Abstract
Information processing models of posttraumatic stress disorder (PTSD) suggest that PTSD is characterized by preferential allocation of attentional resources to potentially threatening stimuli. However, few studies have examined the neural pattern underlying attention and emotion in association with PTSD symptomatology. In the present study, combat veterans with PTSD symptomatology engaged in an emotional oddball task while undergoing functional magnetic resonance imaging (fMRI). Veterans were classified into a high or low symptomatology group based on their scores on the Davidson Trauma Scale (DTS). Participants discriminated infrequent target stimuli (circles) from frequent standards (squares) while emotional and neutral distractors were presented infrequently and irregularly. Results revealed that participants with greater PTSD symptomatology showed enhanced neural activity in ventral-limbic and dorsal regions for emotional stimuli and attenuated activity in dorsolateral prefrontal and parietal regions for attention targets. In the anterior cingulate gyrus, participants with fewer PTSD symptoms showed equivalent responses to attentional and emotional stimuli while the high symptom group showed greater activation for negative emotional stimuli. Taken together, the results suggest that hyperresponsive ventral-limbic activity coupled with altered dorsal-attention and anterior cingulate function may be a neural marker of attention bias in PTSD.
Keywords: PTSD, fMRI, information processing, attentional bias, trauma, oddball task
1. INTRODUCTION
Rates of posttraumatic stress disorder (PTSD) in returning veterans from Iraq and Afghanistan are high, with some estimates showing that close to 20% of Army and Marine troops meet criteria for PTSD three to four months post-deployment (Hoge et al., 2006). PTSD in these veterans is associated with cognitive deficits and functional impairment in everyday life (Hoge et al., 2006; Vasterling et al., 2006). Neuropsychological studies suggest that the nature of these cognitive deficits is more closely related to inattention and interference during the encoding process than retention loss due to amnesia (Vasterling, 2005). One such source of interference in PTSD may be attentional bias to threatening information, which disrupts ongoing cognitive activities by redirecting attentional resources away from the cognitive task at hand. For instance, combat veterans show longer response latencies while naming the color of the ink used to print trauma-related words during an emotional Stroop task (McNally et al., 1990; Kaspi et al., 1995; Constans et al., 2004). It is therefore hypothesized that delayed naming of emotional words represents a diversion of attention away from neutral stimuli toward traumatic stimuli in individuals with PTSD. Further evidence for attentional bias in PTSD comes from ERP studies that show enhanced P3 amplitude responses to threat or novel distracting stimuli (Attias et al., 1996; Kimble et al., 2000; Stanford et al., 2001). These studies employed modified oddball paradigms in which infrequent salient target stimuli were interspersed with frequent standard stimuli. Alterations in P3 amplitudes and latencies for emotional versus neutral stimuli provide evidence for heightened responsivity to potentially threatening stimuli in PTSD.
While there is evidence for attentional bias for threat from behavioral and ERP studies, evidence from neuroimaging studies for threat bias is scarce. The majority of previous neuroimaging studies in PTSD have focused on investigating provocation of trauma symptoms (Rauch et al., 1996; Bremner et al., 1999; Liberzon et al., 1999; Shin et al., 1999; Lanius et al., 2001; Lanius et al., 2002; Pissiota et al., 2002; Lanius et al., 2003a; Gilboa et al., 2004; Shin et al., 2004; Yang et al., 2004; Britton et al., 2005; Sakamoto et al., 2005), while others have examined cognition in PTSD such as working memory impairment without inclusion of emotional stimuli (Clark et al., 2003). However, in order to understand the neural circuitry underlying concentration and attention difficulties in relation to PTSD symptomatology, examination of both emotion and executive processing is necessary. Few neuroimaging studies have examined both emotion and attention within the same paradigm to simulate emotional distraction that occurs during cognitive task performance in real life. Neuroimaging studies that have employed emotional Stroop tasks in patients with PTSD (Shin et al., 2001; Bremner et al., 2004) combine the focus of both emotion and attention systems on the same stimulus, rendering it difficult to separate these two processes.
The goal of the present study was to examine the neural circuitry underlying alterations in attention by emotional distraction in veterans with symptoms of PTSD. We employed a modified emotional oddball paradigm based on our previous work in healthy adults, in which we demonstrated that emotion and attention function segregate into two large-scale neural networks (Yamasaki et al., 2002), with emotional content engaging fronto-limbic regions including the amygdala and inferior prefrontal cortex (IFG) and attentional targets engaging dorsolateral prefrontal cortex and posterior parietal regions. Additionally, emotional distractors and attentional targets both engage the rostral anterior cingulate cortex (ACC) suggesting that this region is important for integrating executive and emotion processing streams (Yamasaki et al., 2002; Fichtenholtz et al., 2004). The present study tested several hypotheses. First, PTSD symptom severity should be related to greater activation in ventral-limbic regions during the emotional condition, including the ventromedial prefrontal cortex (vmPFC) and amygdala. Second, symptom severity should be related to attenuated activity in dorsal frontal and parietal regions during the attention task, such as the middle frontal gyrus (MFG) and supramarginal gyrus (SMG) reflecting the disruption of executive pathways in PTSD. Finally, based on our previous work, we hypothesized that PTSD symptomatology would be related to an altered pattern of ACC activity for emotional and target stimuli.
