Abstract
Background and Purpose
Chronic periodontitis (CP) is associated with stroke and subclinical atherosclerosis, but clinical measurement of CP can be time consuming and invasive. The purpose of this study was to determine whether radiographically assessed CP is associated with nonstenotic carotid artery plaque as an ultrasound measure of subclinical atherosclerosis.
Methods
Panoramic oral radiographs were obtained from 203 stroke-free subjects ages 54 to 94 during the baseline examination of the Oral Infections and Vascular Disease Epidemiology Study (INVEST). CP exposure among dentate subjects was defined either categorically (periodontal bone loss ≥50% [severe] versus <50% bone loss) or via tertile formation (for dose-response investigation), with edentulous subjects categorized separately. In all subjects, high-resolution B-mode carotid ultrasound was performed. Carotid plaque thickness (CPT) and prevalence (present/absent) were recorded. Covariates included age, sex, smoking, diabetes, hypertension, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein cholesterol.
Results
Among dentate subjects with severe periodontal bone loss, mean CPT was significantly greater (1.20±1.00 mm versus 0.73±0.89 mm; P=0.003). CPT increased with more severe bone loss (upper versus lower tertile bone loss; P=0.049; adjusted for age, sex, and hypertension). This apparent dose-response effect was more evident among never-smokers. In a fully adjusted multivariate logistic regression model, severe periodontal bone loss was associated with a nearly 4-fold increase in risk for the presence of carotid artery plaque (adjusted odds ratio, 3.64; CI, 1.37 to 9.65).
Conclusions
Severe periodontal bone loss is associated independently with carotid atherosclerosis. Panoramic oral radiographs may thus provide an efficient means to assess CP in studies of atherosclerosis risk.
Keywords: alveolar bone loss, carotid stenosis, periodonitis, radiography, panoramic, ultrasonography
Increasing evidence attributes a role for chronic infection in the pathogenesis of atherosclerosis.1 Chronic periodontitis (CP), a destructive infection of the periodontal tissues caused primarily by Gram-negative bacteria, has been associated with coronary heart disease, stroke,2–9 and recently subclinical atherosclerosis.10 In the Atherosclerosis Risk in Communities (ARIC) study, Beck et al10 demonstrated that clinically measured severe CP is associated with intima-media thickness (IMT) in 6017 adults. Grau et al8 and Dorfer et al9 recently confirmed an association with clinically and radiographically measured severe CP and ischemic stroke risk.
Radiographic measurement of alveolar bone is an accurate method for assessment of CP11–13 and compares favorably with clinical measures of periodontitis.14 However, to date, radiographs have not been used to assess CP in research studies of subclinical atherosclerosis. The present cross-sectional study was undertaken to measure CP radiographically and to determine the association with carotid artery plaque in a stroke-free community-based cohort.
Materials and Methods
Study Sample
The present study sample comprises a subsample of participants enrolled in the Oral Infections and Vascular Disease Epidemiology Study (INVEST), a prospective population-based cohort study investigating the relationship between oral infections, carotid atherosclerosis, and stroke.15 INVEST participants are also enrolled in the Northern Manhattan Study (NOMAS).15,16 This study was approved by the institutional review board at Columbia University Medical Center. All participants gave informed consent. Methods of subject recruitment and enrollment into INVEST have been described in a previous publication.15 Briefly, subjects were eligible for enrollment if they were: (1) a resident (>3 months) of northern Manhattan; (2) ≥55 years old; (3) without a history of stroke, myocardial infarction, or chronic inflammatory conditions such as systemic lupus erythematosus, Lyme disease, gonococcal arthritis, or bacterial endocarditis; (4) able to come to the clinic; and (5) concurrently enrolled in NOMAS. The present study sample was enrolled between July 2000 and August 2002. The panoramic radiograph was offered to all participants in INVEST during that period.
Index Evaluation of Participants
Information about risk factors was collected through interviews by trained research assistants. Physical and neurological examinations were performed by study physicians. Fasting blood samples were obtained and lipid panels were measured as described.15 Standardized questions were adapted from the Behavioral Risk Factor Surveillance System of the Centers for Disease Control and Prevention regarding the following conditions: hypertension, diabetes, hypercholesterolemia, peripheral vascular disease, transient ischemic attack, cigarette smoking, and cardiac conditions such as myocardial infarction, coronary artery disease, angina, congestive heart failure, atrial fibrillation, other arrhythmias, and valvular heart disease. Hypertension was defined as a systolic blood pressure recording of ≥140 mm Hg or a diastolic blood pressure recording of ≥90 mm Hg or the participant’s self-report of a history of hypertension or antihypertensive use. Diabetes mellitus was defined by a fasting blood glucose level ≥126 mg/dL, the subject’s self-report of such a history, or insulin or oral hypoglycemic use. For the present analysis, smoking status was categorized as never, former, or current.
