Abstract
An important cue for sound localization and separation of signals from noise is the interaural time difference (ITD). Humans are able to localize sounds within 1–2° and can detect very small changes in the ITD (10–20 μs). In contrast, many animals localize sounds with less precision than humans. Rabbits, for example, have sound localization thresholds of ~22°. There is only limited information about behavioral ITD discrimination in animals with poor sound localization acuity that are typically used for the neural recordings. For this study, we measured behavioral discrimination of ITDs in the rabbit for a range of reference ITDs from 0 to ± 300 μs. The behavioral task was conditioned avoidance and the stimulus was band-limited noise (500–1500 Hz). Across animals, the average discrimination threshold was 50–60 μs for reference ITDs of 0 to ± 200 μs. There was no trend in the thresholds across this range of reference ITDs. For a reference ITD of ± 300 μs, which is near the limit of the physiological window defined by the head width in this species, the discrimination threshold increased to ~100 μs. The ITD discrimination in rabbits less acute than in cats, which have a similar head size. This result supports the suggestion that ITD discrimination, like sound localization (see Heffner, 1997, Acta Otolaryngol Suppl 532:46–53, 1997) is determined by factors other than head size.
Keywords: Sound localization, animal psychoacoustics, neural discrimination
INTRODUCTION
One of the most important binaural cues for sound localization and separation of signals is the interaural time difference (ITD). Humans are able to localize sounds with an acuity of 1–2° (Mills, 1958), and can detect changes in the ITD as small as 10–20 μs (Zwislocki and Feldman, 1956; Klumpp and Eady, 1957). In contrast, other mammalian species generally perform less well on tasks of sound localization and ITD discrimination. In cats, for example, the sound localization acuity is ~ 5° and the threshold for ITD discrimination is about 30 μs (Wakeford and Robinson, 1974). The behavioral discrimination of ITDs has not been studied in species with even more limited sound localization acuity than cats, such as the rabbit which has sound localization acuity of about 22° (Gandy et al., 1995; Heffner, 1997). Thus, one goal of the current study was to measure ITD discrimination in rabbits for comparison to species with better sound localization acuity.
A second reason for measuring ITD discrimination in rabbits is that results from neural studies of ITD processing done in animals are typically compared to human performance. A better comparison, of course, would be between the neural and behavioral sensitivity in the same species. Thus, this report provides a description of the behavioral sensitivity to ITD in rabbits with a description of the neural sensitivity to be provided in a subsequent report.
MATERIALS AND METHODS
Subjects
The animals used were adult female Dutch-belted rabbits. All animals were handled according to the standards and protocols of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, and the “Guide for the Care and Use of Laboratory Animals” published by the National Institute of Health.
Behavioral Testing
Our behavioral method was conditioned avoidance as previously used in laboratory experiments with rabbits (Heffner and Heffner, 1995). Three water-deprived rabbits were trained to drink from a spout while in a sound attenuated chamber. On testing days, the rabbits received all of their water during the experiment. The task was to discriminate a safe sound from a warning sound that was followed by a small electrical shock to the water spout. Correct detection (a hit) occurred when the animal withdrew from the spout prior to receiving the shock. The presence of the shock was also indicated by a bright light providing feedback to the animal in cases where withdrawal resulted in shock avoidance. Contact with the spout combined with a ground screw mounted to the animals’ skull completed a circuit that could be monitored by the computer and through which the shock could be delivered. The measure of withdrawal was absence from the spout for more than ½ of a 300 ms period before the onset of the shock. For each animal, the level of the shock was adjusted upwards from zero until the animal showed a rapid withdrawal from the spout when the shock was presented. Thresholds for withdrawal were typically on the order of 0.1 mA. During a session the shock level was manually varied up to 2–3 times threshold to prevent tolerance to a particular level. The stimulus timing was the same for all experiments. The sound duration was 2 seconds, so the animals had 1.7 seconds to detect the warning sound and react. The interstimulus interval was 1 s. The number of safe sounds was randomly varied between 2 and 10 prior to each warning sound. The shock duration was set to 300 ms for hits, and was increased to 3000 ms for each failure to detect (a miss) to provide the additional penalty of a “time-out”. False alarms (withdrawal from the spout during safe trials) were also scored. Stimuli were constructed in MATLAB, and presented by a two-channel digital stimulation system consisting of an array processor, digital to analog converters, anti-aliasing filters and programmable attenuators (Tucker-Davis System II). Behavioral stimulus delivery and response recording was controlled using a National Instruments data acquisition board (Model PCI-6052E) and custom MATLAB software.