2. METHODS
2.1. Participants
Twenty-six recently returned (mean ± standard deviation; 23 ± 14 months) veterans from deployments to post-9/11 military conflicts completed the fMRI procedures. Veterans were recruited from a large recruitment database for the study of post-deployment mental health. Veterans entering the registry completed a neuropsychiatric self-assessment battery which included the Davidson Trauma Scale (DTS; Davidson et al., 1997), Beck Depression Inventory-II (BDI-II; Beck et al., 1996), Combat Exposure Scale (CES; Keane, 1989), Trauma Life Events Questionnaire (TLEQ; Kubany et al., 2000), Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993), and Drug Abuse Screening Test (DAST; Skinner, 1982). Veterans with prior history of combat trauma as assessed by the TLEQ were contacted by phone and screened to exclude those with a history of psychotic symptoms, serious medical illness, head injury resulting in a loss of consciousness, or shrapnel or other metal in their bodies. Twenty-one participants served in either Iraq or Afghanistan, while the remaining subjects served in other regions including Kuwait and Saudi Arabia. Participants provided written informed consent for procedures approved by the Institutional Review Boards at Duke University and the Durham VA Medical Center.
The DTS is a 17-item self report measure that assesses trauma symptoms and has high test-retest reliability, internal consistency, convergent and divergent validity, and predictive validity (Davidson et al., 1997). This measure was administered to participants prior to scanning to obtain a current measure of PTSD symptomatology. A cutoff score of 40, which has previously shown to have good diagnostic accuracy against a clinician-administered interview (Davidson et al., 1997), was used to partition participants into a low DTS group (n = 12; M DTS = 14; SD = 14) and a high DTS group (n = 14; M DTS = 71; SD = 23).
Subject demographics are summarized in Table 1. The groups did not differ on age, education, gender, ethnicity, handedness, alcohol use as assessed by the AUDIT, or drug abuse as assessed by the DAST. The high DTS group reported greater exposure to combat situations than the low DTS group as assessed by the CES. The high DTS group also had significantly higher scores on the BDI than the low DTS group. Three participants (2 high DTS and 1 low DTS) were taking antidepressant medication that included selective serotonin reuptake inhibitors and/or norepinephrine dopamine modulators.
Table 1.
Demographic and clinical characteristics of subject sample
| Characteristic | High DTS n = 14 | Low DTS n = 12 | t/Chi Square | P |
|---|---|---|---|---|
| Age (years), [Standard Deviation] | 35.5 [3.6] | 37.2 [11.6] | 0.35 | > 0.73 |
| Gender, No. (%) of females | 4 (29) | 1 (8.3) | 1.70 | > 0.19 |
| Handedness, No. (%) right-handed | 12 (86) | 12 (100) | 1.86 | > 0.17 |
| Ethnicity, No. (%) of Caucasian subjects | 7 (50) | 8 (66.7) | 0.74 | > 0.39 |
| Education (years), [std dev] | 14.5 [1.6] | 15.8 [3.2] | 1.28 | > 0.21 |
| Davidson Trauma Scale [std dev] | 71 [23] | 14 [14] | 7.30 | < 0.001 |
| Combat Exposure Scale [std dev] | 17.1 [8.8] | 8.7 [9.1] | 2.41 | < 0.03 |
| Beck Depression Inventory [std dev] | 22.9 [9.5] | 9.6 [8.3] | 2.29 | < 0.04 |
| AUDIT [std dev] | 4.0 [3.6] | 5.7 [5.8] | 0.87 | > 0.39 |
| Drug Abuse Screening Test [std dev] | 1.21 [2.0] | 0.33 [1.15] | 1.32 | > 0.20 |
AUDIT = Alcohol Use Disorders Identification Test; DTS = Davidson Trauma Scale
2.2. Stimulus presentation
Four categories of stimuli were displayed: (i) emotional distractor pictures from the International Affective Picture System (IAPS; Lang et al., 2005) (ii) neutral distractor pictures matched with negative pictures for luminance, presence of human figures, and chromatic features (iii) baseline standards consisting of squares of varying colors and sizes (iv) attentional targets consisting of circles of varying colors and sizes.
Negative IAPS pictures were utilized in this study in lieu of Iraq War combat-specific pictures for their known psychometric properties. Pictures included depictions of mutilations, burn victims, attacks and aimed guns, sick or crying children, and medical illnesses. Arousal and valence ratings of each negative picture included in the study are shown in Table 2.
Table 2.
IAPS Valence and Arousal
| Picture ID | Picture Description | Valence | Arousal |
|---|---|---|---|
| 2053 | Baby | 2.47 | 5.25 |
| 2800 | Sad Child | 1.78 | 5.49 |
| 2810 | Boy | 4.31 | 4.47 |
| 2900 | Crying Boy | 2.45 | 5.09 |
| 3000 | Mutilation | 1.45 | 7.26 |
| 3030 | Mutilation | 1.91 | 6.76 |
| 3053 | Burn Victim | 1.31 | 6.91 |
| 3060 | Mutilation | 1.79 | 7.12 |
| 3063 | Mutilation | 1.49 | 6.35 |
| 3071 | Mutilation | 1.88 | 6.86 |
| 3080 | Mutilation | 1.48 | 7.22 |
| 3100 | Burn Victim | 1.60 | 6.49 |
| 3110 | Burn Victim | 1.79 | 6.70 |
| 3120 | Dead Body | 1.56 | 6.84 |
| 3150 | Mutilation | 2.26 | 6.55 |
| 3160 | Eye Disease | 2.63 | 5.35 |
| 3220 | Hospital | 2.49 | 5.52 |
| 3230 | Dying Man | 2.02 | 5.41 |
| 3280 | Dental Exam | 3.72 | 5.39 |
| 3350 | Infant | 1.88 | 5.72 |
| 3400 | Severed Hand | 2.35 | 6.91 |
| 3530 | Attack | 1.80 | 6.82 |
| 3550 | Injury | 2.54 | 5.92 |
| 6190 | Aimed Gun | 3.57 | 5.64 |
| 6212 | Soldier | 2.19 | 6.01 |
| 6243 | Aimed Gun | 2.33 | 5.99 |
| 6244 | Aimed Gun | 3.09 | 5.68 |
| 6250 | Aimed Gun | 2.83 | 6.54 |
| 6510 | Attack | 2.46 | 6.96 |
| 6550 | Attack | 2.73 | 7.09 |
| 6570 | Suicide | 2.19 | 6.24 |
| 9007 | Needles | 2.49 | 5.03 |
| 9040 | Starving Child | 1.67 | 5.82 |
| 9041 | Scared Child | 2.98 | 4.64 |
| 9253 | Mutilation | 2.00 | 5.53 |
| 9400 | Soldier | 2.50 | 5.99 |
| 9404 | Soldiers | 3.71 | 4.67 |
| 9405 | Sliced Hand | 1.83 | 6.08 |
| 9420 | Soldier | 2.31 | 5.69 |
IAPS = International Affective Picture System
Stimuli were presented for 1.5 sec with a 2.0 sec stimulus onset asynchrony in an event-related design. Target circles and emotional and neutral distractors were pseudorandomly distributed throughout each run. The interval between successive rare stimuli (i.e., targets, distractors, or both) was randomized. Eight runs lasted 4 min and 52 sec each. Stimuli consisted of 891 standards (84.4% of all trials), 87 attentional targets (8.2%), and 39 each of emotional and neutral pictures (3.7%). Participants were instructed to press the same button for all standard, emotional and neutral stimuli, but a different button press for target stimuli.