Assessment of Carotid Artery Plaque and Carotid Plaque Thickness
The scanning protocol for assessment of carotid plaque thickness (CPT) was performed as described previously.15 Briefly, CPT was assessed by trained and certified sonographers who were blinded to the participants’ risk factors at the time of performing the scans. Ultrasonography was performed on a GE LogIQ 700 system with a multifrequency 9/13-MHz linear-array transducer. With the subject lying in a supine position, the extracranial carotid arteries were imaged in transverse and longitudinal planes (anterior, lateral, and posterior views). Internal and common carotid arteries and the bifurcations were examined for the presence of atherosclerotic plaque, defined as an area of focal wall thickening. If no atherosclerosis was identified, carotid artery plaque was recorded as 0. If plaque was imaged, the view showing the thickest plaque was frozen, and the intima-medial wall thickness (including the plaque) was measured in a single frame using an electronic caliper. For analyses of carotid artery plaque prevalence, carotid artery plaque was deemed present if either a left or right carotid artery plaque was recorded. For analysis of CPT as a continuous variable, the average of the right and left CPT was log transformed [log (mean CPT+1)].
Panoramic Oral Radiograph Assessments
Panoramic radiographs were obtained by trained dental school personnel, then read and scored by a trained examiner as described by Beck et al.4 The reader was blinded to the participants’ atherosclerosis risk factor status. Severity of alveolar bone loss was measured as a percentage of missing bone at the mesial and distal surfaces of each tooth present using a Schei ruler.17,18 Each tooth surface was assigned 1 of 4 scores (1, 2, 3, or 4) corresponding to bone loss of 0% to 24%, 25% to 49%, 50% to 74%, and 75% to 100%, respectively. These measurements were averaged to yield a single mean bone loss score for each participant. We defined a mean bone loss score of <2 as none/mild/moderate periodontal bone loss, or >2 as severe periodontal bone loss.4 Subjects without teeth were categorized as edentulous. Mean subject periodontal bone loss of >50% corresponds with a diagnosis of severe CP.19 Bone loss tertile groups were also formed among dentate subjects.
Statistical Analysis
Means and SDs were calculated for normally distributed continuous variables. Proportions were calculated for categorical variables. Median and interquartile range values were calculated for non-normally distributed continuous variables. Nonparametric tests were used to compare non-normally distributed continuous variables. Analysis of covariance (ANCOVA) was used to compare normally distributed variables. Logistic regression was used to estimate the odds ratio (OR) for the association of severe periodontal bone loss and the dependent variable carotid artery plaque among dentate subjects. Stata/SE software (Stata Corporation) version 8.2 for Macintosh was used to perform statistical analyses.
Results
A total of 277 subjects, or 26% of all eligible INVEST subjects, were enrolled. Individuals without Doppler ultrasound (n=37), missing lipid data (n=28), or previous myocardial infarction (n=9) were excluded from this analysis. Among the final study sample of 203 participants, the mean age was 67.7±8.8 years; 115 (57%) were women; 34 (17%) were edentulous; and 36 (17.7%) had severe periodontal bone loss. The prevalence of carotid artery plaque was 57%. The distribution of sociodemographic factors and the presence of other atherosclerotic risk factors are shown in Table 1. Subjects who consented to the dental radiograph had more extensive periodontal disease, and tended to be younger with higher diastolic blood pressure but were otherwise similar to the remainder of the INVEST cohort (Table 2).
