Abstract
Objective
Doxorubicin-based treatment is standard therapy for metastatic uterine leiomyosarcoma. There is no standard second-line therapy. We determined activity of fixed-dose rate gemcitabine plus docetaxel as second-line treatment for metastatic uterine leiomyosarcoma.
Methods
Eligible women with unresectable uterine leiomyosarcoma progressing after prior cytotoxic therapy were treated with gemcitabine 900 mg/m2 days one and eight over 90 minutes, plus docetaxel 100 mg/m2 on day 8 of a 21-day cycle with granulocyte growth factor. Patients with prior pelvic radiation received lower doses. Response Evaluation Criteria in Solid Tumors (RECIST) response was assessed by computed tomography (CT).
Results
Forty-eight of 51 women were evaluable for response (one wrong histology, two never treated). Prior therapy was doxorubicin-based in 90%, and ifosfamide-based in 6%. The overall objective response rate is 27%, with complete response in 6.3% (3/48), and partial response in 20.8% (10/48). An additional 50% (24/48) had stable disease (median duration 5.4 months). The median number of cycles per patient was 5.5 (range 1–22); 73% of patients remained progression-free at 12 weeks and 52% at 24 weeks. The predominant toxicity was uncomplicated myelosuppression: thrombocytopenia grade 3 (29%), grade 4 (10.4%); neutropenia grade 3 (12.5%), grade 4 (8.3%) anemia grade 3 (20.8%), grade 4 (4.2%). While pulmonary toxicity was reported, no patient had drug-related pneumonitis/hypoxia-type toxicity. Median progression-free survival (PFS) was 5.6+ months (range 0.7 – 27+ months). The median duration of objective response was 9+ months (range 3.9 – 24.5+ months).
Conclusion
Fixed-dose rate gemcitabine plus docetaxel is active second-line therapy for uterine leiomyosarcoma.
Keywords: uterine leiomyosarcoma, gemcitabine, docetaxel
INTRODUCTION
Uterine leiomyosarcoma accounts for approximately 1% of all uterine malignancies and thus is diagnosed in only a few thousand women each year [1]. Women who present with advanced disease, and women whose disease recurs after initial surgical resection have a poor prognosis; and, except for rare, highly-selected cases with resectable, isolated pulmonary metastases, are not curable [2]. Median survival among women with advanced disease is less than one year.
Current treatment options for recurrent or advanced uterine leiomyosarcoma are limited. Negligible activity was observed in phase II trials testing the following drugs as single agents: cisplatin, mitoxantrone, amonifide, oral etoposide, diazoquone (AZQ), intravenous etoposide, topotecan, paclitaxel, thalidomide, and trimetrexate [3–14]. Single agents that show moderate activity in leiomyosarcoma include ifosfamide (response rate 17.2%), doxorubicin (response rate 25%), and gemcitabine (bolus infusion achieved a 20% response rate among women with uterine leiomyosarcoma who had received 0–1 prior cytotoxic regimen) [15–17]. Trabectedin achieved a response rate of 8% among patients with prior treatment, and 17% as first-line therapy, in patients with soft tissue sarcoma [18–19].
Combination chemotherapy regimens with activity in previously untreated patients include hydroxyurea, dacarbazine and etoposide (overall response rate 18.4%) and doxorubicin plus ifosfamide (response rate 30.3%) [20,21]. Doxorubicin with or without ifosfamide is frequently employed as first line therapy for women with advanced or recurrent leiomyosarcoma. Standard second-line therapy for uterine leiomyosarcoma has not been established.
