990; Malley et al., 1990) were used to study haze formation. Mice have not been used extensively because mouse corneas are relatively resistant to haze generation following normal PRK. This limitation has restricted the researcher’s ability to perform in-depth investigation of genetic factors that may be important in myofibroblast generation. The purpose of this study was to develop a technique that could be used to consistently generate moderate to severe corneal haze in the mouse cornea.
2. Method
2.1. Animals and haze generation
Eight to ten week-old black C57BL/6 mice were used in this study. All procedures in animals were performed in accordance with the tenets of the ARVO Statement for the Use of Animals and approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic, Cleveland, Ohio.
Anesthesia was performed by intramuscular injection of ketamine hydrochloride (130 mg/kg) and xylazine hydrochloride (8.8 mg/kg). In addition, topical proparacaine hydrochloride 1% (Alcon, Ft. Worth, TX, USA) was applied to each eye just before surgery. Euthanasia was performed with an intravenous injection of pentobarbital (100 mg/kg) while the animal was under general anesthesia.
An attempt to induce corneal haze was carried out by performing regular or irregular phototherapeutic keratectomy (PTK) with a 2 mm ablation zone on the central stroma by firing 45 laser pulses (ablation depth ~10 μm) with the Summit Apex excimer laser (Alcon, Ft. Worth, TX) after removing entire epithelium (~4 mm) with #64 surgical blade without injuring the limbus. Irregular PTK was performed by positioning a fine mesh screen in the path of the laser after firing 50% of the pulses, as was previously used successfully in the rabbit (Netto et al., 2006). Animals were divided in six groups and only one eye of each animal was used for experiments. Group 1 animals had epithelial scrape, group 2 animals had neither epithelial scrape nor PTK, group 3 animals had regular PTK with epithelial scrape (45 pulses), and group 4 animals had irregular PTK with epithelial scrape (45 pulses). Each group had four time points (1, 2, 3 or 4 weeks), with six eyes at each time point.
2.2. Biomicroscopic grading of corneal haze
The level of opacity (haze) in the cornea was measured with slit lamp after 1, 2, 3 and 4 weeks of PTK following a method reported previously (Fantes et al., 1990), with animals under general anesthesia. Grade 0 was a completely clear cornea; grade 0.5 had trace haze seen with careful oblique illumination with slit-lamp biomicroscopy; grade 1 was more prominent haze not interfering with visibility of fine iris details; grade 2 was mild obscuration of iris details; grade 3 was moderate obscuration of the iris and lens; and grade 4 was complete opacification of the stroma in the area of the ablation. Haze grading was performed in a masked manner by two independent observers.
2.3. Tissue collection
Eyes were removed with 0.12 forceps and sharp Westcott scissors, embedded in liquid OCT compound (Sakura FineTek, Torrance, CA) within a 15 mm × 15 mm × 5 mm mold (Fisher, Pittsburgh, PA) and snap frozen following previously reported methods (Mohan et al, 2003). The frozen tissue blocks were maintained at −85°C. Tissue sections (7 microns) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany) and maintained frozen at −85°C until staining was performed.
2.4. Immunocytochemistry
Immunofluorescent staining for alpha smooth muscle actin (SMA), a marker for myofibroblasts, was performed using mouse monoclonal antibody for SMA (DAKO, Carpinteria, CA). Tissue sections (7 microns) were incubated at room temperature with the monoclonal antibody for SMA at a 1:50 dilution in 1x PBS for two hours and with secondary antibody Alexa 568 or 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) at a dilution of 1:200 for one hour. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories, Inc.; Burlingame, CA) to allow visualization of all nuclei in the tissue sections. Irrelevant isotype-matched primary antibody, secondary antibody alone and tissue sections from naïve eyes were used for negative controls for each immunocytochemistry experiment. The sections were viewed and photographed with a Nikon Eclipse E800 microscope equipped with a digital camera (SpotCam RT KE, Diagnostic Instruments Inc., Sterling Heights, MI).