Animals were first trained to detect the presence of a sound in the free field. The speaker was a RadioShack supertweeter (model 40–1310). Calibration for sound level was performed at the outset of the experiment with a measuring amplifier connected to a ¼ inch microphone that was placed at the position of the rabbit’s ear (B&K Instruments, Naerum Denmark). Calibrating sounds were bands of noise spanning the range from 60–50,000 Hz in steps of variable bandwidths. Intensities throughout this report are in dB relative to 20 μPascals (dB SPL). For the initial detection experiments, safe trials were silent while warning trials contained a 2-sec noise burst comprised of Gaussian noise bandpass filtered at 2000–4000 Hz. This range of noise was chosen because free-field tones at 2–4 kHz have low thresholds in the rabbit’s audiogram (Heffner and Masterton, 1980). The animals were initially trained in blocks with readily detectable warning sounds (40 dB SPL) until 80% correct responses were consistently obtained, determined as the hit rate – [hit rate × false alarm rate] (Heffner and Heffner, 1995). After this, thresholds of sound detection were determined by a staircase tracking procedure. The step size was 4 dB SPL and the tracking rule was either 1-up/1-down or 2-up/1-down. The tracks were started at a high suprathreshold level (usually 40 dB SPL) so that performance over the course of a typical session would be between 60–70%. We found that keeping animals performing near threshold for an entire session resulted in a degradation of performance over time. With these procedures, detection thresholds of less 10 dB were obtained, in accord with previous behavioral experiments in rabbits over this frequency range (Heffner and Masterton, 1980).
Once consistent thresholds were obtained in the free field, custom-fitted earmolds were introduced and sound detection thresholds were re-measured. To make the earmolds the animal was anesthetized (ketamine: 35 mg/kg and xylazine: 5 mg/kg, IM), a rod was inserted into the ear and impression compound (Hal-Hen Co. Inc., Long Island City, NY) was pressed around the rod. The rod was subsequently replaced with a hollow, 6.5 cm long sound tube that was cemented in the earmold. For these and all subsequent measurements, sounds were delivered independently to each ear through Beyer DT-48 loudspeakers, coupled to the earmolds to form a sealed system. The phase and amplitudes of the stimuli at each ear were calibrated through the ¼ inch microphone fitted with a probe tube that extended to the base of the sound tube, which reached to within about 2 cm of the eardrum. The thresholds with sealed earmolds were higher than in the free-field (by 2–6 dB) as found in previous experiments over this frequency range (Martin et al., 1980; Borg and Engstrom, 1983).
Following the sound detection threshold measurements, the animals were trained to discriminate the side of stimulation using noise (frozen 2-sec Gaussian noise bursts, 500–1500 Hz, 75 dB SPL) delivered as safe sounds to one ear and warning sounds to the other ear. These frequencies encompass the range where sensitivity to ITDs is maximal for rabbit neurons (Fitzpatrick et al., 2002). Training continued until performance greater than 80% correct was reached. Then, binaural stimuli with ITDs lateralized to the left and right ears were introduced (± 200 μs), and the 80% criteria repeated.
After this initial training, which typically took 1–2 months, discrimination thresholds to ITDs were determined by changing the ITD in the safe or warning sound while keeping the other constant at some reference ITD. The noise was recomputed for each track and was frozen throughout a track. Each track began at a suprathreshold ITD (100–200 μs away from the reference ITD), again to maintain an overall level of 60–70% performance throughout a session. The step size was 20 μs in most cases, but 10 μs steps were used in some tracks for better resolution. At least the first two reversals in each track were discarded, and tracks were continued until a stable level was reached. For each reference ITD, at least five training sessions were conducted prior to collecting tracks for analysis.