TABLE 1. Characteristics of Participants.
| Characteristic | No. (%) or mean±SD (unless otherwise indicated) |
Males 88 (43) |
Females 115 (57) |
|---|---|---|---|
| Age, y | 67.7±8.8 | 66.7±8.6 | 68.5±8.9 |
| Hypertension | 108 (53) | 36 (41) | 72 (61) |
| Diabetes | 28 (14) | 15 (17) | 13 (11) |
| Any cardiac disease | 18 (9) | 7 (8) | 11 (10) |
| Current smoker | 27 (13) | 15 (17) | 12 (10) |
| Former smoker | 75 (37) | 39 (44) | 36 (31) |
| Total cholesterol, mg/dL | 196.9±36.5 | 188.8±33.4 | 203.1±37.8 |
| HDL cholesterol, mg/dL | 48.0±14.6 | 42.6±12.7 | 52.2±14.7 |
| LDL cholesterol, mg/dL | 122.8±34.9 | 118.6±36.1 | 126.1±33.7 |
| Median bone loss score | 1.45 (interquartile range 1.12–1.78) | 1.62 (1.17–2.05) | 1.36 (1.17–1.62) |
| No. of teeth (dentate subjects) | 17.5±8.4 | 18.1±8.5 | 16.9±8.3 |
| Edentulous | 34 (17) | 14 (16) | 20 (17) |
| Carotid artery plaque prevalence | 57% | 56% | 57% |
| Median CPT (mm) | 0.6 (interquartile range 0–1.35) | 0.65 (0–1.45) | 0.6 (0–1.3) |
TABLE 2. Characteristics of INVEST Participants With and Without the Panoramic Radiograph.
| Variable | Panoramic Radiograph (n=231) |
No Panoramic Radiograph (n=820) |
P Value |
|---|---|---|---|
| Age | 68 | 70 | 0.03 |
| CP (% sites >3-mm attachment loss) | 70% | 54% | 0.0001 |
| Female | 58% | 60% | 0.45 |
| Presence of carotid artery plaque | 57% | 59% | 0.60 |
| Completed high school | 46% | 48% | 0.64 |
| Diabetes | 18% | 20% | 0.54 |
| Former smokers | 36% | 37% | 0.92 |
| Current smokers | 13% | 14% | |
| Systolic blood pressure (mm Hg) | 142 | 139 | 0.05 |
| Diastolic blood pressure (mm Hg) | 82 | 79 | <0.01 |
| Serum total cholesterol (mg/dL) | 197 | 201 | 0.16 |
| HDL cholesterol (mg/dL) | 48 | 50 | 0.06 |
| LDL cholesterol (mg/dL) | 123 | 125 | 0.44 |
| Race/ethnicity | |||
| Hispanic | 58% | 65% | 0.33 |
| Black | 18% | 22% | |
| White | 15% | 18% | |
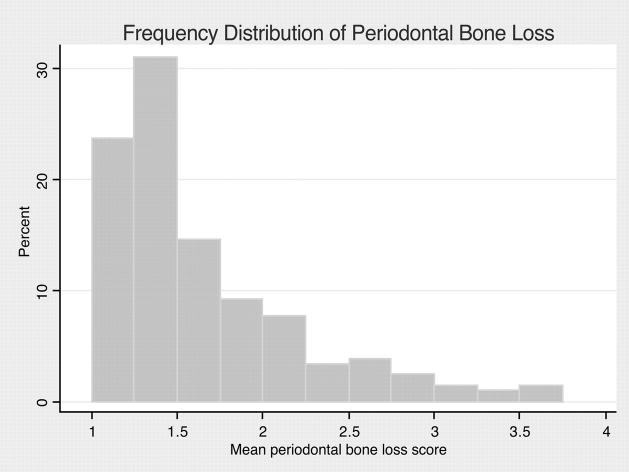
As expected, the frequency distribution of periodontal bone loss in this sample was skewed (Figure 1). Among dentate subjects with severe periodontal bone loss, mean CPT was higher (1.20±1.00 mm versus 0.73±0.89 mm) than those with none/mild/moderate bone loss, and significantly different (P=0.003; 2-sample Wilcoxon rank-sum [Mann–Whitney] test). When edentulous subjects were included in the comparison, mean CPT values in the edentulous group (0.84±0.86 mm) were slightly higher than those with none/mild/moderate bone loss (P=0.18) and lower than those with severe bone loss (P=0.08; Kruskal–Wallis test).
Figure 1.
Frequency distribution of mean whole mouth periodontal bone loss score among dentate subjects (n=169). Median bone loss score 1.45 (interquartile range 1.25 to 1.91).