In a single-institution study, the combination of fixed-dose-rate gemcitabine plus docetaxel achieved objective response rates of 53% among patients with unresectable leiomyosarcoma who had received up to two prior cytotoxic regimens. Nearly all patients had uterine-primary leiomyosarcoma [22]. This high response rate was observed even among the subgroup of patients previously treated with doxorubicin-based chemotherapy. The rationale for using the fixed-dose rate infusion of gemcitabine was based on pre-clinical data which showed that a gemcitabine concentration of 20 umol/liter was optimal for the formation of the active gemcitabine metabolite, and for its subsequent incorporation of into deoxyribonucleic acid (DNA). Delivering gemcitabine at a rate of 10 mg/m2/minute was determined to be the best rate for maintaining the gemcitabine concentration at 20 umol/liter, and thus optimizing in vivo cell kill [23,24]. Pharmacokinetic analyses in the single institution phase II study [22] showed that fixed-dose rate gemcitabine at 10mg/m2/minute increased the duration of time that the gemcitabine metabolite remained above the threshold for incorporation into deoxyribonucleic acid (DNA) compared with bolus gemcitabine infusion in patients. Furthermore, in a randomized phase II trial in pancreatic cancer, fixed-dose-rate gemcitabine (1500 mg/m2 over 150 minutes) achieved longer time-to-treatment-failure and longer median survival than bolus gemcitabine therapy at a higher dose (2200 mg/m2 over 30 minutes) [25].
The Gynecologic Oncology Group (GOG) conducted this phase II trial of fixed-dose-rate gemcitabine plus docetaxel to determine the activity of this regimen as second-line therapy among women with advanced or recurrent uterine leiomyosarcoma.
MATERIALS AND METHODS
Patients
Women with advanced or recurrent uterine leiomyosarcoma who had progressed after treatment with one prior cytotoxic regimen, and who had measurable disease that was not considered resectable, were eligible. Histologic confirmation was accomplished by central review of the GOG Pathology Committee. Prior therapy with gemcitabine or docetaxel was not permitted. Patients were permitted to have had prior pelvic radiotherapy. Patients were required to have GOG performance status of 0–2, and adequate bone marrow function (absolute neutrophil count (ANC) greater than or equal to 1,500/microliter, and platelets greater than or equal to 100,000/microliter); renal function (creatinine less than or equal to 1.5 × institutional upper limit of normal); hepatic function (bilirubin less than or equal to 1.5 × institutional upper limit of normal, and SGOT and alkaline phosphatase less than or equal to 2.5 × institutional upper limit of normal); and neurologic function (baseline neuropathy (sensory and motor) less than or equal to Common Toxicity Criteria grade 1).
All patients signed written, informed consent. The protocol and consent were reviewed and approved annually by participating institutions’ Institutional Review Boards.
Treatment plan
All participants had baseline imaging (CT scan of chest, abdomen, and pelvis) within four weeks of starting therapy, which was repeated following every other cycle of treatment to assess response. History and physical examination, and assessment of toxicities were done each cycle. Complete blood counts were monitored weekly and comprehensive metabolic panels on day one of each cycle.
Participants without a history of pelvic radiation received gemcitabine 900 mg/m2 on days one and eight intravenously over 90 minutes, followed by docetaxel 100 mg/m2 on day eight intravenously over one hour. Granulocyte-colony-stimulating factor (GCSF) 150 micrograms/m2 was given subcutaneously on days 9–15, or pegfilgrastim 6 mg was given subcutaneously on day nine or 10. Participants with a history of prior pelvic radiation received gemcitabine 675 mg/m2 on days one and eight intravenously over 90 minutes, followed by docetaxel 75 mg/m2 on day eight intravenously over one hour, with the same granulocyte growth factor support as above. Treatment cycles were repeated approximately every three weeks.
Recommended pre-medication for the docetaxel was dexamethasone 8 mg orally twice a day starting the day prior to docetaxel and continuing for three days. Early intervention with diuretics was encouraged for signs of docetaxel-related fluid retention. Treatment continued until time of objective progression of disease, or unacceptable toxicity.
Patients received day one treatment of each cycle provided the ANC was greater than or equal to 1500/microliter and platelet count greater than or equal to 100,000/microliter. Patients received full-dose day eight treatment provided the ANC was greater than or equal to 1000/microliter and platelet count greater than or equal to 100,000/microliter. Seventy-five percent of the planned day eight dose was given if the ANC was between 500 and 1000/microliter or the platelet count was between 50,000 and 100,000/microliter, and provided the bilirubin from day one or after was within institutional normal limits. Day eight treatment with docetaxel was omitted if the bilirubin was still above normal on day eight. Day eight gemcitabine and docetaxel were both omitted if the day eight ANC was under 500/microliter or the platelet count was less than 50,000/microliter.