Data analysis
Several criteria were used to identify tracks suitable for further analysis. Tracks were not included if the false alarm rates were greater than 15%. Tracks were also excluded if the hit rate was too low relative to the false alarm rate, as determined by a d’ < 1 for trials where the ITD in the warning sound should have been highly discriminable (>100 μs difference between the ITD in the safe and warning sound in most cases). The metric used was d’ = z[Hits] – z[False Alarms] (Klein, 2001). Other tracks were excluded if there were more than four straight misses, which indicates a lack of attention to the task as misses would have occurred for ITDs well into the discriminable range. For each reference ITD, at least twelve tracks judged acceptable by these criteria were taken for each condition tested, and of these only the best 50% were used to determine thresholds and construct psychometric functions. Our goal was to identify the best that the animals could do, so these procedures minimized the inclusion of less than optimal data.
The threshold for a track was computed as the average ITD of the last six reversals. Psychometric functions were calculated from all of the data in the tracks included for analysis. The psychometric functions were calculated by fitting the proportion of correct responses to a logistic function using a least squares method. The formula used was:
(adapted from Early et al., 2001) where m is the midpoint of the psychometric function, s is the slope and PL is the lapse, or the difference from perfect performance at highly suprathreshold ITDs. Only data points with at least 10 measurements were used for the fitting procedure.
Statistical analyses consisted of one-way analysis of variance and pairwise comparisons via Tukey Test and/or Student’s t-test. Statistical significance was determined prior to the experiment for p values of < 0.05 and a power of 0.80.
RESULTS
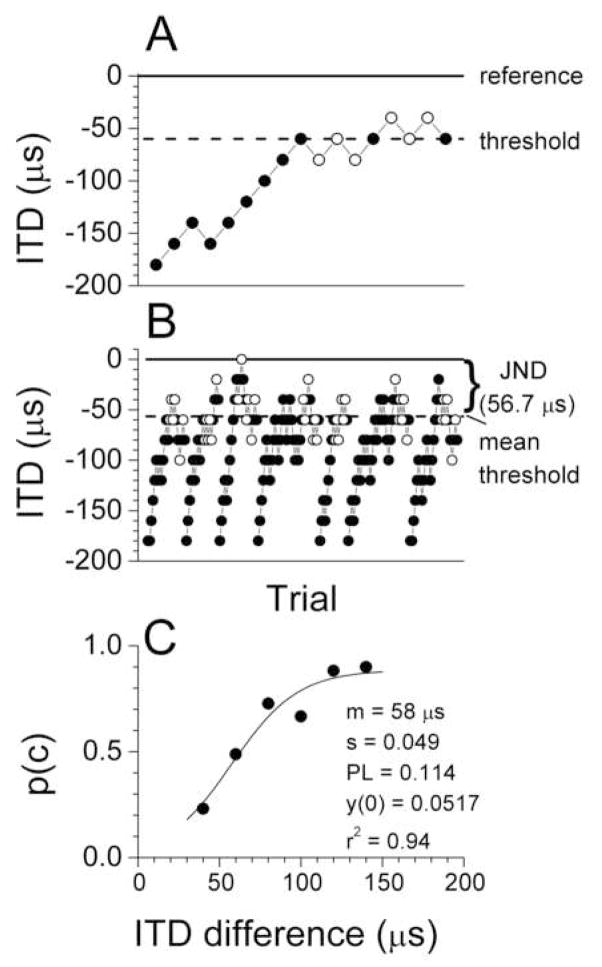
An example of a track produced by varying the ITD in band-limited noise is shown in Fig. 1A. The reference ITD in the safe sound was zero μs (solid line), the ITD in the warning sound began at −200 μs from the reference ITD. By our convention a negative sign indicates a delay to the right ear, corresponding to a sound that would be lateralized to the left side. The ITD increased by 20 μs if the ITD in the warning sound was correctly discriminated or decreased by 20 μs if the animal did not withdraw from the spout. The last six reversals (open circles) were used to compute the threshold for the track (dotted line). The threshold for this track was 60 μs.