2.3. MRI acquisition and analysis
Functional images were acquired on a 3-Tesla GE scanner. fMRI data were collected with a gradient-echo inverse spiral pulse sequence (Guo and Song, 2003) with the following imaging parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms, field of view (FOV) = 24; 34 axial slices parallel to the AC-PC plane, 3.75 × 3.75 × 3.8 mm. High-resolution 3D SPGR images (TR = 12 ms; TE = 5.4 ms; FOV = 24) covering the entire brain were acquired to aid in normalization and coregistration. Functional data sets were preprocessed using FSL version 3.3.5 (Smith et al., 2004). Preprocessing was applied to individual participants’ data in the following steps: (i) brain extraction for non-brain removal (Smith, 2002), (ii) motion correction using MCFLIRT (Jenkinson et al., 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor, and (v) high-pass filtering (Jenkinson et al., 2002; Smith et al., 2004). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images.
Statistical analyses consisting of both voxel-based and region of interest (ROI) analyses were performed using custom software. For each individual, the fMRI signal was selectively averaged as a function of trial type (i.e., emotional, neutral, and target) and two pre-stimulus and eight post-stimulus time points (i.e., image volumes). Epoch averages were correlated at each voxel with a canonical hemodynamic response function. T-statistics from each participant were then submitted to a random effects group analysis to evaluate whether the mean effect from the population differs from zero. For group difference analyses, time points around the peak signal change for each condition were averaged within each individual and then contrasted between the two groups.
Functional ROIs were selected from voxels showing maximum effects in the contrasts of interest as identified by voxel-based analyses. For both voxel- and ROI-based analyses, an intensity threshold of P < 0.002 (two-tailed) and extent threshold of 10 contiguous voxels was used for a priori areas of interest, and P < 0.001 and extent threshold of 10 voxels for all other areas. Selection of a priori areas of interest was guided by hypotheses derived from our previous emotional oddball studies (Yamasaki et al., 2002; Fichtenholtz et al., 2004; Morey et al., 2008) and included IFG, amygdala, vmPFC, orbitofrontal cortex (OFG), MFG, SMG, and the cingulate cortex. Percent signal change at time points 2, 4, 6, and 8 seconds poststimulus for each ROI was analyzed by repeated measures MANOVA. An alpha level of 0.05 was used to determine significant activity in all MANOVA contrasts.
Finally, to investigate individual differences regarding symptom severity and executive and emotion processing, multiple regression analyses between fMRI data and DTS and BDI were performed within ROIs identified by voxel-based analyses of contrasts of interest (i.e., emotion > target and target > emotion).
3. RESULTS
3.1. Behavioral performance
Behavioral analysis is based on 11 high DTS participants and 11 low DTS participants. Three participants were excluded from the behavioral analysis because they did not make a button press to distractor pictures, and one participant was excluded from the behavioral analysis for making the same button press to both targets and distractors. MANOVA for reaction time with condition (emotion, neutral, target, standard) as a within subjects factor and group (low or high DTS) as a between subjects factor yielded a main effect of condition (Wilks’ Lambda = 0.12; F3,18 = 44.99, P < 0.0001). Post hoc analyses revealed that participants had longer reaction time latencies for the emotional condition than for the other conditions (P’s < 0.001 for all). There was no condition by group interaction (Wilks’ Lambda = 0.84; F3,18 = 1.15, P > 0.3).
For response accuracy, MANOVA revealed a main effect of condition (Wilks’ Lambda = 0.35; F3,18 = 11.2, P < 0.001). Participants were less accurate in making a button response to target stimuli in comparison to emotional, neutral and standard conditions (P < 0.0001 for all) and also less accurate for emotion stimuli than neutral stimuli (P = 0.05). There was a marginal condition by group interaction (Wilks’ Lambda = 0.66; F3,18 = 3.04, P = 0.056) suggesting that high DTS participants were less accurate in detecting targets versus the other picture types relative to low DTS participants.
