Summary
Granulysin is a cytolytic protein of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). Serum levels of granulysin are related to host cellular immunity. We used an ELISA to quantify granulysin serum levels in children with tuberculosis (TB), before and after chemotherapy. The study involved children affected by different clinical forms of TB (n = 72) and healthy control children (n = 150) from the same geographical area and of similar socio-economic background.
Serum granulysin levels before the initiation of TB therapy were significantly lower in children with TB compared to controls, with the lowest levels being found in TB patients who were PPD skin test negative. No statistically significant differences were found between serum granulysin levels and clinical severity (mild/moderate or advanced pulmonary TB) or the clinical form (pulmonary or extra-pulmonary) of TB. At four months after completion of therapy, serum granulysin levels in children treated for TB were not significantly different to those observed in control children. This finding was paralleled by the increased in vitro mycobactericidal activity of sera from TB patients after completion of therapy.
We propose that serum granulysin levels may provide a marker of disease activity in childhood TB and might be useful for monitoring improvement after chemotherapy.
Keywords: Serum granulysin, Tuberculosis, Disease activity, Therapy
Introduction
Granulysin is a cytolytic protein localized to granules of human cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells.1 It is the major known cytolytic protein responsible for CD8 CTL mediated antimicrobial activity after granules release upon T-cell receptor (TCR) stimulation. This molecule has also been identified in γδ T cell,2 in CD1-restricted cells3,4 and mycobacteria-specific CD85 and CD46 T cells. The cytolytic protein is synthesized as a 15-kDa precursor form which is then processed into the active 9-kDa granule form and, upon antigen stimulation, is released into the intercellular space between target and effector cells via a granule exocytosis pathway.7
Granulysin is a member of the saposin-like protein (SAPLIP) family of lipid-binding proteins8 which interact with membrane lipids and activate lipid-degrading enzymes, such as glucosylceramidase and sphingomyelinases with production of endogenous ceramide and subsequent induction of cell death through the apoptotic pathway.9
It has been demonstrated that granulysin has tumoricidal and antiviral activities and it is also able to inhibit the growth of pathogenic bacteria, fungi and parasites in vitro. For example, granulysin is able to kill extracellular Mycobacterium tuberculosis (M. tuberculosis) by itself and the intracellular bacteria in the presence of perforin.10 Recent findings have also suggested a possible role for granulysin in the in vitro inhibition of the growth/killing of Plasmodium falciparum by γδ T lymphocytes.11
Recently, an enzyme-linked immunosorbent assay (ELISA) method has been reported which measures serum granulysin: this assay, which only detects the 15-kDa form of the protein whose biological function is still unknown, has shown an association between serum levels of granulysin and the activity of NK cells and CTL, thus representing a soluble biomarker of cell-mediated immune responses.12 Serum granulysin has been also proposed as a marker of Th1/Th2 ratio in pre-eclampsia.13
Moreover, granulysin expression by NK cells is considered a useful prognostic tool of some types of cancer, being related to their positive outcome.14
In leprosy, granulysin-expressing T cells were detected with a six-fold greater frequency in the skin lesions of patients with the localized tuberculoid form than those with the disseminated lepromatous form of disease, correlating the clinical form of disease with the frequency of granulysin-expressing T cells. These data suggest granulysin involvement in cell-mediated immune response against mycobacteria.6
We have previously reported that γδ T cells from children affected by active tuberculosis (TB) have lower levels of intracellular granulysin and produce less IFN-γ when compared to healthy controls. Anti-TB therapy normalizes these responses, suggesting granulysin and IFN-γ involvement in host defensive immune responses against M. tuberculosis.15
As biomarkers of protective immune responses are considered to have important potential in evaluating efficacy of TB interventions, including therapy or vaccination, and would constitute useful tools for clinical monitoring, we decided to measure serum granulysin levels in children affected by TB and healthy controls, in order to assess whether circulating granulysin levels correlated with disease activity and the clinical outcome after successful therapy.