Figure 1.
Examples of individual tracks used to determine the behavioral JND via a staircase tracking procedure. A. Example of a single track. The reference ITD was 0 μs. The last six reversals (open circles) were used to compute the threshold for the track (dashed line at 60 μs). B. All tracks used to compute the JND for this reference ITD. The JND (dashed line) for a given reference ITD was the difference between the reference ITD and the average of the thresholds (arrow), and in this case was 56 μs. C. The psychometric function derived from the tracks in B. The parameters of the function are given, including the midpoint of the function (m), the slope (s), the lapse (PL) the y-intercept (y(0)), and the percent variance accounted for (r2).
The seven tracks used for the determination of the just-noticeable difference (JND) are shown in Fig. 1B. The JND for each condition was taken as the difference between the reference ITD (0 μs in this case) and the mean threshold for tracks. The JND for this condition was 57 ± 2.4 μs (standard error).
The psychometric function for these tracks is shown in Fig. 1C. The midpoint of the curve (m) as determined by the fitting procedure (see Methods) was 58 μs, which is a close match to the JND determined from the average of the thresholds of each track. The lapse (PL) was 0.114 and the intercept was 0.0517, so the midpoint occurred at a p(c) value of 0.468. The intercept represents an approximation of the false alarm rate, and the average false alarm rate over these tracks was 3.1% which is close to the 5% indicated by the fit. The quality of the fit was determined by the r2 value, which indicated that the line accounted for 94% of the variance in the data.
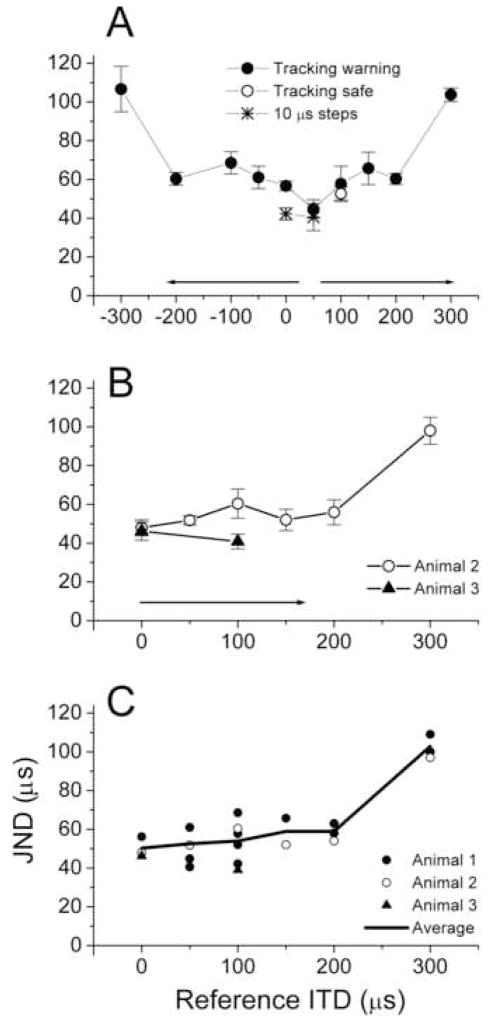
Results of the JND measurements across all conditions for this animal are shown in Fig. 2A. Conditions consisted of different reference ITDs or to the same reference ITD but different tracking rules. Besides zero μs, the reference ITDs used were +/− 50, 100, 150, 200 and 300 μs. Using both positive and negative reference ITDs meant that noises lateralized to both hemispheres were tested. Different tracking rules were 10 μs steps (at reference ITDs of 0 and 50 μs, asterisks) instead of 20 μs (circles) and varying the ITD of the safe sound (at 100 μs, open circle) rather than the warning sound (filled circles). For non-zero reference ITDs, the direction of tracking was such that ITD more central than the reference ITD would be tested (indicated by arrows). In other words, the tracks started with an ITD that would be lateralized across the midline or closer to the midline compared to the reference ITD, rather than at an extreme ITD in the same hemisphere.