3.2. fMRI results
3.2.1. Regions of activation for group
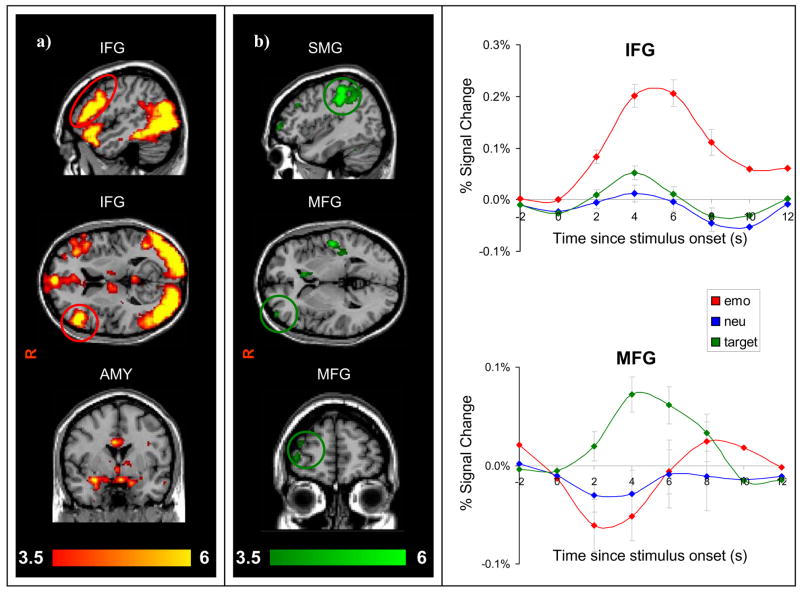
As expected, random effects group analysis for the emotional condition revealed activation in ventral brain regions including IFG, OFG, vmPFC, and amygdala (see Fig. 1). Percent signal change extracted from an IFG ROI shows that this region was activated for emotional stimuli, but not for neutral or target stimuli. MANOVA was highly significant for a main effect of condition (Wilks’ Lambda = 0.24; F2,24 = 37.19, P < 0.00001), time point (Wilks’ Lambda = 0.28; F3,23 = 19.43, P < 0.0001) and a condition by time interaction (Wilks’ Lambda = 0.39; F6,20 = 5.15, P < 0.003). Activation was greater for emotion than neutral and target at all time points examined (P < 0.002 for all).
Figure 1.
Dissociable systems for ventral and dorsal regions in all 26 subjects. (a) Emotional distracters elicited activation in ventral regions including inferior frontal gyrus (IFG) and amygdala (AMY) (b) Target circles elicited activation in dorsal attention regions including middle frontal gyrus (MFG), and supramarginal gyrus (SMG).
Conversely, the target identification task evoked activity in dorsal regions, including the right middle frontal gyrus (MFG) and other frontoparietal regions. In MFG there was a main effect for condition (Wilks’ Lambda = 0.75; F2,24 = 4.05, P < 0.04) and a condition by time interaction (Wilks’ Lambda = 0.39; F6,20 = 5.20, P < 0.003). Planned comparisons revealed that at 4 seconds, percent signal change was higher for targets than emotional (P < 0.0003) and neutral (P < 0.04) distractors.
3.2.2. Between groups: ventral regions
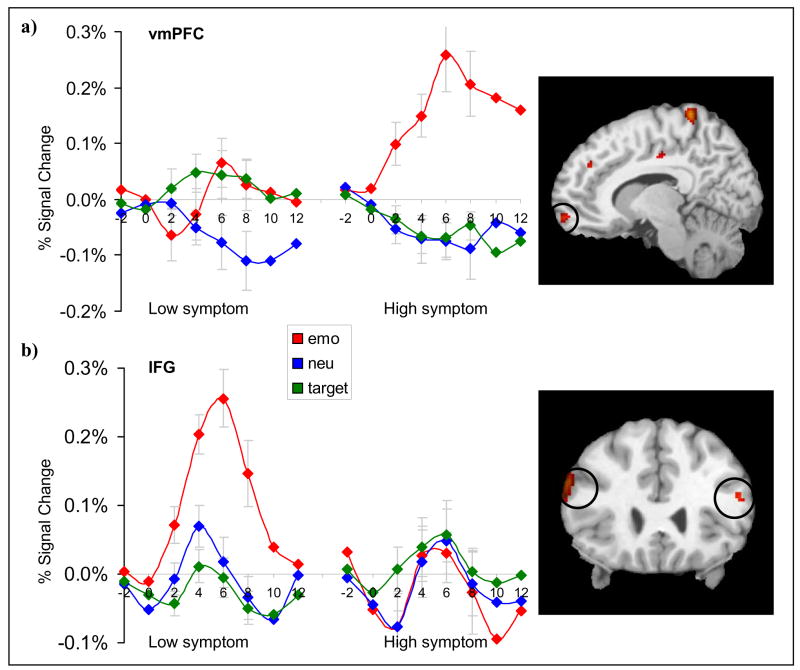
The between-group contrast of emotion > target revealed greater activation in bilateral IFG (414 voxels) in low DTS participants relative to high DTS participants (see Fig. 2). MANOVA yielded a significant condition by group interaction (Wilks’ Lambda = 0.56; F2,23 = 9, P < 0.002). Planned comparisons revealed that emotional distractors elicited greater signal in the low DTS group than the high DTS group at 2 and 6 seconds (P’s < 0.05). A left anterior temporal lobe and left cerebellar region was also activated although we did not hypothesize a role for these regions prior to the study.
Figure 2.
Comparison of mean percent signal change in ventral emotional regions corresponding to emotional, neutral, and target stimuli in the low and high PTSD symptom groups. (a) Activation in the ventromedial prefrontal cortex (vmPFC) was greater for emotional distracters in the high symptom group but not in the low symptom group. (b) Activation in the inferior frontal gyrus (IFG) was greater for emotional distracters in the low symptom group but not the high symptom group.