Doses of both docetaxel and gemcitabine were reduced by 25% in subsequent cycles if a patient experienced grade 3 elevations in SGOT, SGPT, or alkaline phosphatase; and treatment was not resumed until such grade 3 elevations had resolved to grade 1 or less.
Patients who experience grade 2 or worse neurotoxicity had treatment held for a maximum of two weeks, and could resume treatment at 75% of the prior docetaxel dose if the neuropathy had improved. Other non-hematologic toxicities with an impact on organ function of Grade 2 (or greater) required 25% dose reduction and delay in subsequent therapy for a maximum of two weeks until recovered to grade 1, or pre-therapy baseline.
Toxicities were graded according to National Cancer Institution Common Toxicity Criteria version 3.0 (CTC 3.0).
Response was assessed by RECIST. Complete response (CR) is disappearance of all target and non-target lesions and no evidence of new lesions documented by two disease assessments at least four weeks apart. Partial response (PR) is at least a 30% decrease in the sum of longest dimensions (LD) of all target measurable lesions taking as reference the baseline sum of LD. There can be no unequivocal progression of non-target lesions and no new lesions. Documentation by two disease assessments at least four weeks apart is required. In the case where the ONLY target lesion is a solitary pelvic mass measured by physical exam, which is not radiographically measurable, a 50% decrease in the LD is required. Progression of disease requires at least a 20% increase in the sum of LD of target lesions taking as references the smallest sum LD or the appearance of new lesions or death due to disease or global deterioration due to disease. Stable disease (SD) is any condition not meeting the above criteria.
Statistical design
The study employed a two-stage accrual design with an early stopping rule in the event that the treatment demonstrated insufficient activity [26]. During the first stage of accrual, 19–26 patients were to be entered and evaluated. If at least three responses were observed among the first 19–25 patients, or at least four responses out of 26 patients, a second phase of accrual was to be initiated which would increase accrual to 44–51 patients. The regimen would be considered active if at least seven responses were observed among 44–45 patients, or at least eight responses were observed among 46–51 patients. If the true response rate is 10%, the average probability of designing the treatment as designating the treatment as active is limited to 10%. Conversely, if the true response rate was 25%, then the probability of correctly classifying the treatment as active was 90%.
RESULTS
Patient characteristics
Fifty-one women were enrolled on study through 26 participating GOG institutions. The first stage of accrual (23 patients) was achieved over two years, and the second stage (28 patients) was achieved in one year. Forty-eight women were evaluable for response (one patient ineligible due to inadequate pathology to confirm diagnosis, and two patients never treated). The median age was 50 years (range 30–72). All but one patient had a GOG performance status of 0–1. Seventy-nine percent were white; 14.5% black; 6% Asian or Hispanic. Seventeen of 48 patients (35%) had received prior pelvic radiation. All patients had received one prior cytotoxic regimen, and in the majority, the prior therapy had been doxorubicin-based (90%) or ifosfamide-based (6%) (Table 1).
Table 1.
Patient Characteristics (n=48)
| Characteristic | Number of Patients |
|---|---|
| Age | |
| <40 | 6 |
| 40–49 | 18 |
| 50–59 | 17 |
| 60–69 | 4 |
| ≥70 | 3 |
| Performance Status | |
| 0 | 34 |
| 1 | 13 |
| 2 | 1 |
| Race | |
| White | 38 |
| Black | 7 |
| Asian | 2 |
| Hispanic | 1 |
| Prior Chemotherapy | 48 |
| Doxorubicin-based | 43 |
| Ifosfamide-based | 3 |
| Other (paclitaxel-cisplatin, temozolomide) | 2 |
| Prior Radiotherapy | 17 |
| Number of cycles received on study | |
| 1 | 5 |
| 2 | 7 |
| 3 | 3 |
| 4 | 7 |
| 5 | 2 |
| 6 | 5 |
| 7 | 5 |
| >7 | 14 |
Response to treatment and survival
Confirmed CR was observed in 6.3% (3/48), and PR observed in 20.8% (10/48) of patients treated for an overall objective response rate of 27% (95% confidence interval 15.3%–41.8%). An additional 50% (24/48) had stable disease on therapy, for a clinical benefit rate of 77%. Best response was progression of disease in 16.6% (8/48) of patients.