Figure 2.
JNDs for different reference ITDs in different animals. A. JNDs for one subject tested for reference ITDs lateralized to both sides. The arrows indicate the direction of tracking. Most points were taken from tracks using 20 μs steps; two tracks (asterisks) used 10 μs steps. One point (open circle) was taken while tracking the normal instead of the warning sound. B. The JNDs measured in two other animals. C. All of the data from the three animals, plotted as if lateralized to the same side in each case. The average JNDs are shown by the thick line.
For different reference ITDs, the smallest JND was obtained for a reference ITD of 50 μs and was 45 ± 4.7 μs (Standard error, n=7). The JNDs for reference ITDs up to ± 200 μs were all less than 70 μs, before increasing sharply to about 100 μs at reference ITDs of ±300 μs.
The two measurements with 10 μs steps (asterisks at 0 and 50 μs in Fig. 2A) had smaller JNDs than those with the larger step size (42 ± 3.1 compared to 57 ± 5.8 μs, and 41 ± 7.0 μs compared to 45 ± 4.7 μs, respectively). Thus, smaller steps sizes can result in slightly better thresholds, but the minimum threshold remained near 40 μs.
All of the previous results had the reference ITD carried by the safe sounds, and the test ITD carried by the warning sound. In one case with a reference ITD of 100 μs this tracking rule was reversed (open circle). The JND changed little whether the ITD in the warning or safe sound was changed after each presentation of the warning sound.
Measurements from additional animals confirmed these basic trends (Fig. 2B). In one animal (open circles) the full range of reference ITDs was tested for positive values while in the other animal (filled triangles) only two reference ITDs were tested. For the animal with testing across the range of reference ITDs the tracking rule was changed to 2-up/1-down (i.e., two misses were required for the distance from the reference ITD to increase), because with a 1-up/1-down rule responses at high rates (>80%) were not obtained, even for easily detected warning sounds. However, with the different tracking rule high rates of correct responses were obtained. As in the previous animal under similar conditions (20 μs steps, tracking the warning sound), the minimum JNDs obtained were ~45 μs, the JNDs were less than 70 μs for reference ITDs of 0 to 200 μs, and the JND increased to about 100 μs for a reference ITD of 300 μs. The third animal was tested at two reference ITDs (0 and 100 μs, Fig. 2B, triangles) and produced reliable results using a 1-up/1-down tracking rule, as did the first animal. For this animal, the JNDs at the two points (46 μs at 0 ITD and 40 μs at 100 μs ITD) were similar to the minimum values obtained in the previous two animals.
The trend across all animals (Fig. 2C) showed a consistent increase to a reference ITD of 300 μs, compared to reference ITDs closer to 0 μs. For this figure, values at negative reference ITDs obtained from animal one were flipped to positive values. Over the range of reference ITDs from ± 0–200 μs the average discrimination thresholds across animals and conditions varied from 50–60 μs, with no discernable trend across this range. When tested with a one-way ANOVA the only comparisons that were significantly different (p’s < 0.05) were ± 300 μs with all other reference ITDs, while none of the comparisons between 0 and ± 200 μs reached significance.