Conversely, the high DTS group showed greater activation in right vmPFC, right peri-amygdala regions, and right OFG. In the vmPFC (132 voxels), MANOVA indicated a significant condition by group interaction (Wilks’ Lambda = 0.7; F2,23 = 5, P < 0.02). Emotional distractors elicited greater signal in the high DTS than low DTS group at all time points (P’s < 0.03). In peri-amygdala regions (110 voxels), MANOVA revealed a significant condition by group interaction (Wilks’ Lambda = 0.57; F2,23 = 8.63, P < 0.003) and a condition by time by group interaction (Wilks’ Lambda = 0.54; F6,19 = 2.71, P < 0.05). Follow up tests showed greater activity for emotional pictures in the high DTS group in comparison to the low DTS group at 6 and 8 seconds (P < 0.02). In OFG (447 voxels), repeated measures MANOVA revealed a significant condition by group interaction (Wilks’ Lambda = 0.66; F2,23 = 5.84, P < 0.01). Follow up tests showed that the high DTS group had greater activation for emotional stimuli than the low DTS group at 4, 6, and 8 seconds (P < 0.03 for all).
3.2.3. Between groups: dorsal regions
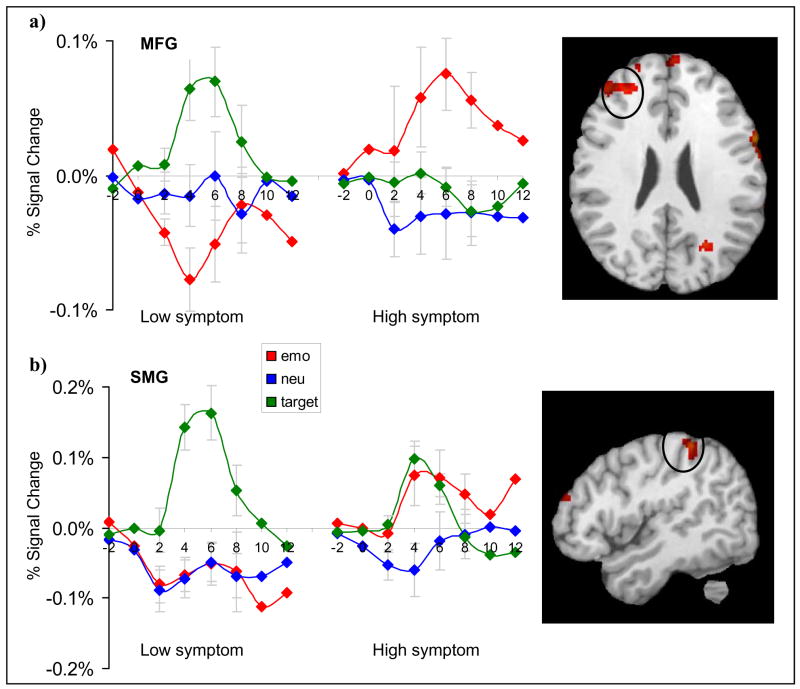
For attentional targets, we hypothesized that high DTS symptoms would be associated with reduced activity in putative dorsal-attention regions. As expected, the high DTS group showed reduced activity in right MFG and right SMG for the target > emotion contrast. In MFG (731 voxels), repeated measures MANOVA indicated a significant condition by group interaction (Wilks’ Lambda = 0.66; F2,23 = 5.83, P < 0.01). Planned comparisons revealed decreased activation in the high DTS group than the low DTS group in MFG for targets at 4 s (P < 0.03) and 6 s (P < 0.02). The high DTS group, however, had greater activation in this region for emotional stimuli at 4 s (P < 0.007), 6 seconds (P < .004), and 8 seconds (P < 0.04).
In right SMG (296 voxels), MANOVA revealed a significant condition by group interaction (Wilks’ Lambda = 0.55; F2,23 = 9.32, P < 0.002), condition by time by group interaction (Wilks’ Lambda = 0.49; F6,19 = 3.33, P < 0.03) and condition by time interaction (Wilks’ Lambda = 0.42; F6,19 = 4.45, P < 0.007) (Fig. 3). Follow up tests showed that the high DTS group had reduced activation for target stimuli in comparison to the low DTS group at 6 s (P < 0.02), but greater activation for emotional stimuli at 4 s (P < 0.02) and 6 s (P < 0.03). There were no regions for which the high DTS group had greater activation than the low DTS group for target stimuli.
Figure 3.
Comparison of mean percent signal change in dorsal executive-attention regions corresponding to emotional, neutral, and target stimuli in the low and high PTSD symptom groups. (a) Activation in the middle frontal gyrus (MFG) prefrontal cortex was greater for circle targets in the low symptom group than the high symptom group. (b) Activation in the supramarginal gyrus (SMG) was also greater for circle targets in the low symptom group than the high symptom group.
3.2.4. Regression by symptom severity
Multiple regression analysis by DTS and BDI scores was performed to examine the relationship between regions of interest and PTSD and depression symptomatology. Based on the between groups IFG ROI contrast, results indicate that the variables entered significantly explained the variation in IFG (F2,23 = 7.52, P < 0.004). However, only the DTS was a significant predictor in the model (t23 = 3.75, P < 0.002). The results suggest that activity in the IFG decreased as PTSD symptom severity increased. Similarly, PTSD symptom severity inversely predicted BOLD signal activity in the MFG (t23 = 3.79, P < 0.002), but again depression symptom severity was not a significant predictor in the model (t23 = 0.79, P > 0.44). This finding is consistent with our hypothesis that greater PTSD symptom severity is related to attenuation of the dorsal-attention network.
