Abstract
Background and Purpose
Atherosclerosis is a complex disorder with hereditary and environmental causes. Carotid artery intima-media wall thickness (IMT) is a useful measure of atherosclerosis. The objective of this study was to determine the association between carotid IMT and functional promoter variants of stromelysin-1 (MMP3: −1612 5A>6A), interleukin-6 (IL6: −174G>C), and hepatic lipase (HL: −480C>T) genes.
Methods
B-mode carotid ultrasound was performed among 87 subjects (mean age, 70 ± 12 years; 55% women; 60% Caribbean-Hispanic, 25% black, and 13% white) from the Northern Manhattan Prospective Cohort Study. Carotid IMT was calculated as a composite measure (mean of the maximum IMT in the bifurcation, the common carotid artery, and the internal carotid artery).
Results
For all polymorphisms, genotype distribution was not significantly different from Hardy-Weinberg equilibrium. The frequencies of the rare alleles were as follows: MMP3 −1612 5A>6A, 0.31 (95% CI, 0.25 to 0.39); IL6 −174 G>C, 0.20 (95% CI, 0.13 to 0.25); and HL −480 C>T, 0.45 (95% CI, 0.35 to 0.50). Carotid IMT in the sample was 0.78±0.18 mm. Subjects with the MMP3 genotype 6A6A had 8% greater mean carotid IMT than the other MMP3 genotypes combined (0.95±0.17 versus 0.87±0.15 mm; P=0.04). Subjects with the IL6 genotype GG had 11% greater IMT (0.85±0.17 versus 0.76±0.16 mm; P=0.03), and those with the HL genotype CC had 13% greater IMT (0.87±20 versus 0.76±0.18 mm; P=0.02) than the other genotypes combined. Adjustment for other risk factors did not change these associations.
Conclusions
Carotid IMT is higher among subjects homozygous for functional variants in genes related to matrix deposition (MMP3 −16126A), inflammation (IL6 −174G), and lipid metabolism (HL −480C). These associations were independent of race-ethnicity and some environmental exposures. Further studies are needed to confirm these genotype-phenotype associations.
Keywords: genetics, interleukin-6, intima-media thickness, lipase, stromelysin 1, ultrasonography
Atherosclerosis is a common, but complex, multifactorial disorder that accounts for much of morbidity and mortality in adult life. Carotid artery intima-media thickness (IMT) measured by high-resolution B-mode ultrasound has been demonstrated to correlate well with pathologically and clinically defined atherosclerosis1,2 and increased risk of myocardial infarction and stroke.3
Several complex mechanisms have been hypothesized to play a key role in the development of atherosclerosis, including metabolic and inflammatory processes. There is some evidence that variants of specific genes are associated with these processes. A variant in the promoter of the stromelysin-1 (MMP3) gene [−1612(5A/6A)] regulates transcription of stromelysin-1, one of the matrix metalloproteinases involved in connective tissue remodeling, an essential part of the atherosclerotic process.4,5 This variant has been associated with carotid IMT and progression of angiographically determined coronary artery disease.4,5 A polymorphism of the interleukin-6 (IL6) gene with a G>C change at position −174 has been recently identified.6 It has been reported that individuals homozygous for the G allele have greater IMT than other subjects.4 For the hepatic lipase (HL) gene, a number of polymorphisms have been reported in the promoter, all showing strong allelic association.7 The C>T variation at −480 results in low HL activity and higher HDL levels.8 No study has yet reported on the impact of the HL promoter variants on carotid IMT.
The aim of this study was to investigate the association between carotid IMT and promoter variants of stomelysin-1, interleukin-6, and hepatic lipase genes.
Subjects and Methods
The study population of 87 stroke-free subjects was randomly derived from the Northern Manhattan Prospective Cohort Study.9 The mean age was 70±12 years; 55% were women; 60% were Caribbean-Hispanic, 25% black, and 13% white.
Carotid IMT was assessed by high-resolution B-mode ultrasound (Diasonics 2D-Gateway, 7-MHz probe) with the use of the Atherosclerosis Risk in Communities (ARIC) protocol.2 The sonographers and the readers were certified for this protocol. Interreader and intrareader reliability values for carotid IMT in our laboratory were similar to those published in other studies.2,3 In a sample of 88 stroke-free community subjects, mean absolute difference of carotid IMT between 2 readers was 0.19±0.36 mm, with a variation coefficient of 7.5%, a correlation coefficient of 0.77, and a percent error of 10.6%. Intrareader mean absolute IMT difference was 0.07±0.04 mm, with a variation coefficient of 5.4%, a correlation coefficient of 0.94, and a percent error of 5.6%.
