Abstract
Multifunctional probes are synthesized in a single step using peptide scaffold-based Multifunctional Single-Attachement-Point (MSAP) reagents.
The design of new nanomaterials with ever higher levels of multifunctional capability, and consequent chemical complexity, is a common challenge to the fields of drug delivery, in vivo molecular imaging or sensor development.1-3 Multifunctionality is relatively easy to achieve with nanoparticles or polymers that afford a large number of similar reactive sites. Multifunctional probes can then be obtained using different chemically reactive functional reagents sequentially, see Figure 1a. Examples of sequential modification strategies abound with quantum dots4, gold and magnetic iron oxide nanoparticles5,6 and polymers like polylysines7. However, sequential syntheses of multifunctional probes allow the stoichiometric ratios of functional groups to vary since each group is attached independently. As the number of functional groups increases from two to three or more, the problem of non-stoichiometric functional group attachment escalates. When multifunctional probes are considered for clinical use, a frequent rationale for animal experiments, fixed functional group ratios in each preparation becomes a necessity. In addition, the extent of substrate modification with non-light absorbing functional groups (e.g. chelate, biotin, polymer) sometimes cannot be determined by simple analytical procedures. Yet, another limitation of multifunctional probe syntheses using multiple reagents occurs when they are considered for substrates possessing a single reactive center, a situation more frequently encountered as substrate size descreases from nanoparticles (>500 kDa) to small macromolecules (5-50 kDa, e.g. small proteins) or low molecular weight molecules (<5 kDa, e.g. drugs, peptides, hormones). Addition of multiple functional goups is also impossible with substrates like annexin V (32 kDa) which, though affording multiple reactive amines, loses activity after modification of a single amine.8
Fig. 1. Strategies to obtain multifunctional probes and the design of multifunctional single-attachment-point reagents (MSAPs).
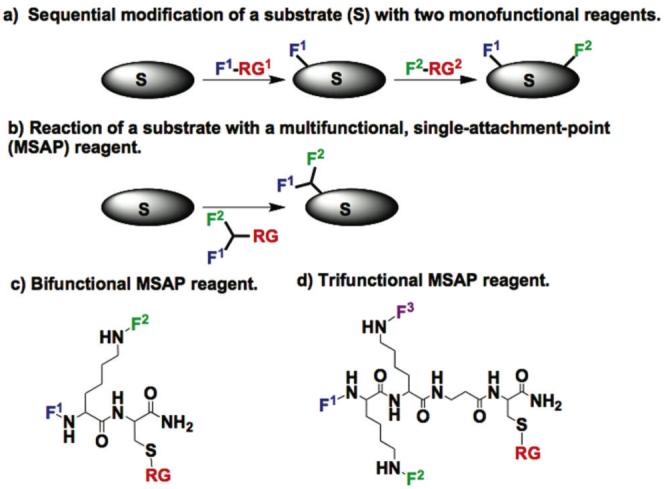
a) A multifunctional probe can be obtained when a substrate is reacted with two chemically reactive functional groups in sequence. F1=Functional group 1; RG1= reactive group 1. b) A substrate can be reacted with an MSAP reagent to obtain a multifunctional probe in single step. c) A bifunctional MSAP scaffold consists of a Lys-Cys dipeptide to which two functional groups (F1 and F2) are attached. d) A trifunctional MSAP scaffold consists of a Lys-Lys-βAla-Cys tetrapeptide to which three functional groups (F1, F2 and F3) are attached. A “probe” consists of a “substrate” modified by one or more “functional groups”.
The need for multifunctional probes, together with the limitations of current syntheses, lead us to consider the development of a new class of reagents designed for the simplified and reproducible syntheses of multifunctional probes. We termed such compounds “multifunctional single-attachment-point” (MSAP) reagents. The MSAP concept is shown schematically in Figure 1b. An MSAP featuring various functional groups (F1, F2) and a single chemically reactive group (RG) is reacted with a substrate, to obtain a multifunctional probe in one step. Functional groups can be chelates, fluorochromes, polymers, affinity tags, etc.. MSAP reagents are based on peptide scaffolds to which the different functional groups and a single reactive group are attached (Figure 1c and 1d). Bifunctional MSAPs employed a Lys-Cys scaffold for the presentation of two functional groups (F1, F2) (Figure 1c), while trifunctional MSAPs (F1, F2, F3) were built on a Lys-Lys-βAla-Cys scaffold (Figure 1d). The syntheses of a bifunctional MSAP and of a trifunctional MSAP are given (Schemes 1 and 2, respectively) and an example of their application for molecular imaging and nanoparticle surface chemistry provided (Figures 2 and 3, respectively).