In the vmPFC ROI, DTS again was a significant predictor in the model (t23 = 2.75, P < 0.02), whereas BDI was not (t23 = 0.47, P > 0.64). This result indicates a significant positive relationship between vmPFC and PTSD symptom severity but not depression symptom severity.
3.2.5. Anterior cingulate
To investigate the putative role of the anterior cingulate in emotion and attention integration, we examined regions of overlap where both emotion and target stimuli evoked greater activation than neutral stimuli in the entire group of participants. Three cingulate regions were activated as shown in Fig. 4, including a rostral ACC region (region 1; 70 voxels), a dorsal ACC region (region 2; 41 voxels), and a posterior ventral region (region 3; 116 voxels). These regions were submitted to ROI analysis and compared across and within the two groups.
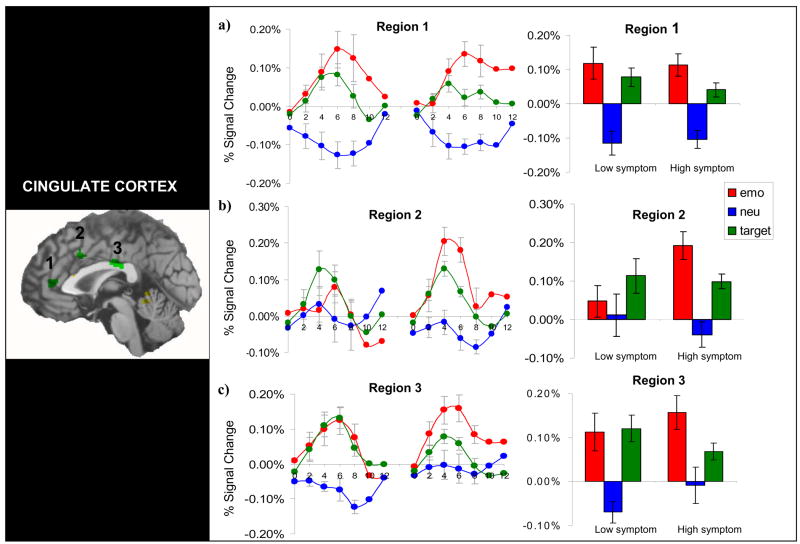
Figure 4.
Regions of overlap where both emotion and target stimuli evoked greater activation than neutral stimuli in the entire group of subjects. (a) In region 1, the low symptom group showed equivalent activity for emotion and target whereas the high symptom group showed the greatest activation for emotion. (b) In region 2, the low symptom group did not differentiate between target, emotion, and neutral stimuli. The high symptom group had greater activity to emotional stimuli than the low symptom group. (c) In region 3, the low symptom group again showed equivalent activity for emotion and target stimuli. In the high symptom group, there was a trend for greater activity for emotion than target stimuli.
A 3-way MANOVA using condition (emotion, target, and neutral) and region (1, 2, and 3) as repeated variables and group (high, low DTS) as the between subjects factor at peak time points yielded a significant condition by region by group interaction (Wilks’ Lambda = 0.65; F4,21 = 2.87, P < 0.05). Follow up tests revealed that in region 2, the high DTS group showed greater activation to emotion distractors than the low DTS group.
The low DTS group showed two patterns of responses across the cingulate regions. In region 1, MANOVA revealed a significant condition by time interaction (F6,66 = 3.6, P < 0.004). Follow up tests showed that emotion and target stimuli evoked greater activation than neutral stimuli at 6 and 8 s, but emotion and target signals were not different from each other. In region 2, differential activation for picture type was not observed. In region 3 MANOVA revealed a significant condition by time interaction (F6,66 = 2.3, P < 0.05). Both target and emotion stimuli evoked greater activation than neutral stimuli at 4, 6, and 8 seconds, but target and emotion did not differ from each other at any time point.
The high DTS group showed three patterns of activation. In region 1, there was a significant condition by time interaction (F6,78 = 2.96, P < 0.02). A graded activation pattern emerged such that target stimuli evoked greater activation than neutral distractors at 4 and 6 s, and in turn emotion distractors evoked greater activation than target stimuli at 6 seconds (P < 0.05). In region 2, the high DTS group again showed a graded activation pattern. MANOVA revealed a significant condition by time interaction (F6,78 = 4.72, P < 0.001). Follow up tests showed that target stimuli evoked greater activation than neutral stimuli at 4, 6, and 8 s, and emotion stimuli evoked greater activation than both target and neutral stimuli at 6 seconds (P < 0.05). In region 3, the high DTS group showed a main effect for condition (F2,26 = 6.07, P < 0.008). Across all time points, emotion stimuli evoked greater activation than neutral stimuli (P < 0.002).
In summary, these results demonstrated that the high DTS group showed greater activation for the emotion distractors in the dorsal ACC, whereas the low DTS group generally showed equivalent activation for emotion and target stimuli.
4. DISCUSSION
Examination of emotion and attention in a group of recently returned combat veterans revealed alterations in the neural circuitry associated with posttraumatic stress symptomatology indicating an attentional bias to emotional stimuli in PTSD. Integration of the findings within the broader emotion and PTSD literature is discussed below.
4.1. Ventral processing stream
Differential activation was observed in the ventral regions for the low and high DTS groups during the emotion condition in accordance with previous neuroimaging findings of PTSD symptomatology (Morey et al., 2008). High DTS symptoms were associated with activation in the vmPFC, peri-amygdala regions, and OFG. This result is largely consistent with a number of studies examining the neural pathways of trauma processing in PTSD (Liberzon and Martis, 2006).