The carotid artery IMT protocol consisted of scanning the carotid arteries in 3 segments, defined as follows (Figure 1): (1) near wall and far wall of the segment extending from 10 to 20 mm proximal to the tip of the flow divider into the common carotid artery (CCA); (2) near wall and far wall of the carotid bifurcation beginning at the tip of the flow divider and extending 10 mm proximal to the flow divider tip; and (3) near wall and far wall of the proximal 10 mm of the internal carotid artery (ICA). Measurements of IMT were performed off-line with the use of IMAGE-Pro (Microsoft) image analysis software.
Figure 1.

Measurement of carotid IMT. ICA indicates internal carotid artery; ECA, external carotid artery; and CCA, common carotid artery. Maxj is maximum distance between 2 lines measured in j points of define segment.
The carotid IMT was calculated as a composite measure (mean of the 12 sites) that combined the near and the far wall of the maximal CCA IMT, the maximal bifurcation IMT, and the maximal ICA IMT of both sides.
Genetic Analysis
Blood samples taken at the time of IMT examination were stored at −20°C until extraction of DNA with the use of the Signal Sequence Trap method, as described.10 DNA was transported to University College London (London, UK) for genotyping. Detailed descriptions of the methods used to determine the genotypes examined in this study have been provided previously.4,6,11–13
Statistical Analysis
The frequencies of alleles of the specific candidate genes were compared between various race-ethnicity groups by χ2 test with Bonferroni correction. The association of gene polymorphisms and carotid IMT was analyzed with a multiple linear regression model (SAS version 6.12) adjusted for demographics, hypertension, cholesterol, and current smoking.
Results
Despite the small numbers of subjects, the genotype distributions were consistent with that expected from Hardy-Weinberg equilibrium (Table 1). For MMP3 gene among blacks, 82% were homozygous for 6A6A in comparison to 46% of whites and 39% of Caribbean-Hispanics (P<0.05), although there was no significant evidence of heterogeneity of allele frequency. For IL6, as reported previously,6 the frequency of the −174C allele among blacks was slightly lower than among other ethnicities (P=0.09). However, 92% of blacks were homozygous for GG of the IL6 gene, in comparison to 66% of Caribbean-Hispanics and 42% of whites (P<0.05). For HL, the −480C allele frequency was lowest in whites, but overall there was no evidence for heterogeneity of frequency. For the HL gene, however, 73% of whites but only 27% of Caribbean-Hispanics and 25% of blacks were homozygous for CC of the HL gene (P<0.05). The frequencies of the rare alleles were as follows: MMP3 −1612 5A>6A, 0.31 (95% CI, 0.25 to 0.39); IL6 −174 G>C, 0.20 (95% CI, 0.13 to 0.25); and HL −480 C>T, 0.45 (95% CI, 0.35 to 0.50). For MMP3 and IL6, but not HL, these frequencies were significantly lower than previously reported in white subjects.4
TABLE 1.
Distributions of Genotypes of Stromelysin-1 (MMP3), Interleukin-6 (IL6), and Hepatic Lipase (HL) Genes With Respect to Race-Ethnicity Among 87 Stroke-Free Subjects
| Gene and Polymorphism | Overall | Caribbean-Hispanic | Black | White |
|---|---|---|---|---|
| Stromelysin-1 (MMP3)* | ||||
| 5A5A | 7 (11) | 3 (7) | 1 (9) | 3 (27) |
| 5A6A | 27 (41) | 22 (54) | 1 (9) | 3 (27) |
| 6A6A | 32 (48) | 16 (39) | 9 (82) | 5 (46) |
| Frequency of 6A allele | 0.69 (0.61–0.77) | 0.66 (0.56–0.76) | 0.86 (0.72–1.00) | 0.59 (0.39–0.80) |
| Interleukin-6 (IL6)† | ||||
| GG | 47 (66) | 29 (66) | 11 (92) | 5 (42) |
| GC | 18 (26) | 12 (27) | 1 (8) | 5 (42) |
| CC | 5 (8) | 3 (7) | 0 (0) | 2 (16) |
| Frequency of C allele | 0.20 (0.13–0.27) | 0.21 (0.12–0.29) | 0.04 (0.00–0.12) | 0.38 (0.18–0.57) |
| Hepatic lipase (HL)‡ | ||||
| CC | 23 (34) | 11 (27) | 3 (25) | 8 (73) |
| CT | 28 (42) | 8 (44) | 7 (58) | 2 (18) |
| TT | 16 (24) | 12 (29) | 2 (17) | 1 (9) |
| Frequency of T allele | 0.45 (0.36–0.53) | 0.51 (0.39–0.64) | 0.46 (0.26–0.66) | 0.18 (0.02–0.34) |
Values are number (percent) or (95% CI). Denominators vary because not all subjects had genotypes determined for all gene polymorphisms.
χ2=11.31, P<0.05.
χ2=7.14, P<0.05.