Scheme 1.
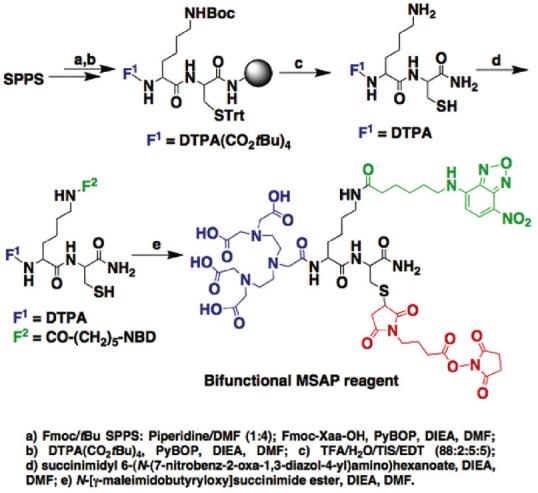
Synthesis of a bifunctional MSAP
Scheme 2.
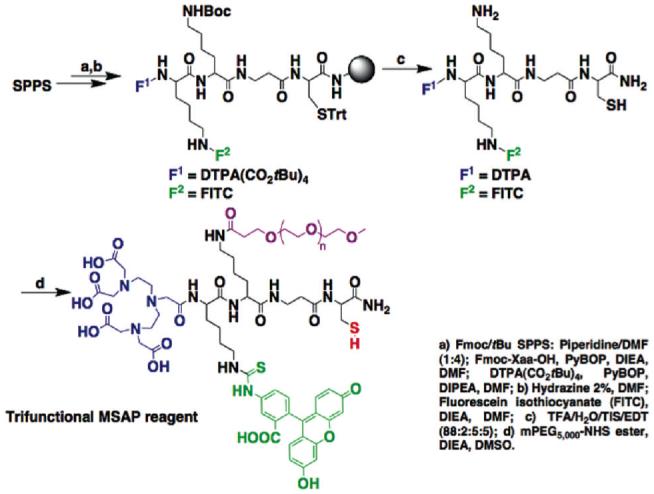
Synthesis of a trifunctional MSAP
Fig. 2.
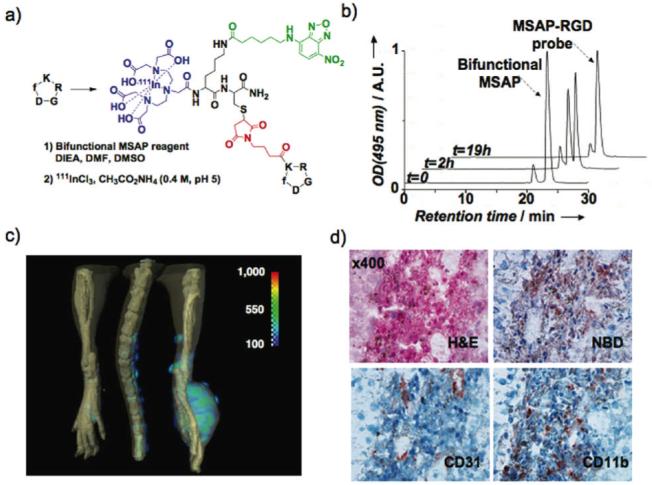
Molecular imaging with a bifunctional MSAP-RGD probe. a) The bifunctional MSAP (Scheme 1) was attached onto the lysine side chain of the cyclo[-RGDfK-] tumor-targeting peptide. b) The reaction between the bifunctional MSAP and the RGD substrate was monitored by RP-HPLC using NBD’s absorbance. c) After complexation with 111InCl3, the bifunctional MSAP-RGD probe was injected into a tumor-bearing mouse and monitored by SPECT-CT. d) Distribution of the probe within the tumor was visualized by immunohistochemistry with an antibody to the NBD hapten and compared to the distribution of CD31 (marker for endothelial cells) and CD11b (marker for monocytes/macrophages)
Fig. 3.