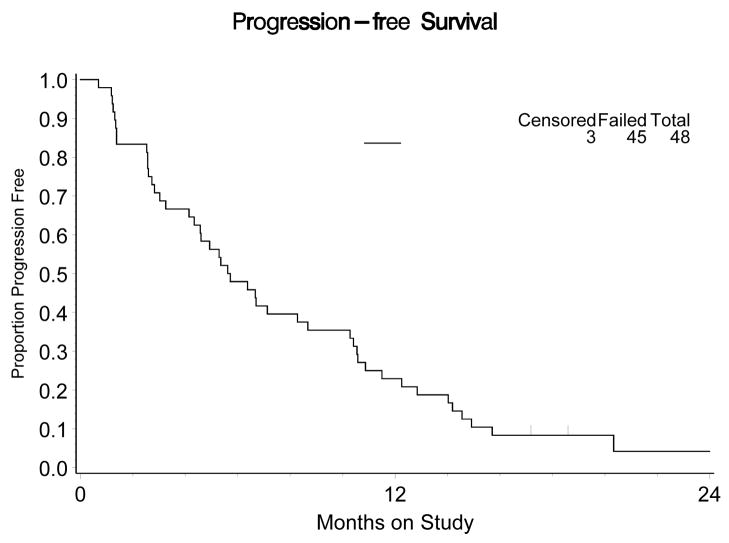
Fifty percent (24/48) of patients received six or more cycles of therapy. The median number of cycles per patient was 5.5 (range 1–22). Median PFS for all 48 patients was 6.7+ months (range 0.7 – 27+ months) (Figure 1). Among the 13 patients with objective response, the median duration of response was nine plus months (range 3.9 – 24.5+ months). One patient had sustained response for over two years, and two others had response durations of over one year (13.3 months and 20.5 months). The median PFS for patients whose best response was stable disease was 5.4 months. The percent of patients remaining free from progression at 12 weeks is 73% (95% confidence interval 58.2%-84.7%) and the percent progression-free at 24 weeks is 52% (95% confidence interval 37.2%–66.7%). Median overall survival (OS) is 14.7 months (.8–50.9+).
Figure 1.
Progression-free survival, n=48 patients with advanced uterine leiomyosarcoma
Adverse events
A summary of all adverse events of all grades is provided in Table 3. The predominant toxicity was myelosuppression: leukopenia grade 3 (14.5%), grade 4 (8.3%); thrombocytopenia grade 3 (29%), grade 4 (10.4%); neutropenia grade 3 (12.5%), grade 4 (8.3%) anemia grade 3 (20.8%), grade 4 (4.2%). Complications of myelosuppression were uncommon: one patient with hemorrhage grade 3 (2%); two patients with neutropenic fever grade 3 (4.2%). Fluid retention syndrome is a known toxicity of docetaxel and was grade 3 in nine patients (19%). Grade 3 constitutional symptoms were reported in five patients (10%), while grade 3 fatigue was reported in only one (2%). Half of the patients received at least one red blood cell transfusion, and six (12.5%) received at least one platelet transfusion. Patients with a history of prior pelvic radiation were more likely to require red blood cell transfusion but not platelet transfusion: 12 of the 17 patients with prior pelvic radiation received red blood cell transfusion (71%), and one of 17 received platelets (6%); whereas 12 of the 31 patients with no history of pelvic radiation received red blood cells (39%), and five of 31 received platelets (16%).
Table 3.