Materials and methods
Patient population
The study involved children affected by different clinical forms of TB (n = 72) and healthy control children (n = 150) from the same geographical area (Sicily, southern Italy) and of similar socio-economic background. None of the children had been vaccinated with BCG in infancy. None of the case or control patients had evidence of human immunodeficiency virus (HIV) infection, or was being treated with steroids or anti-tubercular drugs at the time of diagnosis and first sampling. The baseline characteristics of TB patients and control children are shown in Table 1. Diagnosis of TB was established by the presence of clinical symptoms of TB, by chest radiography and by the positivity of the PPD skin test, and was confirmed by symptomatic improvement after chemotherapy. Pulmonary TB cases were classified according to international classification. Mild TB was defined by the presence of scattered and non-confluent pulmonary infiltrates of slight to moderate density, in one or both lungs with the total volume less than one lung, without cavities. Moderate TB was defined by pulmonary infiltrates present in one or both lungs with: (i) scattered lesions of slight to moderate density, not involving more than one lung, (ii) dense, confluent lesions, not involving more than one third of the volume of one lung, and (iii) a total diameter of cavities <4 cm. In our study, mild TB and moderate TB were grouped together. Advanced TB was defined by lesions exceeding the above criteria. Positive cultures of M. tuberculosis or M. tuberculosis detection by polymerase chain reaction were obtained in 5/7 TB meningitis, 1/1 pleural and 1/1 renal TB cases, and further supported the diagnosis. Although most of the TB cases are likely to represent primary TB, the possibility that some cases represent reactivation cannot be excluded. Tuberculous children were treated with two weeks of daily isoniazid, rifampin, and pyrazinamide; then six weeks of the same combination twice weekly, followed by 16 weeks of twice-weekly isoniazid and rifampin without pyrazinamide. Response to therapy was defined as improvement in symptoms, weight gain, improvement by physical examination and chest radiography. The PPD+ healthy children included in this study were not household contacts of known TB cases. PPD skin test was considered positive when the induration diameter was larger than 5 mm at 72 h after injection of 1 U of PPD (Statens SerumInstitut, Copenaghen, Denmark). The study was approved by the ethical committee of the University Hospital in Palermo, and informed consent was given by children’s parents.
Table 1.
Baseline characteristics of TB patients and control children
| TB patients |
Healthy controls |
|||
|---|---|---|---|---|
| PPD+ | PPD− | PPD+ | PPD− | |
| Number | 53 | 19 | 113 | 37 |
| Males | 33 | 11 | 75 | 21 |
| Females | 20 | 8 | 38 | 16 |
| Mean age (y) (mean ± SE) | 8.2 ± 4.1 | 6.8 ± 2.7 | 7.3 ± 4.3 | 6.9 ± 3.7 |
| Age range (y) | 1–12 | 2–12 | 1–14 | 3–14 |
| Clinical forms of TB | ||||
| Pulmonary | 38 | 14 | ||
| Lymphadenitis | 5 | 0 | ||
| Meningitis | 7 | 3 | ||
| Renal | 1 | 1 | ||
| Pleural | 1 | 1 | ||
| Pleuro-pulmonary | 1 | 0 | ||
NOTE. TB, tuberculosis; PPD+, purified protein derivative positive; PPD−, purified protein derivative negative.
ELISA for measurement of serum granulysin
A sandwich ELISA system was developed using the anti-granulysin mAbs DH5 as the capturing Ab and DH4 as the detecting Ab. Both DH4 and DH5 mAbs recognize the 9-and the 15-KDa forms in human sera, as confirmed by Western Blotting analyses on 10 sera from healthy children and 10 sera from TB patients (data not shown). Generation of anti-granulysin mAbs is described elsewhere.7 Recombinant 9-kDa granulysin, produced as previously described9 was used to set a standard curve.
Sera were collected before initiation of TB therapy and four months after completion of therapy, and stored at −80 °C. All samples were titrated two-fold in the same solution and assayed in triplicate on the ELISA plate. An ELISA plate (Nunc MaxiSorp) was coated overnight at 4 °C with 100 μl of 4 μg/ml anti-granulysin DH5 mAb in tris buffered saline (TBS). After removing capture antibody solution, plates were blocked with 200 μl of 4% (w/v) non-fat dry milk in TBS containing 0.1% tween 20 (TBST) for 1 h at 37 °C, washed once with TBST and incubated for 1 h at 37 °C with 100 μl of plasma samples or recombinant 9-kDa granulysin for assay calibration. Plates were washed three times with TBST, and incubated for 1 h at 37 °C with 100 μl of anti-granulysin DH4 biotinylated mAb (5 μg/ml) and for 45 min at RT with avidine–peroxidase (1:300) (Sigma) both diluted in TBST, with three washes of TBST in between and after. The plates were then developed colorometrically with 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, Sigma) in the presence of H2O2 (1:100), and read at OD 405 nm with an ELISA multi-well reader (Sigma Diagnostics). This ELISA system could specifically detect granulysin with a detection limit around 25 pg/ml.