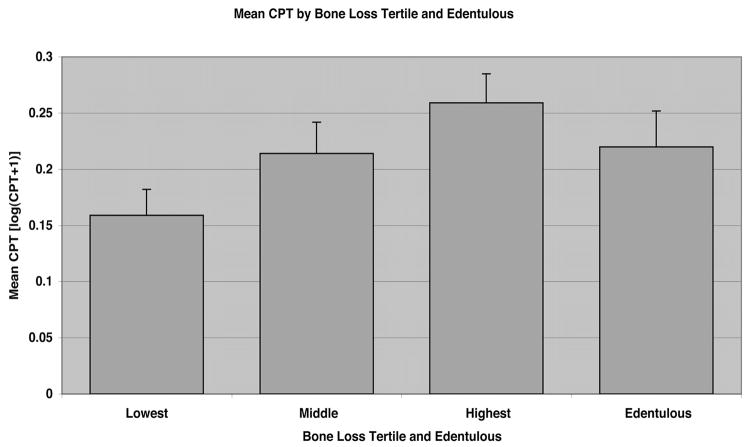
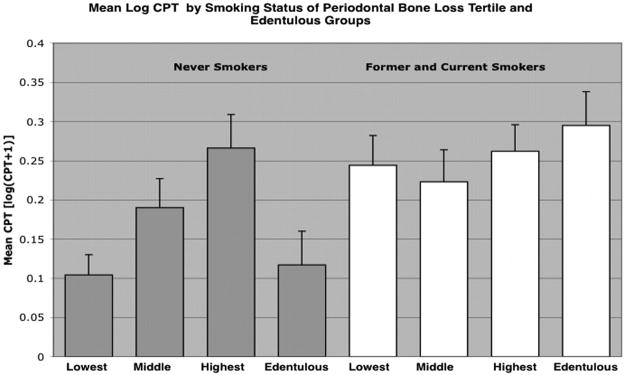
We examined log-transformed mean CPT values by periodontal bone loss tertile for evidence of a dose-response effect (Figure 2). Mean CPT was significantly greater among subjects in the highest tertile of periodontal bone loss compared with the lowest (ANCOVA; P=0.049) but not with the middle tertile (P=0.52), adjusting for age, sex, smoking, and hypertension. Age and smoking status were associated with periodontal bone loss (data not shown). Among smoking status subgroups, former and current smokers had significantly greater mean CPT than never-smokers (P<0.0001), adjusted for age, sex, and hypertension (Figure 3). Among former and current smokers, the apparent dose-response effect observed between periodontal bone loss and CPT was not evident (highest versus lowest tertile bone loss; P=0.70). Among never-smokers, subjects in the upper bone loss tertile had significantly greater mean CPT than those in the lower tertile (P=0.015), or edentulous (P=0.018), adjusted for age, sex, and hypertension.
Figure 2.
Mean carotid plaque thickness [log(CPT+1)] by periodontal bone loss tertile, and edentulous status. Normalized CPT values were significantly greater for subjects in the highest bone loss tertile versus the lowest bone loss tertile, adjusting for age, sex, smoking, and hypertension (P=0.049 by ANCOVA).
Figure 3.
Normalized CPT values were significantly greater among former and current smokers, P<0.0001. Among never smokers, normalized CPT values were significantly greater for subjects in the highest bone loss tertile versus lowest bone loss tertile, P=0.015, and highest bone loss tertile versus edentulous, P=0.018, adjusted for age, sex, and hypertension.
In a logistic regression model, severe periodontal bone loss was associated with the dependent variable CPT, defined dichotomously as presence or absence of carotid artery plaque (unadjusted OR, 2.87; CI, 1.34 to 6.13). After adjusting for age and smoking status, periodontal bone loss remained a significant independent predictor of carotid artery plaque (OR, 2.88; CI, 1.18 to 7.04). In the final model, adjustments were made for age, hypertension, history of cardiovascular disease, diabetes, smoking, high-density lipoprotein (HDL), and low-density lipoprotein (LDL; Table 3). Severe periodontal bone loss remained significantly associated with carotid artery plaque (OR, 3.64; CI, 1.37 to 9.65). Age, current smoking status, and HDL, but not hypertension, also showed a significant association with carotid artery plaque. Additional analysis with education and race/ethnicity as covariates (data not shown) did not change the result.