2.5. Quantification of SMA-positive cells
Six corneas for each time point were used to quantify SMA-positive cells in the tissues. The SMA-positive cells in six randomly selected, non-overlapping, full-thickness central corneal columns extending from the anterior stromal surface to the posterior stromal surface were counted following a method reported previously (Mohan et al, 2003). The diameter of each column was a 400X microscope field.
2.6. Statistical analysis
Statistical analysis was performed with StatView software 4.5 (Abacus Concepts, Berkeley, CA). Variance was expressed as the standard error of the mean (SEM). Statistical comparisons between the groups were performed using analysis of variance (AVOVA) with the Bonferonni-Dunn adjustment for repeated measures.
3. Results
3.1. Biomicroscopic evaluation of haze
The PTK-treated corneas of both the groups collected at the 4-week time point showed different levels of clinical haze. The mouse corneas that had irregular PTK after epithelial scrape (group 4) showed significantly higher levels of haze than the corneas that underwent regular PTK after epithelial scrape (group 3). The biomicroscopic grading of the clinical corneal haze in the animals 4 weeks after irregular PTK with epithelial scrape (group 4) showed prominent haze compared to the corneas of regular PTK with epithelial scrape (group 3) that showed less haze (Figure 1). No corneal haze was observed during biomicroscopic studies in epithelial scrape only (group 1) or naïve (group 2) corneas. Also, none of the mouse corneas examined at the 3 weeks or earlier time points after PTK had corneal haze (data not shown).
Figure 1.
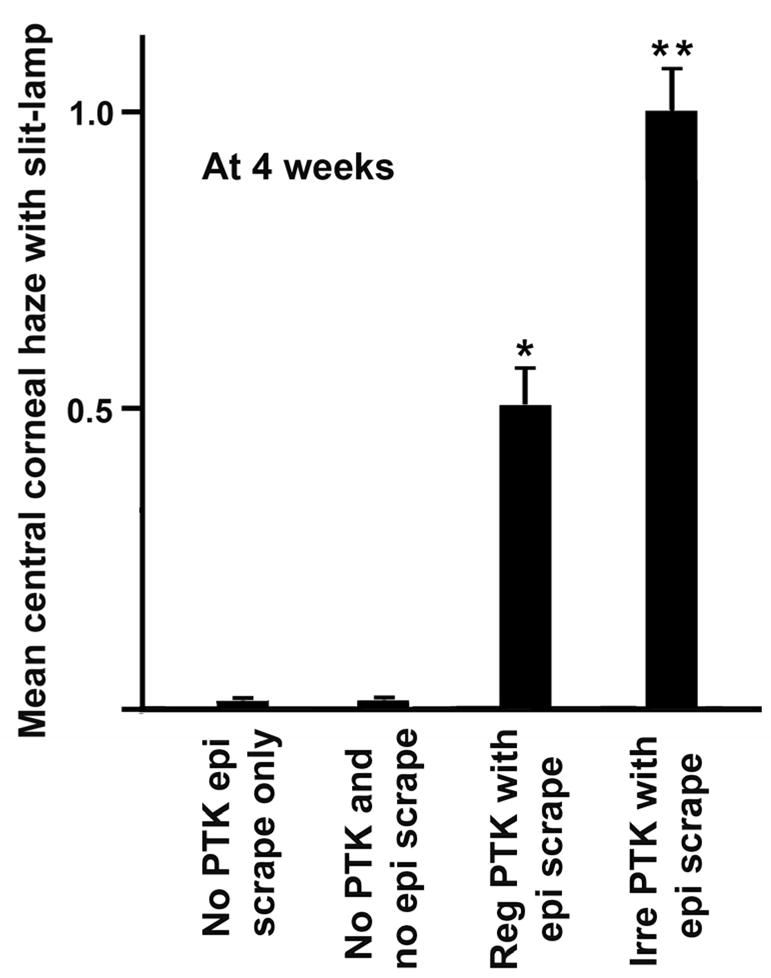
Biomicroscopic quantification of haze in the central corneas of the mouse eyes of different groups at 4 weeks after PTK/epithelial scrape. None of the corneas showed haze at time points earlier than 4 weeks.