Coculture of sera with extracellular M. tuberculosis
The effects of sera from TB patients before and after therapy, on the viability of extracellular M. tuberculosis were assessed according to our previously described method.2,10 Briefly, sera were added to 104 M. tuberculosis bacilli in 7H9 medium supplemented with ADC for 72 h at 37 °C. Cultures of sera with free M. tuberculosis bacilli were tested at the end of incubation without further treatment. Serial 10-fold dilutions were made in 7H9 broth and were plated on 7H10 agar plates. Plates were sealed in plastic and were kept at 37 °C, and the colonies were counted after 14–21 days.
Statistical analysis
Data are presented as median + interquartile (IQ) range. Differences among group were evaluated by Mann–Whitney test. Values of p < 0.05 were considered significant.
Results
Serum granulysin levels in tuberculosis
Serum granulysin levels before the initiation of TB treatment were significantly lower in children with TB compared to controls (Fig. 1A): before therapy, median granulysin levels in TB patients were 0.45 + 0.21 ng/ml (range 0.33–2.98), whereas controls had values of 1.4 + 1.1 ng/ml (range 0.22–6), significant with p value <0.005. Amongst children with TB, those who were PPD skin test negative at the time of diagnosis had the lowest serum levels of granulysin (0.36 + 0.09 ng/ml; range 0.30–1.5; n = 19), when compared to skin test positive TB patients (0.48 + 0.20 ng/ml; range 0.33–2.98; n = 53), but this difference was not statistically significant (p = 0.081). Conversely, no such difference was found between the corresponding healthy controls: serum granulysin levels in PPD positive (1.40 ± 1.3 ng/ml; range 0.22–6; n = 113) and negative children (1.40 ± 0.80 ng/ml; range 0.41–6, n = 37), were comparable and statistically not different (p = 0.061; Fig. 1B).
Fig. 1.
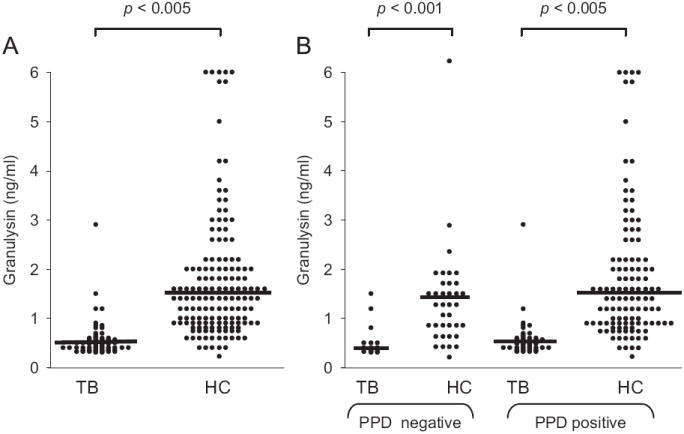
(A) Serum granulysin levels in children with tuberculosis (TB) and healthy control (HC) subjects. (B) Serum granulysin levels in PPD skin test positive or negative TB children and healthy controls.
Serum granulysin levels and clinical severity
No statistically significant differences were found when granulysin levels were analyzed either in relation to clinical severity (mild/moderate TB versus advanced TB; Fig. 2A) as assessed by X-ray, or in relation to the pulmonary or extrapulmonary forms of TB (Fig. 2B). Similarly, serum granulysin levels were also not associated with age and sex of patients or controls (data not shown).
Fig. 2.