TABLE 3. Logistic Regression Model for Carotid Artery Plaque Among Dentate Subjects (n=169).
| OR | 95% CI | P Value | |
|---|---|---|---|
| Periodontal bone loss | |||
| None/mild/moderate | 1.00 (referent) | ||
| Severe | 3.64 | 1.37–9.65 | 0.01 |
| Age | 1.10 | 1.05–1.15 | 0.0002 |
| Male sex | 0.61 | 0.28–1.33 | 0.28 |
| Hypertension | 1.40 | 0.68–2.87 | 0.36 |
| Coronary artery disease | 0.86 | 0.24–3.09 | 0.82 |
| Diabetes | 0.49 | 0.18–1.35 | 0.18 |
| Smoking | |||
| Never | 1.00 (referent) | ||
| Former | 2.27 | 0.70–7.30 | 0.17 |
| Current | 2.40 | 1.11–5.21 | 0.03 |
| Lipids | |||
| HDL | 0.97 | 0.95–0.99 | 0.049 |
| LDL | 1.00 | 0.99–1.01 | 0.43 |
Discussion
We used panoramic radiographs to measure periodontal bone loss and duplex Doppler ultrasound to measure carotid artery plaque of participants in an epidemiological study of stroke risk. Our cross-sectional data demonstrate a strong independent association between severe periodontal bone loss and the presence of carotid artery plaque in an elderly stroke-free urban population. Severe periodontal bone loss remained a significant determinant of carotid artery plaque after adjusting for established atherosclerosis risk factors. Further, we observed a dose-response effect between periodontal bone loss severity and carotid artery plaque thickness. These findings confirm previous cross-sectional studies that showed a direct association between CP and atherosclerosis and suggest that panoramic radiography may be an efficient method for assessing CP in studies of atherosclerosis and stroke.
To our knowledge, the present study is the first cross-sectional study to investigate the association between carotid artery plaque and periodontal bone loss. A smaller retrospective study (n=83) observed a positive association between subjects with periodontal bone loss and the degree of carotid artery stenosis.20 Our findings also support those of Beck et al,10 who reported an association (OR, 2.09; CI, 1.73 to 2.53) between clinically measured severe CP and IMT ≥1 mm in 6017 stroke-free adults. Desvarieux et al15 reported an association between tooth loss and carotid artery plaque in 711 subjects from the same INVEST cohort. The association between CP and carotid artery plaque we observed may have been expected, given that CP and tooth loss are highly correlated.
Others have used periodontal bone loss as a measure of CP in studies of stroke risk. Dörfer et al9 and Grau et al8 used radiographs to assess CP among 303 ischemic stroke cases and 300 controls. That study reported an increased risk (OR, 3.62; CI, 1.58 to 8.28) for ischemic stroke among individuals with severe periodontal bone loss. Although the present study did not examine stroke outcome, carotid plaque is a strong predictor for ischemic stroke.21 It is interesting that our study found a similar association (OR, 3.64; CI, 1.37 to 9.65) between severe periodontal bone loss and carotid artery plaque in 203 stroke-free adults. Our findings are inconsistent with those of Elter et al,22 who reported a higher odds for ischemic stroke (OR, 1.4; CI 1.1 to 1.7) among 1491 edentulous subjects compared with 9415 dentate subjects.
Because of the cross-sectional nature of this study, no causal inferences may be made. Although INVEST subjects were chosen randomly from the northern Manhattan population, our study comprised a subset of subjects who agreed to receive the dental radiograph. Comparisons between these responders and nonresponders showed significantly more CP among those who received the dental radiograph (Table 2).15 Another potential limitation of the present report is that gingival inflammation was not assessed. Dörfer et al9 and Grau et al8 demonstrated that gingivitis may also be an important risk factor for ischemic stroke. Because periodontitis may be influenced by adult and childhood socioeconomic factors, another limitation to this study is that childhood factors were not assessed.
In our study, the OR of hypertension was modest and not statistically significant for carotid artery plaque. Consistent with our findings, Weber23 did not find a significant association between hypertension and carotid plaque. Although hypertension is an important risk factor for cardiovascular disease, its association with subclinical markers of atherosclerosis is less clear. Most studies found hypertension to be a strong risk factor for IMT but not for carotid plaque.23,24,25 Carotid plaque and IMT are related26 but may represent different pathological stages in the atherosclerosis process,25,27 or different phenotypes with different risk factor determinants, as suggested by Spence and Hegele.28,29 In this study, we did not measure carotid IMT and cannot test for the possible different determinants of IMT compared with carotid plaque.