3.2. Immunofluorescence detection of myofibroblasts
The formation of haze in mouse corneas was also confirmed by the immunocytochemical detection of myofibroblasts (Figure 2) and quantification of SMA-positive cells in tissue sections (Figure 3). All tissue sections of control and PTK treated mouse eyes collected after 1, 2 or 3 weeks following PTK showed 4 or less SMA-positive cells/400x column in the stroma (data not shown). In contrast, mouse corneas collected 4 weeks after regular or irregular PTK, with epithelial removal, (groups 3 and 4), demonstrated higher myofibroblast cell densities (p value 0.001 or <0.0001). The highest numbers of SMA-positive cells (34 ± 7.9) were detected in the corneas having irregular PTK after epithelial scrape (group 4). The corneas having regular PTK with epithelial scrape (group 3) also showed significantly higher SMA-positive cells (22 ± 6.6) than the control, but less than the mouse corneas that had irregular PTK. The most remarkable feature of the PTK technique was that it selectively induced haze in the anterior stroma, similar to the . Careful evaluation of corneal sections from the different groups showed that 70% or more of the SMA-positive cells were present in the anterior stroma beneath the epithelium and the remaining 10–30% SMA-positive cells in the central or posterior stroma. A few of the corneas also showed 1 to 4 SMA-positive cells in the epithelium and/or endothelium but these cells were excluded from the counting.
Figure 2.
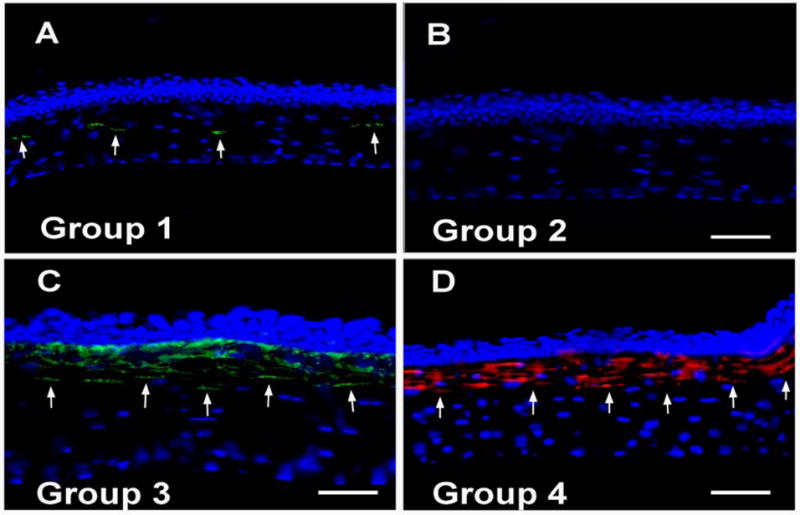
Alpha-smooth muscle actin staining in the mouse corneas of groups 1 to 4 collected 4 weeks after PTK, epithelial scrape or no treatment. Cell nuclei were stained blue with DAPI and SMA-positive cells were stained green or red. Panels A to D show representative images of the SMA-positive cells detected in the corneas of groups 1 to 4, respectively, collected 4 weeks after treatment. In some experiments, secondary antibody with green fluorescence was used, and in others, secondary antibody with red fluorescence was used. The bar is 50 μm. Magnification 200×.
Figure 3.