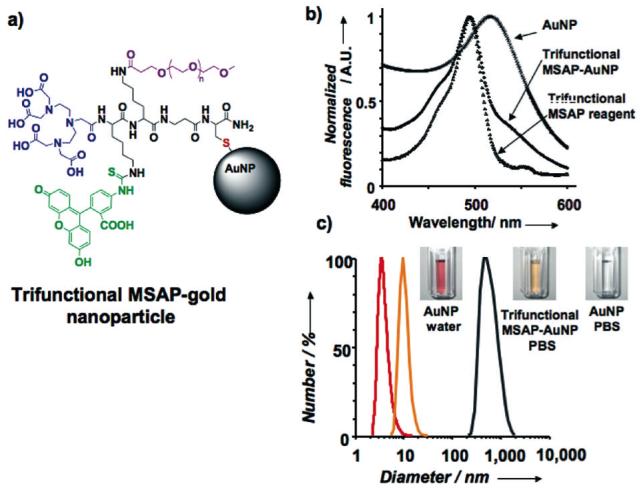
Gold nanoparticle modification and stabilization with a trifunctional MSAP. a) The trifunctional MSAP (Scheme 2) was reacted to the surface of a gold nanoparticle (AuNP) to obtain a trifunctional MSAP-AuNP probe. b) Absorption spectra of the trifunctional MSAP reagent, AuNP and MSAP-AuNP probe. The latter presents absorption maxima at 494 nm (FITC) and 517 nm (AuNP). c) The PEG functional group of the trifunctional MSAP-AuNP stabilized the nanoparticles in physiological buffer as measured by light scattering.
A bifunctional MSAP (F1=DTPA derivative for metal chelation, F2=NBD fluorochrome (7-nitrobenz-2-oxa-1,3-diazol-4-yl), RG=NHS ester) was prepared as shown in Scheme 1. A partially protected DTPA derivative, diethylenetriamine-N,N,N”,N”-tetra-tert-butyl acetate-N’-acetic acid (DTPA(CO2tBU)4), was reacted at the N-terminus of the Lys(Boc)-Cys(Trt) sequence on a solid support (steps a and b). After full deprotection and cleavage (step c), the differential reactivity of the primary amine and thiol groups of the DTPA-Lys-Cys intermediate enabled two consecutive site selective reactions in solution. An NHS ester activated NBD derivative was firstly attached to the lysine side chain (step d). Finally, a commercially available thiol to amine crosslinking agent (maleimidobutyryloxysuccinimide ester, step e) was reacted with the thiol group of the cysteine to endow the bifunctional MSAP with an NHS ester reactive group.
A trifunctional MSAP (F1=chelate, F2=fluorescein, F3=PEG, RG=thiol) was synthesized as shown in Scheme 2. DTPA(CO2tBu)4 was again reacted at the N-terminus of the Lys(ivDde)-Lys(Boc)-βAla-Cys(Trt) sequence on a solid support (step a). FITC was attached after selective removal of the ivDde protecting group (step b). After cleavage and deprotection (step c), the DTPA-Lys(FITC)-Lys-βAla-Cys intermediate was reacted with a 5 kDa NHS ester-activated methoxypolyethyleneglycol.
To demonstrate the advantage of the bifunctional MSAP reagent, we used as a model drug the cyclo[-RGDfK-] peptide currently exploited for its high affinity for integrins.9 The latter presents a single modifiable amine outside the -RGD-binding motif. The bifunctional MSAP (Scheme 1) was then reacted with the peptide to obtain in one step the bifunctional probe (Figure 2a). NBD’s absorbance allowed ready monitoring of the reaction by HPLC (Figure 2b). After the chelation of 111In, the MSAP-RGD probe was administered intraveneously to a mouse bearing an integrin-expressing B16F0 melanoma10 and the probe disposition was determined by SPECT-CT (Figure 2c). To visualize the distribution of the MSAP-RGD probe within the tumor, an antibody to the NBD hapten was employed. Accumulation of the MSAP-RGD probe occurred in parts of the tumor with high CD31 (endothelial cell maker) levels and CD11b (monocytes/macrophages marker) levels (Figure 2d). Thus, a peptide featuring a single modifiable center was reacted with the MSAP to provide, in only one synthetic step, a probe whose disposition could be monitored concurrently by two modalities: tumoral uptake and gross tumor distribution were determined by SPECT and disposition within the tumor tissue was determined by immunohistochemistry. Since both tumor and endothelial cells express RGD binding integrins11,12, the association of the probe with endothelial cell-rich areas suggests that tumor-associated radioactivity results from the binding of the probe to endothelial cell rather than tumor cell integrins.