In previous studies, a positive association between CP and atherosclerosis/thrombotic disorders has generally been found.2,4,6 –10,30 –33 Therefore, CP measures appear to be an important part of atherosclerosis risk assessment. However, consensus is lacking as to which measurement of CP exposure most accurately reflects systemic exposure. Recently, several different measures of CP were compared in a case-control study of CP and acute myocardial infarct.34 In that study, radiographic evidence of bone loss and not probing pocket-depth measures was the best individual measure of CP for predicting myocardial infarction cases. Radiographic measurement of alveolar bone is an accurate method for assessment of periodontitis11–13 and compares favorably with clinical measures of periodontitis.14 The panoramic radiograph can be performed in a few minutes in relative patient comfort compared with 20 to 30 minutes for a complete clinical oral evaluation. Interestingly, Ravon et al have shown a high degree of correlation between carotid artery calcifications and CP severity, both determined from the same panoramic radiograph.20 Thus, the oral radiograph may prove to be useful as a reliable CP measure and as a screening tool for calcifications of the carotid artery.
Although gingivitis can be successfully treated, CP bone loss generally cannot be reversed. However, CP can be stabilized through appropriate surgical and nonsurgical therapies. Antimicrobial and anti-inflammatory strategies, as well as host-modulating agents,35 have proven to be useful adjunctive therapies for CP. However, to date, there have been no clinical trials of CP as it relates to atherosclerosis. Future studies are needed to assess whether the treatment of CP may slow the progression of carotid atherosclerosis and reduce ischemic stroke events. In this context, measurement of carotid artery plaque area change36 may prove a useful method for monitoring atherosclerosis progression.
In summary, this study adds to the growing body of evidence that CP is an important and independent risk factor for atherosclerosis. Panoramic oral radiographs provide an efficient means to assess CP in studies of atherosclerosis risk.
Acknowledgments
This work was supported by grants from the American Heart Association (grant in aid 0050543N to I.B.L.); Columbia University Office of Clinical Trials (pilot award to S.P.E.); National Institute of Dental and Craniofacial Research (K23 DE00449 to S.P.E.); National Institute of Neurological Disorders and Stroke (R01 NS 29993 to R.L.S.); National Institute of Dental and Craniofacial Research (R01 DE-13094 to M.D.); and K23 NS 42912 to M.S.V.E.); American Heart Association (grant in aid 256205T to P.N.P.); the General Clinical Research Center (2 M01 RR00645); and the Hazel K. Goddess Fund for Stroke Research in Women (to T.R.). R.T.D. is supported by T32 HL-07779. We thank the INVEST staff; George Loo, Dr Shantanu Lal, Janet DeRosa, and Drs Romel Ramas, Sam Trocio, and Oscar Ramos, for performing the ultrasound scans. Participants were seen at the Columbia University General Clinical Research Center (National Institutes of Health grant RR-00645).
References
- 1.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 2.Syrjanen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, Huttunen JK. Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med. 1989;225:179–184. doi: 10.1111/j.1365-2796.1989.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Persson RE, Hollender LG, Powell VL, MacEntee M, Wyatt CC, Kiyak HA, Persson GR. Assessment of periodontal conditions and systemic disease in older subjects. II. Focus on cardiovascular diseases. J Clin Periodontol. 2002;29:803– 810. doi: 10.1034/j.1600-051x.2002.290903.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 5.Grau AJ, Buggle F, Ziegler C, Schwarz W, Meuser J, Tasman AJ, Buhler A, Benesch C, Becher H, Hacke W. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28:1724–1729. doi: 10.1161/01.str.28.9.1724. [DOI] [PubMed] [Google Scholar]
- 6.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 7.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688– 691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, Dorfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- 9.Dorfer CE, Becher H, Ziegler CM, Kaiser C, Lutz R, Jorss D, Lichy C, Buggle F, Bultmann S, Preusch M, Grau AJ. The association of gingivitis and periodontitis with ischemic stroke. J Clin Periodontol. 2004;31:396– 401. doi: 10.1111/j.1600-051x.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 10.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21:1816–1822. doi: 10.1161/hq1101.097803. [DOI] [PubMed] [Google Scholar]