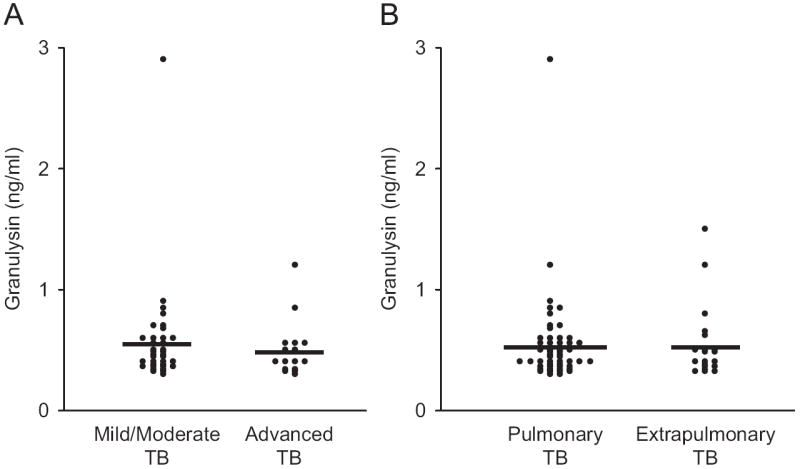
(A) Serum granulysin levels in children with mild/moderate versus advanced pulmonary TB. (B) Serum granulysin levels in children with pulmonary TB versus extra-pulmonary TB.
Follow-up study
At four months after completion of anti-TB therapy, serum granulysin levels were significantly increased in children with TB (from 0.45 + 0.21 up to 1.20 + 0.95 ng/ml) and this occurred both in PPD skin test positive and negative TB patients, not significantly different to values observed in control children (Fig. 3). Granulysin levels remained particularly low in one child with advanced pulmonary TB and two children with TB meningitis. Of these three patients, only one patient had documented treatment failure, while the remaining two patients apparently had been treated successfully as judged by improvement of clinical conditions.
Fig. 3.
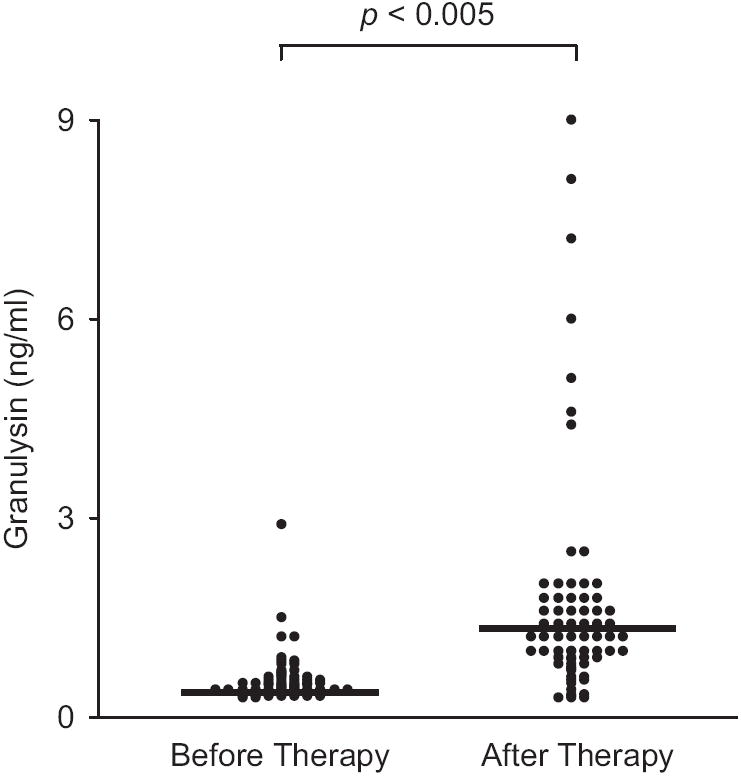
Serum granulysin concentration in children with TB, before therapy and four months after completion of therapy.
Granulysin levels were unchanged in five children with pulmonary TB and one child with renal TB, although none of them showed treatment failure.
Thus, our results clearly indicate that measurement of serum granulysin levels provide a marker of TB disease activity, but not TB disease severity, in children.
Anti-mycobacterial activity of sera from TB patients
To assess the anti-mycobacterial activity of sera from patients during active disease and after therapy, we tested the ability of sera from 10 patients at the time of diagnosis (i.e. starting therapy) and four months after completion of therapy, to reduce in vitro the viability of extracellular M. tuberculosis. As shown in Fig. 4A, sera taken from patients before therapy only slightly reduced the viability of extracellular M. tuberculosis (7.4 ± 3.6%), but sera taken from the same patients at four months after completion of therapy significantly reduced the viability of M. tuberculosis (33 ± 11.2%) and the differences in M. tuberculosis CFU counts after incubation with pre- and post-therapy sera were statistically significant (p = 0.0027). Additionally, as shown in Fig. 4B, a very good correlation was found between granulysin concentration in sera and their ability to inhibit the growth of M. tuberculosis (r = 0.9458; p < 0.0001).