The utility of the trifunctional MSAP (Scheme 2) for nanoparticle surface design was demonstrated by coating gold colloids (AuNP), as shown on Figure 3a. The thiol RG of the MSAP was reacted with the surface of gold nanoparticles, since thiols react strongly with this surface.13 Here the MSAP provided two functional groups (DTPA, Fluorescein) for detection and one for stabilization (PEG). By fluorescein absorbance (Figure 3b), there were 410 MSAPs per nanoparticle. The increased stability of the nanoparticle in PBS due to MSAP attachment is shown in Figure 3c. While uncoated nanoparticles aggregated in PBS (size increased from 4.1 nm to 500 nm after 2 h), the 10 nm MSAP-AuNP remained stable for up to three days.
Although it may seem that any functional group(s), singly or in combination, can be attached to peptide scaffolds to obtain MSAP reagents (Figures 1c and 1d), our experience was that it was frequently not the case. When attaching functional groups to the solid phase peptide, functional groups with multiple chemically reactive centers must be avoided since they crosslink peptides; hence we employed DTPA(CO2tBu)4 rather than DTPA or DTPA anhydride. Second, the functional groups must survive the harsh conditions of peptide deprotection and release. Near infrared fluorochromes lacked the necessary stability, which fluorescein exhibited. After cleavage and deprotection, the peptide intermediates obtained (DTPA-Lys-Cys or DTPA-Lys(FITC)-Lys-βAla-Cys) offered a single primary amine and a single thiol, allowing two additional chemoselective reactions in solution. Finally solid phase peptide scaffolds were manually synthesized on a scale sufficient (0.2-0.5 mmol) to permit the use of peptide intermediates for the preparation of multiple MSAPs from a common intermediate. This afforded a labor savings and gave considerable flexibility to MSAP reagent design. Our preferred MSAP synthetic strategy was therefore to attach robust functional groups to the solid phase peptide, and complete the synthesis with solution phase reactions run under mild conditions.
The advantages of MSAPs for the synthesis of multifunctional probes (fixed functional group stoichiometry, multimodal detection in biological systems, multifunctional modification of substrates bearing a single reactive group, facile monitoring of chemical reactions), may lead to their commercial development. In much the way that bifunctional crosslinking agents where developed in academic centers and are now commercially available, selected MSAPs may become commercially available for the efficient and reproducible design of ever more complex multifunctional nanomaterials.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
Notes and references
- 1.Ferrari M. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Rhyner MN, Smith AM, Gao X, Mao H, Yang L, Nie S. Nanomed. 2006;1:209–217. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Chen X. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 4.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Aaron J, Sokolov K. Nat Protoc. 2008;3:314–320. doi: 10.1038/nprot.2008.1. [DOI] [PubMed] [Google Scholar]
- 7.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 8.Schellenberger EA, Reynolds F, Weissleder R, Josephson L. Chembiochem. 2004;5:275–279. doi: 10.1002/cbic.200300713. [DOI] [PubMed] [Google Scholar]
- 9.Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stocklin G, Schwaiger M. J Nucl Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 10.Sancey L, Ardisson V, Riou LM, Ahmadi M, Marti-Batlle D, Boturyn D, Dumy P, Fagret D, Ghezzi C, Vuillez JP. Eur J Nucl Med Mol Imaging. 2007;34:2037–2047. doi: 10.1007/s00259-007-0497-z. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualini R, Koivunen E, Ruoslahti E. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 12.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Oliver C. Methods Mol Biol. 1999;115:331–334. doi: 10.1385/1-59259-213-9:331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