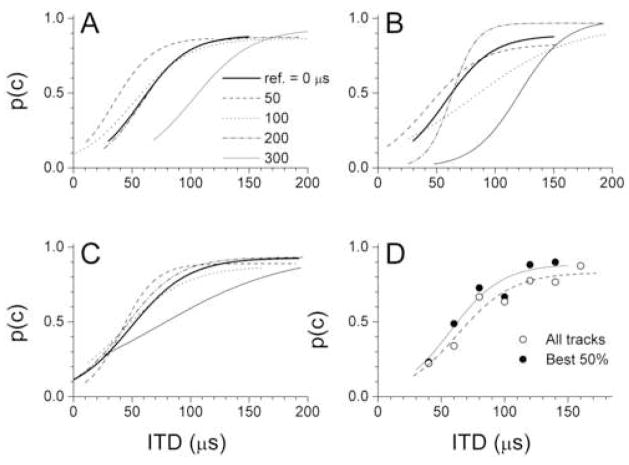
The psychometric functions supported the trends described for the JND data. The functions for the left and right hemispheres for the first animal are shown in Figs. 3A and B, respectively, and for the second animal in Fig. 3C. To compute the function for the second animal where a one/down, two/up tracking rule was used, each “miss” was the two consecutive misses that resulted in a change of ITD while a single miss that did not result in a change was not scored. In Figs. 3A–C the functions for reference ITD of ± 300 μs were consistently shifted to the right along the ITD axis indicating a higher threshold. For all other reference ITDs there was no consistent trend. Although in Fig. 3A the curve at 50 μs with a 20 dB step size is shifted to the left, this result was not seen in the other animals. Of the remaining curves there was no ordering by the reference ITD.
Figure 3.
Psychometric functions. A. Functions for the positive reference ITDs for animal 1. B. Functions for the negative reference ITDs for animal 1. C. Functions for the positive reference ITDs for animal 2. D. Functions for a reference ITD at 0 μs for animal 1 using either the best 50% of tracks (filled circles) or all of the tracks (open circles).
A justification for excluding the lower 50% of tracks even though they met the other criteria for inclusion is shown in Fig. 3D. In this case, the function for a reference ITD of 0 μs using only the upper 50% of tracks (n=7, from Fig. 1C) was compared to the function computed from all tracks (n=14). In addition to a shift to the right, the curve with all tracks has a shallower slope and lower upper asymptote (or PL). These features are consistent with a decrease in general attentiveness, as will be further considered in the Discussion.
DISCUSSION
The ITD discrimination thresholds of noise stimuli were measured in rabbits, a species with a small head and poor sound localization compared to humans. The minimum discriminable ITD was determined to be 40–60 μs for reference ITDs of 0–200 μs, and about 100 μs for a reference ITD of 300 μs. These results can be compared to the sound localization acuity in rabbits, to the ITD discrimination in species with better sound localization, and with neuronal discrimination of ITDs in a variety of species. First, however, we will consider the technical merits of the measurements.
Technical considerations
As with most behavioral measurements in animals, it is difficult to know if the measured JNDs are actually the best values that the animals can achieve. Several procedures were adopted to try to determine the limits of ITD discrimination in the rabbits. First, the animals were very highly trained in the conditioned avoidance task. Each was taken though a series of easier tasks leading up to the tests of ITD discrimination. The ITD discrimination experiments were then continued for an extended period, at least several months for each animal. Tracks were rejected if they had high false alarm rates (> 15%), if the hit rates were too low relative to the false alarm rate (d’ <1 at ITDs that should have been easily discriminable), or if four straight misses were obtained, indicating a loss of attentiveness to the task. Furthermore, after passing these criteria, only the 50% of tracks yielding the lowest thresholds were used in the calculation of final thresholds. It is our experience with the conditioned avoidance technique that some tracks produce thresholds well below what the animal is able to detect. From a signal processing standpoint, it is possible that these low thresholds are the result of a change in the behavioral criterion used to decide that a given change in the ITD is a hit rather than a miss. If so, we would expect that stimuli well above the criterion would be identified at the same rate regardless of the criterion itself. This was not the case, as demonstrated by the example where we compared all tracks with the best 50% of tracks. In this and other examples not shown, both the slope and asymptote of the psychometric function changed, rather than the function simply shifting to the right. This result indicates that what our observations of the animals suggested was correct, which was that for motivational reasons (usually being too thirsty such that the small shock was an insufficient deterrence) the animals sometimes did not give correct responses for signals they clearly detected. Because of this, it was deemed appropriate to focus on the best results obtained from the animals, rather than from an average of all results.