Adverse events, all grades, by number of patients experiencing the event.
| Grade | ||||||
|---|---|---|---|---|---|---|
| Adverse Event | 0 | 1 | 2 | 3 | 4 | Total |
| Leukopenia | 15 | 9 | 13 | 7 | 4 | 48 |
| Thrombocytopenia | 8 | 11 | 10 | 14 | 5 | 48 |
| Neutropenia | 19 | 9 | 10 | 6 | 4 | 48 |
| Anemia | 4 | 6 | 26 | 10 | 2 | 48 |
| Hemorrhage | 44 | 3 | 0 | 1 | 0 | 48 |
| Coagulation | 46 | 0 | 0 | 2 | 0 | 48 |
| RBC transfusion | 24 | 0 | 0 | 24 | 0 | 48 |
| Platelet transfusion | 42 | 0 | 0 | 6 | 0 | 48 |
| Nausea/vomiting | 29 | 12 | 6 | 0 | 1 | 48 |
| Gastrointestinal | 18 | 15 | 12 | 2 | 1 | 48 |
| Genitourinary | 45 | 2 | 1 | 0 | 0 | 48 |
| Alkaline phosphatase | 39 | 9 | 0 | 0 | 0 | 48 |
| SGOT | 41 | 7 | 0 | 0 | 0 | 48 |
| Alopecia | 21 | 1 | 26 | 0 | 0 | 48 |
| Dermatologic | 27 | 9 | 10 | 2 | 0 | 48 |
| Neurotoxicity | 26 | 15 | 7 | 0 | 0 | 48 |
| Renal | 45 | 3 | 0 | 0 | 0 | 48 |
| Infection | 41 | 0 | 4 | 2 | 1 | 48 |
| Fatigue | 40 | 2 | 5 | 1 | 0 | 48 |
| Pain | 22 | 14 | 11 | 1 | 0 | 48 |
| Hepatic | 38 | 6 | 3 | 1 | 0 | 48 |
| Cardiovascular/Edema | 24 | 10 | 5 | 9 | 0 | 48 |
| Metabolic | 29 | 11 | 7 | 1 | 0 | 48 |
| Lymphocytopenia | 46 | 0 | 0 | 2 | 0 | 48 |
| Fever | 43 | 2 | 1 | 2 | 0 | 48 |
| Musculoskeletal | 46 | 1 | 0 | 1 | 0 | 48 |
| Pulmonary | 36 | 4 | 4 | 3 | 1 | 48 |
| Ocular | 44 | 3 | 1 | 0 | 0 | 48 |
| Dyspnea | 47 | 0 | 1 | 0 | 0 | 48 |
| Endocrine | 45 | 3 | 0 | 0 | 0 | 48 |
| Allergy | 46 | 0 | 2 | 0 | 0 | 48 |
| Constitutional | 14 | 13 | 16 | 5 | 0 | 48 |
| DVT | 47 | 0 | 0 | 1 | 0 | 48 |
The median WBC nadir for those 33 patients experiencing leukopenia was 2200 (range: 200–3750).
Pulmonary toxicity has been reported with gemcitabine, docetaxel, and the combination. In this study pulmonary toxicity was reported as grade two in four patients (8.3%), grade 3 in three patients (6.2%), and grade 4 in one patient (2%). Details of these pulmonary events suggest that none were typical drug-related pneumonitis-hypoxia type pulmonary toxicities. The reported grade 3 events are: one patient had pneumonia without neutropenia or hypoxia during cycle 6, requiring a three-day hospitalization for intravenous antibiotics; one patient had grade 3 dyspnea without significant hypoxia due to new bilateral effusions, cytology was negative for malignancy; one patients had acute dyspnea and hypoxia during gemcitabine infusion on cycle 6 day one with work-up negative for pulmonary embolism, cardiac event, or disease progression. She improved clinically and received her day eight treatment but came off study approximately two weeks later for evidence of disease progression on CT scan. The grade 4 event was bilateral pneumonia without neutropenia in a 71-year-old woman with lung metastases, which occurred after cycle 3 of study treatment. The CT scan after cycle two had shown stable disease. The pneumonia was associated with hypoxia requiring intubation. This grade 4 pulmonary toxicity was attributed to both disease and treatment. There were no grade 3 or 4 neurotoxicity events.
DISCUSSION
For decades doxorubicin, with or without ifosfamide, has been the mainstay of treatment for unresectable leiomyosarcoma, with no established second-line therapy. This large phase II trial demonstrates that fixed-dose rate gemcitabine plus docetaxel achieves high objective response rates, including complete responses, as second-line therapy for advanced uterine leiomyosarcoma. Objective responses were sustained in duration (median duration of response was greater than nine months) with some patients having responses that lasted more than two years.