Fig. 4.
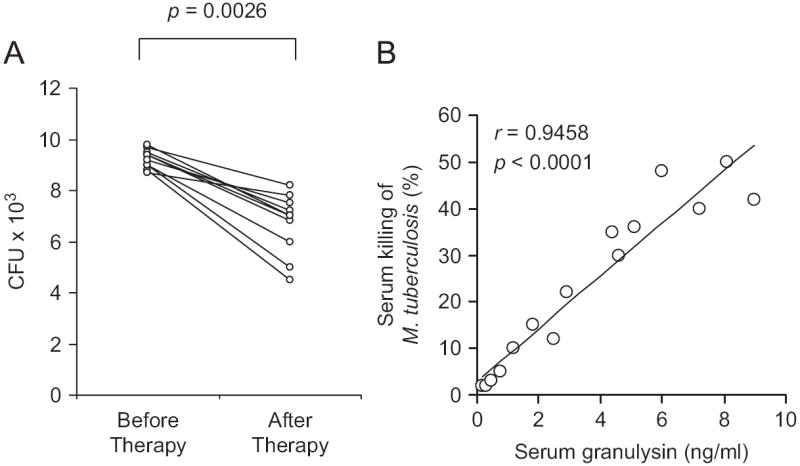
(A) Ability of sera from children with TB before therapy and four months after completion of therapy, to reduce in vitro the viability of M. tuberculosis. Results with individual sera are shown and data are expressed as numbers of M. tuberculosis bacilli CFU. (B) Correlation between serum granulysin levels and the ability of serum to reduce in vitro the viability of M. tuberculosis.
Discussion
Granulysin is a cytolytic granule protein with a broad range of antimicrobial and tumoricidal activities. Recently, two short synthetic peptides derived from granulysin, have been shown to inhibit the in vitro growth of clinical isolates of both multidrug resistant and drug susceptible strains of M. tuberculosis.16 Relevant to M. tuberculosis infection, most T cell subsets with anti-mycobacterial activity express granulysin2-6 and, at least for some of them, requirement for granulysin has been found as a requisite to kill both intracellular and extracellular M. tuberculosis,2 inferring an important role of granulysin in the protective immune response against M. tuberculosis. Accordingly, recent in vivo mouse studies have demonstrated that delivery of a recombinant plasmid containing full-length cDNA of granulysin significantly reduced the numbers of viable bacilli in the lung lesions indicating that granulysin has a therapeutic effect against M. tuberculosis.17 In another study, a viable therapeutic vaccine of recombinant M. smegmatis containing the IL-12 and the granulysin genes induced efficient protective immune response against M. tuberculosis.18
Because of the importance of correlates of protective immune responses as potential tools to evaluate the efficacy of TB interventions, including therapy or vaccination, we decided to measure serum granulysin levels in children affected by TB and healthy controls, in order to assess whether serum granulysin levels correlated with disease activity and the clinical outcome after therapy.
Results here reported show that serum granulysin levels before the initiation of TB therapy were significantly lower in children with TB compared to controls, with the lowest levels being found in TB patients who were PPD skin test negative at the time of diagnosis. Interestingly, we did not find any significant differences between serum granulysin levels and clinical severity (mild/moderate or advanced) of pulmonary TB or the clinical form (pulmonary or extrapulmonary) of TB. At four months after completion of therapy, serum granulysin levels were increased in children with TB, not significantly different to values observed in control children.
In this study, we were unable to determine granulysin levels in relation to treatment failure, due to the low number of failure cases in our cohort (one case). However, after TB therapy, granulysin levels decreased in three patients and remained unchanged in additional six patients. Thus, a total of 9 out of 72 (12.5%) did not show any increase in serum granulysin. In the accompanying paper by Sahiratmadja et al., granulysin remained low in 17.5% of patients with mild/moderate TB and 23.7% of patients with advanced TB. Therefore, additional longer follow-up studies are necessary to obtain better insight into the dynamics of serum granulysin levels after anti-TB therapy.