The low DTS group showed greater bilateral IFG activity than the high DTS group. Although IFG activity in response to emotional stimuli in healthy samples has been reported in several studies (Shin et al., 1999; Yamasaki et al., 2002; Fichtenholtz et al., 2004), the function of this region in emotion remains unclear. In prior studies, the IFG –particularly in the right hemisphere— has been implicated in inhibitory functions, which allow one to carry out thoughts and actions with minimal interruption from external, distracting stimuli (Aron et al., 2004). Impaired inhibition in PTSD may be contributory towards attentional bias to emotionally relevant information (Amir et al., 2002). The present results are consistent with the IFG’s role in inhibition of distracting stimuli and the growing literature suggesting that a failure to engage this putatively inhibitory IFG activity is decreased in participants with posttraumatic symptomatology.
4.2. Dorsal processing stream
There is now abundant evidence that in healthy individuals, attentional targets elicit activity in dorsal regions including the MFG and parietal cortex (Kirino et al., 2000; Yamasaki et al., 2002; Fichtenholtz et al., 2004; Morey et al., 2008). In the present study, attentional targets were associated with attenuated signal in the dorsal network in combat veterans with greater PTSD symptomatology relative to combat veterans with fewer symptoms. This neural finding coincided with lowered behavioral performance of the high symptom group for target stimuli as compared to other picture types. However, while neutral targets elicited attenuated signal in MFG in the high DTS group, emotional distractors elicited the greatest activity in MFG. There is some evidence that the MFG plays an active role in attention to salient target stimuli regardless of the emotional content (Fichtenholtz et al., 2004). Therefore, it is possible that while the low DTS group applied greatest attention to circle (target) stimuli, the high DTS group applied greater attention to the emotional distractors. These results yield findings complementary to ERP studies in which P3 signal is attenuated for neutral targets and enhanced for threat stimuli (Stanford et al., 2001). The differential MFG signal observed between groups could be a corresponding neural marker for threat bias in PTSD.
Interestingly, Bryant et al. (2005) demonstrated that PTSD was related to increased activity in dorsal-attention systems including parietal cortex during an oddball task that did not employ emotional distractors. The authors suggested that attentional systems may be enhanced in PTSD patients in the absence of emotional stimuli, reflecting generalized hypervigilance. In light of these findings, it is possible that dorsal-attention regions may be enhanced in PTSD during processing of salient neutral stimuli whereas within the context of threat, these regions are attenuated for neutral stimuli in favor of processing emotional stimuli. This finding parallels the ERP literature, in which studies that included emotional probes found increased responsivity for emotional stimuli (Stanford et al., 2001), while those that did not found increased responsivity for salient target stimuli (Kimble et al., 2000).
4.3. Role of the ACC
The ACC has reciprocal connections with both dorsal-attention regions and ventral-affective regions and therefore this region may be ideally suited to regulate interactions between dorsal-ventral pathways. However, there is some conflicting evidence in the neuroimaging literature regarding the ACC’s role in PTSD. The majority of studies have shown that this region either deactivated in patients with PTSD or failed to activate in comparison to control participants (Bremner, 1999; Bremner et al., 1999; Shin et al., 1999; Lanius et al., 2001; Shin et al., 2001; Lanius et al., 2003b; Shin et al., 2004; Shin et al., 2005; Etkin and Wager, 2007). However, some studies have also demonstrated the opposite, that the ACC is in fact activated or correlated with PTSD symptomatology. For instance, activation in this region is associated with PTSD symptomatology during a script-driven symptom provocation study (Rauch et al., 1996), and while viewing trauma scenes (Morey et al., 2008), negative pictures, (Shin et al., 1997), and listening to combat sounds (Zubieta et al., 1999). Activity in this region is also heightened for salient non-threatening targets in an oddball paradigm (Bryant et al., 2005). The conflicting evidence therefore raises the concern that other factors likely account for the discrepancy of results including task differences, differences in dissociative states (see Lanius et al., 2002), and depression comorbidity, among others.
The present study supports the hypothesis that the ACC serves as an intermediary between attention and emotion processing. Compared to low-symptom individuals, participants with high levels of PTSD symptomatology showed greater activity for emotional distractors than attentional targets in a rostral ACC region, and greater activity in a dorsal ACC region for emotional distractors. Thus, the greater activity may reflect a twofold effort to integrate emotion and attention streams, although with limited success, as observed by attenuated activity for target stimuli and poorer behavioral performance. These results are also consistent with our previous work in healthy adults which demonstrated that activity in the ACC increased when subjects are instructed to apply greater attention to emotional stimuli versus nonemotional stimuli (Fichtenholtz et al., 2004).
4.4. Limitations and strengths
A limitation of the present study is that a clinician administered measure was not used to classify groups. Although the DTS has good diagnostic accuracy against a clinician-administered interview (Davidson et al., 1997), use of the Clinician Administered PTSD Scale or another clinician administered measure may help to more accurately diagnose whether the symptoms participants report meet DSM-IV PTSD criteria.
Another limitation of the present study is that several of the high DTS participants had significant depression scores on the BDI, leaving open the possibility that our results could be influenced by depressive symptomatology. Comorbidity rates between PTSD and other mood disorders are strikingly high. By one estimate, lifetime comorbidity with another DSM disorder was over 80% in men with PTSD (Kessler et al., 1995). This issue raises the concern that the current nosology of the DSM-IV does not adequately acknowledge depressive symptoms as a characteristic of PTSD (Simms et al., 2002). Nonetheless, the present results suggest that activation in ventral-limbic regions increased as a function of PTSD symptoms, but not depressive symptoms, suggesting that these two aspects of the disorder may have distinct neural effects. Additionally, we observed heightened activation in the dorsal ACC whereas this region is hypothesized to be attenuated in depression (Mayberg, 1997; Wang et al., in press). At least two other studies have examined neural differences in PTSD patients with and without depression (Kemp et al., 2007; Lanius et al., 2007). The findings appear to be mixed; Lanius et al. (2007) reported increases in ACC activity in PTSD patients with comorbid depression, while Kemp et al. (2007) reported increases in ACC in PTSD patients without depression. These differences may be due to additional factors including the nature of the task employed and differences in dissociative states (see Lanius et al., 2002). Additional studies directly investigating the role of depression in PTSD are warranted.