Compared with other agents that have been tested in the second-line metastatic sarcoma setting, the fixed-dose rate gemcitabine plus docetaxel response rate is encouraging. Gemcitabine as bolus infusion, single-agent therapy for patients with 0–1 prior cytotoxic regimen (17% had had no prior therapy) achieved response in 20% of patients; however, the median duration of response was only 4.9 months, and the clinical benefit rate (CR, PR, or stable disease) was 36% [27]. Trabectedin achieved objective response in 8% of patients as second-line therapy [19]. In a phase II study of sorafenib for patients with soft tissue sarcoma who had had 0–1 prior therapies, sorafenib achieved response in two of 37 (5%) patients with leiomyosarcoma [28].
Fixed-dose rate gemcitabine plus docetaxel has been prospectively evaluated in other sarcoma treatment settings. The objective response rate of 53% in the single-institution phase II study is somewhat higher than the objective response in this cooperative group study, a finding that is typical for single-institution versus cooperative group studies [22]. In the randomized trial of fixed-dose rate gemcitabine v. fixed dose-rate gemcitabine plus docetaxel for patients with soft tissue sarcoma who had received zero to three prior regimens, response rate, PFS, and OS were all superior for the gemcitabine-docetaxel arm [29]. The objective response rate across all sarcoma histologic types was 16% with gemcitabine-docetaxel. This lower response rate, compared with the 27% observed in this GOG phase II study, is likely due to the varied-histology sarcoma patient population treated on the randomized trial, and the eligibility criteria permitting up to three prior regimens [22].
The side effect profile for fixed-dose rate gemcitabine plus docetaxel is acceptable. Myelosuppression is common but is only rarely complicated by neutropenic fever. Although 50% of patients received at least one red blood cell transfusion, only 20% of patients had grade 3 anemia (hemoglobin < 8 gm/dl), suggesting that the threshold for recommending red cell transfusion was low among treating physicians. Pulmonary toxicity, described as interstitial lung toxicity with dyspnea and hypoxia that may be severe, has been reported with gemcitabine, docetaxel, and with combinations of gemcitabine plus taxanes [30–34]. Although grade 3 pulmonary toxicity was reported in three patients, and grade 4 in one, none of these four patients appears to have had the typical chemotherapy-related interstitial lung toxicity described with gemcitabine plus taxane therapy. The patient with grade 4 pulmonary toxicity had bilateral pneumonia on CT scan with hypoxia requiring intubation. The event was attributed to both disease and treatment, and it seems likely that there was a component of treatment-related interstitial lung toxicity. The PFS rate at 12 weeks and 24 weeks has been suggested as an endpoint for phase II trials in soft tissue sarcoma [35]. Among patients with prior therapy, chemotherapy agents considered to be active were associated with progression-free rates of 39% at 12 weeks and 14% at 24 weeks. By this measure, fixed dose-rate gemcitabine plus docetaxel meets criteria to be considered active by achieving progression-free rates of 73% at 12 weeks, and 52% at 24 weeks, respectively.
Fixed-dose rate gemcitabine plus docetaxel achieves the highest objective response rates as second line therapy for metastatic uterine leiomyosarcoma of any regimen studied to date. The duration of objective response exceeded nine months, and OS for the whole cohort was 14.7 months in this second-line treatment population. Participating sites enrolled an average of two patients per site, yet toxicity was limited toxicity and response rates high, suggesting that this relatively intense treatment regimen can be easily delivered in clinical practice. A separate GOG trial has recently been completed evaluating the efficacy of this regimen as first-line therapy. Fixed dose-rate gemcitabine plus docetaxel regimen should be considered as a treatment option for second-line therapy for women with uterine leiomyosarcoma.
Table 2.