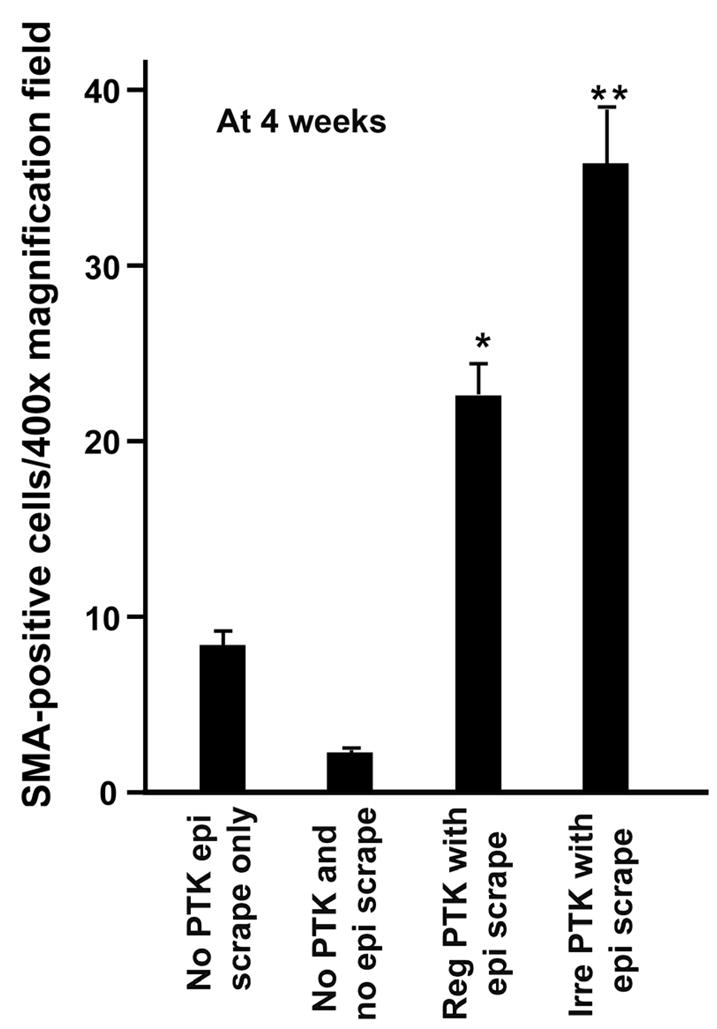
Quantification of SMA-positive cells/400x column in the mouse corneas of groups 1 to 4 collected 4 weeks after PTK, epithelial scrape or no treatment. ** represents the mouse corneas that received irregular PTK after epithelial scrape (group 4), and showed significantly higher SMA-positive cells (p <0.0001) in the anterior stroma compared to the control corneas (group 1 and 2). * denotes mouse corneas that received regular PTK with epithelial scrape (group 3) that showed statistically significant (p 0.001) higher number of SMA-postive cells in the stroma compared to control tissues (group 1 and 2). The error bars represent the SEM.
4. Discussion
Corneal haze is a common complication that occurs in patients after excimer laser photorefractive keratectomy (Seiler and McDonnell, 1995; Lipshitz et al., 1997; Sakimoto et al., 2006). Clinically significant corneal haze has been observed in the eyes after excimer laser PRK (Lipshitz et al., 1997; Hersh et al., 1997; Shah et al, 1998; Siganos et al., 1999; Kuo et al., 2004) and the frequency and intensity of the complication is related to the level of attempted correction. The incidence of corneal haze following PRK in humans (Kuo et al., 2004) or rabbits (Mohan et al., 2003) increases with increasing attempted correction and increasing volume of stromal tissue removal (Moller-Pedersen et al., 1998). Myofibroblasts, and the disordered extracellular matrix materials these cells produce, are the primary causes of corneal haze following PRK (Jester et al., 1999b; Stramer et al., 2003, Mohan et al., 2003; Netto et al., 2006).