χ2=10.64, P<0.05.
Gene Polymorphisms and Carotid IMT
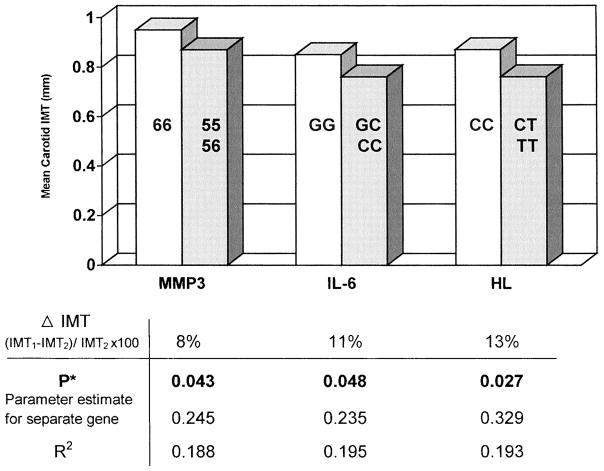
The genotypes with a minor allele were combined to increase statistical power (the 2 genotypes with a minor allele were not significantly different, as seen in Table 2). Subjects with the genotypes MMP3 6A6A (Figure 2) had 8% greater mean carotid IMT than the other genotypes combined (0.95±0.17 versus 0.87±15 mm; P=0.044). Subjects with IL6 GG had 11% greater IMT (0.85±0.17 versus 0.76±0.16 mm; P=0.034), and those with HL CC had 13% greater IMT (0.87±0.20 versus 0.76±0.18 mm; P=0.025). After adjustment these associations remained significant, each explaining 19% of the variance in carotid IMT.
TABLE 2.
Association of Carotid IMT and Alleles of MMP3, IL6, and HL Genes
| Gene and Polymorphism | n (%) | Mean Carotid IMT, mm | t | P* |
|---|---|---|---|---|
| Stromelysin-1 (MMP3) | ||||
| 5A5A | 8 (12) | 0.8130.15 | ||
| 5A6A | 27 (40) | 0.8930.20 | ||
| 6A6A* | 32 (48) | 0.9530.17 | 2.046 | 0.044 |
| Interleukin-6 (IL6) | ||||
| CC | 5 (7) | 0.7230.15 | ||
| GC | 19 (27) | 0.7830.21 | ||
| GG* | 47 (66) | 0.8530.17 | 2.152 | 0.034 |
| Hepatic lipase (HL) | ||||
| TT | 16 (24) | 0.6930.19 | ||
| CT | 28 (42) | 0.7730.17 | ||
| CC* | 23 (34) | 0.8730.20 | 2.286 | 0.025 |
P values correspond to the comparisons of IMT means between 6A6A vs 5A6A/5A5A (0.95±0.17 vs 0.87±0.15); GG vs GC/CC (0.85±0.17 vs 0.76±0.16); CC vs CT/TT (0.87±0.20 vs 0.76±0.18).
Figure 2.
Carotid IMT and MMP3, IL6, and HL gene polymorphism. *Multiple linear regression model adjusted for race-ethnicity, hypertension, cholesterol, and current smoking.
Discussion
We observed that subjects homozygous for specific candidate genes related to matrix deposition (MMP3 gene), inflammation (IL6 gene), and lipid metabolism (HL gene) had an increased carotid artery wall thickness. Although the biology of the vessel wall and development of atherosclerosis are most likely regulated by multiple genes, the investigated genes may explain some aspects of vascular physiology. All known environmental risk factors account for only approximately half of all cases of atherosclerotic diseases.14 The rest may be explained by some newly recognized genetic factors, including specific gene polymorphisms investigated in our study.
Stromyelysin-1, as a key regulator of matrix remodeling, may be involved in development of atherosclerosis. Individuals who produce less MMP3 would have a lower ability to remodel and degrade the matrix components, leading to faster arterial wall thickening and plaque growth.4,5 We observed that 6A homozygous subjects had an 8% increase in carotid IMT compared with other genotypes combined. This strongly confirms the association between the 6A6A genotype and higher carotid IMT reported in Finnish men, in whom IMT was 15% higher in this group than in other genotypes.4 The inflammatory response may be modulated by polymorphisms in the promoter of the IL6 gene. We observed that carotid IMT was 11% greater among subjects homozygous for the G allele in comparison to other genotypes, confirming previous results.4 Genetic polymorphism in the promoter region of the HL gene plays a major role in the control of HDL2 cholesterol.15 High HL activity is associated with an increase in small dense LDL particles and increased risk of vascular events.8 We observed that individuals homozygous for the C allele of the HL gene had 13% higher carotid IMT than other genotypes.