A major strength of the present study is that participants comprised a relatively homogeneous group of post-9/11 combat veterans who were exposed to similar war zone experiences. Additionally, we were able to assess acute PTSD symptomatology on average less than two years had elapsed between development of PTSD symptoms and date enrolled in the study. By contrast, other studies in the literature are often hampered by chronicity of symptoms.
4.5. Conclusions
Understanding the challenges post-9/11 soldiers face as they attempt to re-integrate into civilian life is an important public health concern, as cognitive deficits can have negative implications on work and school related productivity. In the present study, participants with high levels of PTSD symptomatology showed attenuated activity in dorsolateral prefrontal cortex and parietal regions for neutral targets but enhanced activity for emotional distractors, which coincided with lowered capacity to detect attentional targets. Furthermore, PTSD symptom severity was related to greater ventral-limbic activation during the emotional task. Taken together, the results suggest that hyperresponsive ventral-limbic activity coupled with altered function of dorsal attention systems and increased activation for emotional stimuli in the dorsal ACC may be a neural marker of inattention and threat bias in PTSD. Additionally, this study provides testable hypotheses about the role of the IFG in inhibiting and managing distracting emotions in PTSD, and the role of the ACC in integrating attention and emotion processes. Future studies should include the contribution of these regions towards a comprehensive understanding of the neural circuitry of PTSD.
Acknowledgments
This research was supported by the Department of Veterans Affairs, Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health and by the National Institute of Mental Health Grant K23 MH073091. We would like to thank Debra Cooper, Srishti Seth, Lihong Wang, Larry Tupler, as well as the Journal editors and reviewers for their helpful comments and contributions to the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, Coles ME, Foa EB. Automatic and strategic activation and inhibition of threat-relevant information in posttraumatic stress disorder. Cognitive Therapy and Research. 2002;26:645–655. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Attias J, Bleich A, Furman V, Zinger Y. Event-related potentials in post-traumatic stress disorder of combat origin. Biological Psychiatry. 1996;40:373–381. doi: 10.1016/0006-3223(95)00419-X. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown G. Beck Depression Inventory-II Manual. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bremner JD. Alterations in brain structure and function associated with post-traumatic stress disorder. Seminars in Clinical Neuropsychiatry. 1999;4:249–255. doi: 10.153/SCNP00400249. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetter E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney D. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Clark CR, McFarlane AC, Morris P, Weber DL, Sonkkilla C, Shaw M, Marcina J, Tochon-Danguy HJ, Egan GF. Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biological Psychiatry. 2003;53:474–481. doi: 10.1016/s0006-3223(02)01505-6. [DOI] [PubMed] [Google Scholar]
- Constans JI, McCloskey MS, Vasterling JJ, Brailey K, Mathews A. Suppression of attentional bias in PTSD. Journal of Abnormal Psychology. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg M, Mellman T, Beckham JC, Smith RD, Davison RM, Katz R, Feldman ME. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychological Medicine. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Cognitive Brain Research. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. Spiral-in-and-out functional image acquisition with embedded z-shimming for susceptibility signal recovery. Journal of Magnetic Resonance Imaging. 2003;18:389–395. doi: 10.1002/jmri.10355. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. Journal of the American Medical Association. 2006;295:1023–1032. doi: 10.1001/jama.295.9.1023. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaspi SP, McNally RJ, Amir N. Cognitive processing of emotional information in posttraumatic stress disorder. Cognitive Therapy & Research. 1995;19:433–444. [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–55. [Google Scholar]
- Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, Williams LM. Influence of comorbid depression on fear in posttraumatic stress disorder: An fMRI study. Psychiatry Research: Neuroimaging. 2007;155:265–269. doi: 10.1016/j.pscychresns.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biological Psychiatry. 2000;47:880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville, Florida: 2005. International Affective Picture System (IAPS): Digitized photographs, instruction manual and affective ratings. [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: A functional MRI investigation. Psychiatry Research: Neuroimaging. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Hopper JW, Menon RS. Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. American Journal of Psychiatry. 2003a;160:667–669. doi: 10.1176/appi.ajp.160.4.667. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological Psychiatry. 2003b;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Kaspi SP, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. Journal of Abnormal Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, LaBar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Research: Neuroimaging. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. European Archives of Psychiatry & Clinical Neuroscience. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Archives of General Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simms LJ, Watson D, Doebbeling BN. Confirmatory factor analyses of posttraumatic stress symptoms in deployed and nondeployed veterans of the Gulf War. Journal of Abnormal Psychology. 2002;111:637–647. doi: 10.1037//0021-843x.111.4.637. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl. 2004;1:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Vasterling JJ, Mathias CW, Constans JI, Houston RJ. Impact of threat relevance on P3 event-related potentials in combat-related post-traumatic stress disorder. Psychiatry Research. 2001;102:125–137. doi: 10.1016/s0165-1781(01)00236-0. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. Journal of the American Medical Association. 2006;296:519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K. Neuropsychological findings in adults with PTSD. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, Cognitive, and Clinical Perspectives. Guilford Press; New York: 2005. pp. 178–207. [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Psychiatry Research: Neuroimaging. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wu M, Hsu C, Ker J. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neuroscience Letters. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. Journal of Psychiatric Research. 1999;33:259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]