The finding of increased serum levels of granulysin at four months after completion of therapy was paralleled by the increased in vitro mycobactericidal activity of sera from TB patients, which correlates with granulysin concentration. These results are worth of additional comments. Granulysin has been shown to kill M. tuberculosis at higher concentrations than those found in sera.10 Therefore, it is possible that other components in sera contribute to the killing of M. tuberculosis. Antibacterial activity of sera does not appear to be attributable to administration of antituberculous drugs on the grounds that (a) sera were collected four months after completion of therapy to allow washout of drugs from serum and (b) we did not find any difference in serum granulysin in 40 healthy PPD+ children before, and four months after completion of a nine-month isoniazide prophylaxis (our unpublished observations). Thus, it is likely that the increased serum anti-tuberculous activity post therapy could be attributable to increased concentration of peptides with anti-mycobacterial activity, other than granulysin. Further studies with neutralization of granulysin activity by specific antibodies are required to confirm this hypothesis.
Previous studies have correlated granulysin levels with other pathological states. These include the analysis of granulysin expression in leprosy model as a relevant marker for effective host defence.6 T cells expressing granulysin, especially CD4 cells, were found more abundant (8–15%), in the skin lesions of patients with the localized tuberculoid form as compared to those with the disseminated lepromatous form of disease, suggesting a correlation between the clinical form of disease and the frequency of T cell expressing granulysin: localized disease is associated with increased granulysin expression. Similarly, children affected by TB have lower levels of serum granulysin than healthy contacts, i.e. those subjects who control M. tuberculosis infection.
In addition, high granulysin serum levels were found in primary viral infections, such as those with parvovirus B19 and Epstein-Barr virus,12 especially during the acute phase of disease and thereafter granulysin levels rapidly decrease to normal range during the convalescent phase.12 A transient granulysin increase was also found in both lung19 and kidney20 of transplant rejection and measurement of serum granulysin concentration has been suggested as a useful marker of graft-versus-host reaction in patients with hematopoietic stem-cell transplantation.21 Also in preeclamptic patients, serum levels of granulysin were significantly elevated when compared to those of normal pregnancy subjects.10 By contrast, very low granulysin serum levels have been detected in patients with severe immunodeficiencies12 and a reduced expression of this protein in different cell types or even in serum is observed in carcinoma patients, which could be correlated with tumor progression.14,22,23
The reason for the reduction of serum granulysin concentration during active TB and its recovery after therapy is unknown. One possibility is that granulysin is rapidly consumed during active disease, because of the ongoing effector immune response. Another possibility is that serum granulysin is reduced during active disease because of a reduction of the T cell subset dedicated to its production. In agreement with this possibility, we previously reported that γδ T cells expressing granulysin are decreased during active TB, but they recover after therapy.15 Similarly, effector CD8 T cells expressing perforin (and presumably also granulysin) specific for the mycobacterial antigen 85A, are also decreased during active TB, but recover after therapy.24 Thus the decrease, or even the loss, of many different T cell subsets which secrete granulysin during active disease, might well explain the low granulysin concentration observed in such patients. Alternatively, we must also consider the possibility that the increased serum granulysin levels after therapy may represent increased production of granulysin from the same number of cells, rather than an increase in numbers of granulysin-producing cells.
However, further analysis of granulysin-producing cells during active TB and after completion of therapy are required to define the underlying mechanisms.
In conclusion, results reported in this paper clearly indicate that granulysin is not a marker of TB severity but rather a marker of TB disease activity. The potential use of serum granulysin levels to monitor TB disease activity and response to treatment merits further investigation.
Acknowledgments
We thank Prof. T.H.M. Ottenhoff and Dr. E. Sahiratmadja for critically reviewing the manuscript. This work has been supported by grants from the Ministry for Instruction, University and Research (MIUR-PRIN to FD), the University of Palermo (60% to FD) and the European Commission (FP6 EU-TBVAC, to FD).