The roles of laser-induced stromal surface irregularity, cytokines and growth factors released from corneal epithelium and tears, influx of bone marrow-derived cells, extracellular matrix, stromal remodeling, and corneal crystallins has been studied in haze development following PRK in vivo using various animal models (Mohan et al., 2003; Wilson et al., 2003; Jester et al., 1999a; Jester et al., 2002; Jester et al., 1995; Jester et al, 1999b; Funderburgh et al., 2001; Javier et al., 2006). Recently, the structural and functional integrity of the regenerating epithelial basement membrane has been shown to play a critical role in determining whether a particular cornea develops haze (Netto et al., 2006).
One of the major limiting factors restricting further investigation of molecular mechanisms and the function of select genes controlling corneal wound healing and basement membrane repair is the lack of a suitable model to provide a broad range of genetic testing. The mouse is the preferred host for completing such studies and for developing new strategies for treating various disorders and diseases. However, the mouse model is not currently suitable for studying corneal myofibroblast biology because an appropriate technique for inducing haze is not available. Thus, haze does not typically develop in the mouse cornea following epithelial scrape, photorefractive keratectomy or other mechanical injuries.
The excimer laser (193nm) commonly generates stromal irregularity and haze in human and rabbit corneas, especially when used for higher corrections (Netto et al., 2006; Sakimoto et al., 2006). The mouse cornea is unsuitable for performing photorefractive keratectomy (PRK) with excimer laser because its thickness (~100–130 micron) is approximately 3–4 times thinner than rabbit cornea (400–450 microns) or human (~500–700 microns) cornea. It was, therefore, postulated that phototherapeutic keratectomy could be used to generate stromal irregularity and corneal haze in the mouse eye. Our previous studies showed that stromal surface irregularity induced with a fine mesh and the excimer laser significantly increased formation of haze in rabbit corneas associated with structural and functional defects in the epithelial basement membrane (Netto et al., 2006a; Netto et al., 2006b). This knowledge led us to hypothesize that surface irregularity produced by phototherapeutic keratectomy could induce opacity in the mouse cornea to provide a useful mouse model for studying myofibroblast biology. This study confirms that stromal surface irregularity promotes haze formation in the mouse—presumably by triggering structural and functional defects in the epithelial basement membrane.
The biomicroscopic and immunocytochemical analyses performed in this study showed that haze can be induced in the anterior stroma of mouse cornea, especially with a PTK technique that generates stromal surface irregularity. Appearance of significant haze and detection of SMA-positive cells in the PTK-treated corneas (group 3 and 4) was noted at the 4 week time point and not at earlier tested time points (1, 2 or 3 weeks). This is an interesting observation, as our earlier studies performed in rabbit eyes showed the appearance of SMA-positive cells and haze around 2–3 weeks after photo refractive keratectomy (PRK), with a peak around 4 weeks after PRK (Netto et al., 2006a; Netto et al., 2006b; Mohan et al., 2003). The delayed appearance of SMA-positive cells in the mouse corneas after excimer laser surgery could be due to the difference in surgical technique or species wound healing differences. Additional study is needed to determine what factors are most important. We speculate that differences in the timing of myofibroblast appearance in the wounded corneas of different species are due to differences in marker protein expression, such as alpha smooth muscle actin, in the stroma of different species, based on the study performed by Jester and his coworkers (Jester et al. 2005) that showed human, mouse, rabbit, chicken and pig corneal keratocytes have diverse protein expression patterns.
The data from this study provides optimal PTK excimer laser conditions for producing haze preferentially in the anterior stroma of the mouse cornea. Irregular PTK performed after epithelial scraping was found to be the most effective method for generating myofibroblasts in the mouse cornea, and thus for studying corneal biology—particularly corneal haze formation associated with myofibroblast generation in vivo. The availability of mutant and genetically altered strains of mice will substantially enhance the capacity for investigating the roles of selected genes in this myofibroblast biology and haze development.
Acknowledgments
This work was supported by the EY10056 (SEW), EY15638 (SEW) and EY17294 (RRM) grants from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. Dr. Wilson is the recipient of a Research to Prevent Blindness Physician-scientist Award.