RECIST-defined responses to treatment
| Response Category | Number of Patients | % |
|---|---|---|
| Complete Response | 3 | 6.3 |
| Partial Response | 10 | 20.8 |
| Stable Response | 24 | 50.0 |
| Increasing Disease | 8 | 16.6 |
| Inevaluable | 3 | 6.3 |
| Total | 48 | 100.0 |
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, University of Mississippi Medical Center, University of Washington, Milton S. Hershey Medical Center, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Tufts-New England Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, Women’s Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, Tacoma General Hospital, Gynecologic Oncology Network, Yale University, and Community Clinical Oncology Program.
Footnotes
CONFLICT OF INTEREST STATEMENT: Dr. Peter Rose has received honoraria from Lilly Pharmaceuticals. All other authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. Cancer. 1993;71:1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 2.Levenback C, Rubin SC, McCormack PM, Hoskins WJ, Atkinson EN, Lewis JL., Jr Resection of pulmonary metastases from uterine sarcomas. Gynecol Oncol. 1992;45:202–5. doi: 10.1016/0090-8258(92)90286-r. [DOI] [PubMed] [Google Scholar]
- 3.Thigpen JT, Blessing JA, Beecham J, Homesley H, Yordan E. A Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol. 1991;9:1962–6. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 4.Thigpen JT, Blessing JA, Wilbanks GD. Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1986;9:18–20. doi: 10.1097/00000421-198602000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Bundy BN, Adcock L, Beecham J. Mitoxantrone in the treatment of advanced uterine sarcoma: A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1990;13:32–4. doi: 10.1097/00000421-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Asbury R, Blessing JA, Buller R, Malfetano JH, Walker J, Bernd-Uwe S. Amonafide in patients with leiomyosarcoma of the uterus. Am J Clin Oncol. 1998;21:145–6. doi: 10.1097/00000421-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Rose PG, Blessing JA, Soper JT, Barter JF. Prolonged oral etoposide in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1998;70:267–71. doi: 10.1006/gyno.1998.5080. [DOI] [PubMed] [Google Scholar]
- 8.Slayton R, Blessing J, Look K, Anderson B. A Phase II clinical trial of diazoquone (AZQ) in the treatment of patients with recurrent leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Invest New Drugs. 1991;9:207–8. doi: 10.1007/BF00175091. [DOI] [PubMed] [Google Scholar]
- 9.Slayton R, Blessing J, Angel C, Berman M. A Phase II trial of etoposide in the management of advanced or recurrent leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Cancer Treat Rep. 1987;71:1303–4. [PubMed] [Google Scholar]
- 10.Miller DS, Blessing JA, Kilgore LC, Mannel R, van Le L. Phase II trial of topotecan in patients with advanced, persistent, or recurrent uterine leiomyosarcomas: a Gynecologic Oncology Group Study. Am J Clin Oncol. 2000;23:355–7. doi: 10.1097/00000421-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Gallup DG, Blessing JA, Anderson W, Morgan MA. Evaluation of paclitaxel in previously treated leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;89:48–51. doi: 10.1016/s0090-8258(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 12.Sutton G, Blessing JA, Ball H. Phase II trial of paclitaxel in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;74:346–9. doi: 10.1006/gyno.1999.5463. [DOI] [PubMed] [Google Scholar]
- 13.McMeekin DS, Sill M, Benbrook D, Darcy K, Webster K, Waggoner S. A Phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;105:508–16. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith HO, Blessing JA, Vaccarello L. Trimetrexate in the treatment of recurrent or advanced leiomyosarcoma of the uterus: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2002;84:140–4. doi: 10.1006/gyno.2001.6482. [DOI] [PubMed] [Google Scholar]