References
- 1.Pena SV, Krensky AM. Granulysin. A new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol. 1997;9:117–25. doi: 10.1006/smim.1997.0061. [DOI] [PubMed] [Google Scholar]
- 2.Dieli FM, Troye-Blomberg J, Ivanyi JJ, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9Vδ2 T lymphocytes. J Infect Dis. 2001;184:1082–5. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 3.Spada FM, Grant EP, Peters PJ, et al. Self-recognition of CD1 by γδ T cells: implications for innate immunity. J Exp Med. 2000;191:937–48. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 5.Gansert JL, Kiessler V, Engele M, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–61. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa MT, Stenger S, Sieling PA, et al. T-cell release of granulysin contributes to host defense in leprosy. Nat Med. 2001;7:174–9. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]
- 7.Hanson DA, Kaspar AA, Poulain FR, Krensky AM. Biosynthesis of granulysin, a novel cytolytic molecule. Mol Immunol. 1999;36:413–22. doi: 10.1016/s0161-5890(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 8.Pushkareva M, Obeid LM, Hannun YA. Ceramide: an endogenous regulator of apoptosis and growth suppression. Immunol Today. 1995;16:294–7. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 9.Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. Involvement of at least two distinct pathways. J Immunol. 1998;161:1758–64. [PubMed] [Google Scholar]
- 10.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 11.Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M. γδ T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol. 2004;342:2248–56. doi: 10.1002/eji.200424861. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa K, Takamori Y, Suzuki K, et al. Granulysin in human serum as a marker of cell-mediated immunity. Eur J Immunol. 2003;33:1925–33. doi: 10.1002/eji.200323977. [DOI] [PubMed] [Google Scholar]
- 13.Sakai M, Ogawa K, Shiozaki A, et al. Serum granulysin is a marker for Th1 type immunity in pre-eclampsia. Clin Exp Immunol. 2004;136:114–9. doi: 10.1111/j.1365-2249.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishi A, Takamori Y, Ogawa K, et al. Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol Immunother. 2002;50:604–14. doi: 10.1007/s002620100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieli F, Sireci G, Caccamo N, et al. Selective depression of interferon-gamma and granulysin production with increase of proliferative response by Vγ9Vδ2 T cells in children with tuberculosis. J Infect Dis. 2002;186:1835–9. doi: 10.1086/345766. [DOI] [PubMed] [Google Scholar]
- 16.Toro JC, Hoffner S, Linde C, Andersson M, Andersson J, Grundstrom S. Enhanced susceptibility of multidrug resistant strains of Mycobacterium tuberculosis to granulysin peptides correlates with a reduced fitness phenotype. Microbes Infect. 2006;8:1985–93. doi: 10.1016/j.micinf.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Liu S, Qu X, Liu J. Construction of a eukaryotic expression system for granulysin and its protective effect in mice infected with Mycobacterium tuberculosis. J Med Microbiol. 2006;55:1389–93. doi: 10.1099/jmm.0.46706-0. [DOI] [PubMed] [Google Scholar]
- 18.Yi Z, Fu Y, Yang C, et al. Recombinant M. smegmatis vaccine targeted delivering IL-12/GLS into macrophages can induce specific cellular immunity against M. tuberculosis in BALB/c mice. Vaccine. 2007;25:638–48. doi: 10.1016/j.vaccine.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Soccal PM, Doyle RL, Jani A, et al. Quantification of cytotoxic T-cell gene transcripts in human lung transplantation. Transplantation. 2000;69:1923–7. doi: 10.1097/00007890-200005150-00030. [DOI] [PubMed] [Google Scholar]
- 20.Sarwal MM, Jani A, Chang S, et al. Granulysin expression is a marker for acute rejection and steroid resistance in human renal transplantation. Hum Immunol. 2001;62:21–31. doi: 10.1016/s0198-8859(00)00228-7. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa M, Isoda T, Itoh S, et al. Analysis of serum granulysin in patients with hematopoietic stem-cell transplantation: its usefulness as a marker of graft-versus-host reaction. Am J Hematol. 2006;81:340–8. doi: 10.1002/ajh.20570. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa M, Kawamoto H, Tsuji Y, Mizutani S. Transient increase of serum granulysin in a stage IVs neuroblastoma patient during spontaneous regression: case report. Int J Hematol. 2005;82:456–7. doi: 10.1532/IJH97.05091. [DOI] [PubMed] [Google Scholar]
- 23.Pages F, Berger A, Camus M, et al. Effector memory Tcells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 24.Caccamo N, Meraviglia S, La Mendola C, Guggino G, Dieli F, Salerno A. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. J Immunol. 2006;177:1780–5. doi: 10.4049/jimmunol.177.3.1780. [DOI] [PubMed] [Google Scholar]