Footnotes
Proprietary interest statement: The authors have no proprietary or financial interest in relation to this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery. Acta Ophthalmol Scand. 2002;80:238–247. doi: 10.1034/j.1600-0420.2002.800303.x. [DOI] [PubMed] [Google Scholar]
- Del Pero RA, Gigstad JE, Roberts AD, Klintworth GK, Martin CA, L’Esperance FA, Jr, Taylor DM. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990;15:419–429. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes FE, Hanna KD, Waring GO, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MT, Matsubara M, Fini ME. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest Ophthalmol Vis Sci. 1991;32:2441–2454. [PubMed] [Google Scholar]
- Hersh PS, Stulting RD, Steinert RF, Waring GO, Thompson KP, O’Connell M, Doney K, Schein OD. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmol. 1997;104:1535–1553. doi: 10.1016/s0161-6420(97)30073-6. [DOI] [PubMed] [Google Scholar]
- Javier JA, Lee JB, Oliveira HB, Chang JH, Azar DT. Basement membrane and collagen deposition after laser subepithelial keratomileusis and photorefractive keratectomy in the leghorn chick eye. Arch Ophthalmol. 2006;124:703–709. doi: 10.1001/archopht.124.5.703. [DOI] [PubMed] [Google Scholar]
- Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46:2369–2378. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997;16:177–187. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999a;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999b;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Kuo IC, Lee SM, Hwang DG. Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK) Cornea. 2004;23:350–355. doi: 10.1097/00003226-200405000-00007. [DOI] [PubMed] [Google Scholar]
- Lipshitz I, Loewenstein A, Varssano D, Lazar M. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmol. 1997;104:369–73. doi: 10.1016/s0161-6420(97)30306-6. discussion 373–374. [DOI] [PubMed] [Google Scholar]
- Malley DS, Steinert RF, Puliafito CA, Dobi ET. Immunofluorescence study of corneal wound healing after excimer laser anterior keratectomy in the monkey eye. Arch Ophthalmol. 1990;108:1316–1322. doi: 10.1001/archopht.1990.01070110132037. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia MC, Merayo-Lloves J, Blanco-Mezquita T, Mar-Sardana S. Wound healing following refractive surgery in hens. Exp Eye Res. 2006;83:728–735. doi: 10.1016/j.exer.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power WJ, Kaufman AH, Merayo-Lloves J, Arrunategui-Correa V, Foster CS. Expression of collagens I, III, IV and V mRNA in excimer wounded rat cornea: analysis by semi-quantitative PCR. Curr Eye Res. 1995;14:879–886. doi: 10.3109/02713689508995127. [DOI] [PubMed] [Google Scholar]
- Sakimoto T, Rosenblatt MI, Azar DT. Laser eye surgery for refractive errors. Lancet. 2006;367:1432–1447. doi: 10.1016/S0140-6736(06)68275-5. [DOI] [PubMed] [Google Scholar]
- Seiler T, McDonnell PJ. Excimer laser photorefractive keratectomy. Surv Ophthalmol. 1995;40:89–118. doi: 10.1016/s0039-6257(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Shah SS, Kapadia MS, Meisler DM, Wilson SE. Photorefractive keratectomy using the summit SVS Apex laser with or without astigmatic keratotomy. Cornea. 1998;17:508–516. doi: 10.1097/00003226-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Siganos DS, Katsanevaki VJ, Pallikaris IG. Correlation of subepithelial haze and refractive regression 1 month after photorefractive keratectomy for myopia. J Refract Surg. 1999;15:338–342. doi: 10.3928/1081-597X-19990501-10. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hutcheon AE, Mohan RR, Ambrosio R, Zieske JD, Hong J, Lee J. Effect of ectopic epithelial tissue within the stroma on keratocyte apoptosis, mitosis, and myofibroblast transformation. Exp Eye Res. 2003;76:193–201. doi: 10.1016/s0014-4835(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]