In summary, our study showed a genetic effect on carotid IMT in the 3 candidate genes despite small sample size, which did not allow for the analysis of combined genotypes or test for gene×environment interaction. Increased carotid IMT may be an important link between genetic predisposition, environmental exposures, and stoke. Our study confirms the associations seen between the MMP3 and IL6 gene variants and carotid IMT in Finnish men4 and extends these associations to Hispanic subjects, who made up the majority of this sample. This is, according to our knowledge, the first report on association between the HL gene promoter variants and carotid IMT. Further studies with increased power are needed to confirm these genotype-phenotype associations.
Acknowledgments
Tanja Rundek is supported by the Hazel K. Goddess Fund for Stroke Research in Women.
Mitchell S. Elkind is supported by Centers for Disease Control Cooperative Agreement U50/CCU216543. John Pittman, Bernadette Boden-Albala, and Ralph L. Sacco are supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant NS-R01-29993. Suh-Hang Hank Juo is supported by National Institutes of Health/National Human Genome Research Institute grant IPA-5-20562. Steve Martin and Steve E. Humphries are supported by the British Heart Foundation (RG2000015). We would like to thank Dr J.P. Mohr for providing access to the ultrasound laboratory and supporting carotid atherosclerosis research. We also thank Drs Romel Ramas, Sam Trocio, and Oscar Ramos for performing the ultrasound scans.
Footnotes
Presented in part at the 26th American Heart Association International Conference on Stroke and Cerebral Circulation, Ft Lauderdale, Fla, February 14–16, 2001.
References
- 1.Wong M, Edelstein J, Wollman J, Bond MG. Ultrasonic-pathological comparison of the human arterial wall: verification of intima-media thickness. Arterioscler Thromb. 1993;13:482–486. doi: 10.1161/01.atv.13.4.482. [DOI] [PubMed] [Google Scholar]
- 2.Burke GL, Evans GW, Riley WA, Sharret AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr for the Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 4.Rauramaa R, Väisänen SB, Luong L-A, Schmidt-Trücksäss A, Penttilä IM, Bouchars C, Töyry J, Humphries SE. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2657–2662. doi: 10.1161/01.atv.20.12.2657. [DOI] [PubMed] [Google Scholar]
- 5.Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM. Preliminary report: genetic variation of the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73:209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Huphries SE, Wo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene: their effect on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra R, Wang J, Grundy SM, Cohen JC. A hepatic lipase (LIPC) allele associated with high plasma concentrations of high density lipoprotein cholesterol. Proc Natl Acad Sci U S A. 1997;94:4532–4537. doi: 10.1073/pnas.94.9.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen H, Verhoeven AJ, Weeks L, Kastelein JJ, Halley DJ, van den Ouweland A, Jukema JW, Seidell JC, Birkenhager JC. Common C-to-T substitution at position −480 of the hepatic lipase promoter associated with a lowered lipase activity in coronary artery disease patients. Arterioscler Thromb Vasc Biol. 1997;17:2837–2842. doi: 10.1161/01.atv.17.11.2837. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, Berglund L, Hauser WA, Shea S, Paik M. Race-ethnicity and determinants of carotid atherosclerosis in a multi-ethnic population: the Northern Manhattan Stroke Study. Stroke. 1997;28:929–935. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 10.Breslow JL, Ross D, McPherson J. Isolation and characterization of cDNA clones for human apolipoprotein A-I. Proc Natl Acad Sci U S A. 1982;79:6861–6865. doi: 10.1073/pnas.79.22.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolla MK, Martin SG, Wood NA, Humphries SE. Genotyping of the stromelysin-1 5A/6A promoter polymorphism using a heteroduplex generator. Thromb Haemost. 2000;83:790–792. [PubMed] [Google Scholar]
- 12.Talmud PJ, Berglund L, Hawe EM, Waterworth DM, Isasi CR, Deckelbaum RE, Starc T, Ginsberg HN, Humphries SE, Shea S. Age-related effects of genetic variation on lipid levels: the Columbia University BioMarkers Study. Pediatrics. 2001;108:E50. doi: 10.1542/peds.108.3.e50. [DOI] [PubMed] [Google Scholar]
- 13.Day INM, Humphries SE. Electrophoresis for genotyping: microtitre array diagonal gel electrophoresis (MADGE) on horizontal polyacryl-amide (H-PAGE) gels, Hydrolink or agarose. Anal Biochem. 1994;222:389–395. doi: 10.1006/abio.1994.1507. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–587. doi: 10.1001/jama.285.5.581. [DOI] [PubMed] [Google Scholar]
- 15.Juo SH, Han Z, Smith JD, Colangelo L, Liu K. Promoter polymorphisms of hepatic lipase gene influence HDL(2) but not HDL(3) in African American men: CARDIA study. J Lipid Res. 2001;42:258–264. [PubMed] [Google Scholar]