- 15.Sutton GP, Blessing JA, Barrett FJ, McGehee R. A Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992;166:556–9. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 16.Omura GA, Major FJ, Blessing JA, Sedlacek TV, Thigpen JT, Creasman WT, et al. A randomized study of adriamycin with and without dimethyl triazenolimidazole carboxamide in advanced uterine sarcomas. Cancer. 1983;52:626–32. doi: 10.1002/1097-0142(19830815)52:4<626::aid-cncr2820520409>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Look KY, Sandler A, Blessing JA, Lucci JA, Rose PG. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92(2):644–7. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Carbonero R, Supko JG, Maki RG, Manola J, Ryan DP, Harmon D, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol. 2004;23:5484–92. doi: 10.1200/JCO.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Delaloge S, Yovine A, Taamma A, Riofrio M, Brain E, Raymond E, et al. Ecteinasidin-743: a marine-derived compound in advanced, pre-treated sarcoma patients—preliminary evidence of activity. J Clin Oncol. 2001;19:1248–55. doi: 10.1200/JCO.2001.19.5.1248. [DOI] [PubMed] [Google Scholar]
- 20.Currie JL, Blessing JA, Muss HB, Fowler MD, Berman M, Burke TW. Combination chemotherapy with hydroxyurea, dacarbazine (DTIC), and etoposide in the treatment of uterine leiomyosarcoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;61:27–30. doi: 10.1006/gyno.1996.0091. [DOI] [PubMed] [Google Scholar]
- 21.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:226–9. doi: 10.1006/gyno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 22.Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, et al. Gemcitabine and Docetaxel in Patients with Unresectable Leiomyosarcoma: Results of a Phase II Trial. J Clin Oncol. 2002;20:2824–31. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2′,2′-difluorodeoxycytidine (Gemcitabine) administration in leukemia. Cancer Res. 1990;50:6823–6. [PubMed] [Google Scholar]
- 24.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–8. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 25.Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty minute infusion and fixed dose rate infusion in patients with pancreatic carcinoma. J Clin Oncol. 2003;21:3402–8. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 26.Chen TT, Ng T. Optimal flexible designs in phase II clinical trials. Statistics in Medicine. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Look KY, Sandler A, Blessing JA, Lucci JA, III, Rose PG. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92:644–7. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 28.D’Adamo DR, Keohan M, Schuetze S, Undevia S, Livingston M, Cooney M, et al. Clinical results of a phase II study of sorafenib in patients with non-GIST sarcoma (CTEP study #7060) Proc Amer Soc Clin Oncol. 2007;25 doi: 10.1200/JCO.2008.20.4495. abstr 10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel versus gemcitabine alone in patients with metastatic soft-tissue sarcomas: results of Sarcoma Alliance for Research through Collaboration study 002. J Clin Oncol. 2007;25:2755–63. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 30.Katakami N, Takiguchi Y, Yoshimori K, Isobe H, Bessho A, Yoshimura A, et al. Docetaxel in combination with either cisplatin or gemcitabine in unresectable non-small cell lung carcinoma: a randomized phase II study by the Japan Lung Cancer Cooperative Clinical Study Group. J Thorac Oncol. 2006;1:447–53. [PubMed] [Google Scholar]
- 31.Veltkamp SA, Meerum Terwogt JM, van den Heuvel MM, van Boven HH, Schellens JH, Rodenhuis S. Severe pulmonary toxicity in patients with leiomyosarcoma after treatment with gemcitabine and docetaxel. Invest New Drugs. 2007;25:279–81. doi: 10.1007/s10637-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 32.Belknap SM, Kuzel TM, Yarnold PR, Slimack N, Lyons EA, Raisch DW, et al. Clinical features and correlates of gemcitabine-associated lung injury: findings from the RADAR project. Cancer. 2006;106:2051–7. doi: 10.1002/cncr.21808. [DOI] [PubMed] [Google Scholar]
- 33.Kouroussis C, Mavroudis D, Kakolyris S, Voloudaki A, Kalbakis K, Souglakos J, et al. High incidence of pulmonary toxicity of weekly docetaxel and gemcitabine in patients with non-small cell lung cancer: results of a dose-finding study. Lung Cancer. 2004;44:363–8. doi: 10.1016/j.lungcan.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Leimgruber K, Negro R, Baier S, Moser B, Resch G, Sansone S, et al. Fatal interstitial pneumonitis associated with docetaxel administration in a patient with hormone-refractory prostate cancer. Tumori. 2006;92:542–4. doi: 10.1177/030089160609200614. [DOI] [PubMed] [Google Scholar]
- 35.Van Glabbeke M, Verweij J, Judson I, Nielson OS on behalf of the EORTC Soft Tissue and Bone Sarcoma Group. Progression-free rate as the principal end-point for phase II trials in soft tissue sarcoma. Eur J Cancer. 2002;38(4):543–9. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]