- 11.Papapanou PN, Wennstrom JL. Radiographic and clinical assessments of destructive periodontal disease. J Clin Periodontol. 1989;16:609– 612. doi: 10.1111/j.1600-051x.1989.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 12.Reddy MS. The use of periodontal probes and radiographs in clinical trials of diagnostic tests. Ann Periodontol. 1997;2:113–122. doi: 10.1902/annals.1997.2.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Machtei EE, Hausmann E, Grossi SG, Dunford R, Genco RJ. The relationship between radiographic and clinical changes in the periodontium. J Periodontal Res. 1997;32:661– 666. doi: 10.1111/j.1600-0765.1997.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Hausmann E, Allen K, Norderyd J, Ren W, Shibly O, Machtei E. Studies on the relationship between changes in radiographic bone height and probing attachment. J Clin Periodontol. 1994;21:128–132. doi: 10.1111/j.1600-051x.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 15.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Papapanou PN, Sacco RL. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34:2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842– 849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 17.Schei O. Alveolar bone loss as related to oral hygiene and age. J Periodontol. 1959;30:7–16. [Google Scholar]
- 18.Stabholz A, Mann J, Agmon S, Soskolne WA. The description of a unique population with a very high prevalence of localized juvenile periodontitis. J Clin Periodontol. 1998;25:872– 878. doi: 10.1111/j.1600-051x.1998.tb02384.x. [DOI] [PubMed] [Google Scholar]
- 19.Parameter on chronic periodontitis with advanced loss of periodontal support. American Academy of Periodontology. J Periodontol. 2000;71:856– 858. doi: 10.1902/jop.2000.71.5-S.856. [DOI] [PubMed] [Google Scholar]
- 20.Ravon NA, Hollender LG, McDonald V, Persson GR. Signs of carotid calcification from dental panoramic radiographs are in agreement with Doppler sonography results. J Clin Periodontol. 2003;30:1084–1090. doi: 10.1046/j.0303-6979.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 21.Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35:477– 481. doi: 10.1161/01.STR.0000110981.96204.64. [DOI] [PubMed] [Google Scholar]
- 22.Elter JR, Offenbacher S, Toole JF, Beck JD. Relationship of periodontal disease and edentulism to stroke/TIA. J Dent Res. 2003;82:998–1001. doi: 10.1177/154405910308201212. [DOI] [PubMed] [Google Scholar]
- 23.Weber F. Risk factors for subclinical carotid atherosclerosis in healthy men. Neurology. 2002;59:524–528. doi: 10.1212/wnl.59.4.524. [DOI] [PubMed] [Google Scholar]
- 24.Zureik M, Temmar M, Adamopoulos C, Bureau JM, Courbon D, Thomas F, Bean K, Touboul PJ, Ducimetiere P, Benetos A. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens. 2002;20:85–93. doi: 10.1097/00004872-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32:830– 835. doi: 10.1161/01.str.32.4.830. [DOI] [PubMed] [Google Scholar]
- 26.Zureik M, Ducimetiere P, Touboul PJ, Courbon D, Bonithon-Kopp C, Berr C, Magne C. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol. 2000;20:1622–1629. doi: 10.1161/01.atv.20.6.1622. [DOI] [PubMed] [Google Scholar]
- 27.Hegele RA. The pathogenesis of atherosclerosis. Clin Chim Acta. 1996;246:21–38. doi: 10.1016/0009-8981(96)06224-9. [DOI] [PubMed] [Google Scholar]
- 28.Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis: similar windows but different views. Stroke. 2004;35:649– 653. doi: 10.1161/01.STR.0000116103.19029.DB. [DOI] [PubMed] [Google Scholar]
- 29.Spence JD, Hegele RA. Non-invasive assessment of atherosclerosis risk. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:125–128. doi: 10.2174/1568006043336294. [DOI] [PubMed] [Google Scholar]
- 30.Grau AJ. Infection, inflammation, and cerebrovascular ischemia. Neurology. 1997;49:S47–51. doi: 10.1212/wnl.49.5_suppl_4.s47. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: The first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 32.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 33.Mattila KJ. Dental infections as a risk factor for acute myocardial infarction. Eur Heart J. 1993;14(suppl K):51–53. [PubMed] [Google Scholar]
- 34.Renvert S, Ohlsson O, Persson S, Lang NP, Persson GR. Analysis of periodontal risk profiles in adults with or without a history of myocardial infarction. J Clin Periodontol. 2004;31:19–24. doi: 10.1111/j.0303-6979.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 35.Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Treatment with suban-timicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 36.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–2922. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]