In two animals, a 1-up/1-down tracking method was used, which theoretically tracks the 50% correct position of the psychometric function (Levitt, 1971). In one animal, a 2-up/1-down procedure was used, which theoretically tracks the 29.3% correct position. However, there was no consistent difference in the results in the different animals. This similarity might suggest extremely sharp psychometric functions, but instead the slopes of the psychometric functions were relatively shallow, so consistent differences in excess of 10 μs could be expected. This difference was not seen, so it is more likely that the similarity was due to the less reliable performance in the animal where the 2-up/1-down tracking rule was used. When this animal was tested with a one up/one down configuration it did not produce correct responses at a rate high enough to produce reliable tracks, even for easily detectable ITDs. Thus, it is most likely that the differences in tracking methodology in the two animals compensated for performance differences in the conditioned avoidance task between the animals rather than revealing different points on the psychometric function.
The step size used for most experiments (20 μs) was large relative to human performance, but was about ½ that of the best performance achieved in rabbits. To determine if the choice of step size influenced the results we tested the effect of a smaller step size (10 μs) for two reference ITDs, and found that JND improved by only a small amount (4 μs) for one case and more substantially (15 μs) in the other. However, even in the second case the final value achieved was not less than the minimum JND of about 40 μs achieved in some conditions with the larger step size. Thus, we think it unlikely that a smaller step size by itself would have greatly improved performance. However, steeper psychometric functions and a higher degree of accuracy in the measurements might have been achieved had logarithmic steps with a smaller final step size been used (Saberi, 1995; Saberi and Green, 1996). Our bias was to use relatively large steps so that the animal would never be far from achieving a suprathreshold stimulus, as performance seemed to decline when too much time was spent near threshold. In addition, for consistency, once stable performance was achieved we employed the same procedures for most other conditions. Future experiments could test the effects of optimizing the tracking rules to see if the rabbits can be coaxed into better performance.
In general, the psychometric functions obtained had higher thresholds, a shallower slope and a lower maximum response rate than comparable curves in humans (Smoski and Trahiotis, 1986). Similar differences are found in psychometric functions between adults and infants for sound detection with level. These differences have been at least partly attributed to a lessening of “general attentiveness” (Werner and Marean, 1991; Bargones et al., 1995). When we constructed functions based on all of the tracks rather than the best 50% the thresholds increased, the curves became even more shallow and the lapse was greater, consistent with a decrease in the general attentivenss of tracks that were excluded.
Behavioral measurement of the physiological range
The “physiological range” of ITDs is the range that would be encountered by an animal in the environment. Using a spherical model of the head width a maximum ITD of approximately +/− 250 μs was calculated for domestic rabbits (Heffner and Masterton, 1980), which are similar in size to Dutch-Belted rabbits. In other species, measurements of the ITD as a function of azimuth typically report a maximum ITD greater than that expected from the head width and a frequency dependence where the maximum ITD decreases with frequency (human: Kuhn, 1977; cat: Roth et al., 1980; rhesus monkey: Spezio et al., 2000; guinea pig: Sterbing et al., 2003; gerbil: Maki and Furukawa, 2005). Recently, similar measurements have been made in the Dutch-Belted rabbit, where the maximum ITD at 90 degrees was ~300 μs at 1500 Hz, ~350 μs at 1000 Hz, and may be as high as 500 μs at 500 Hz (S. Kuwada, personal communication). The physiological range can also be defined behaviorally. In humans, an increase in ITD thresholds occurred at a reference ITD of 700 μs, which reasonably approximates the maximum ITD of 600 μs measured for a manikin with head and torso (Kuhn, 1977). Our results in rabbits showed an increased behavioral threshold for a reference ITD of 300 μs. An increase between 200–300 μs is as expected from a spherical model, but is on the low side compared to the measured ITDs in real rabbits.
Comparison of ITD discrimination with sound localization acuity in rabbits and other species
The minimum detectable azimuthal angle in rabbits is 22° for a left/right discrimination (Gandy et al., 1995). The experiments were free-field using noise as stimuli (bandwidth 2–40 kHz, duration 100 ms). Performance in this case would have depended primarily on the interaural level difference cue, as the upper limit for ITD sensitivity to low frequency sounds in rabbit neurons is about 2 kHz (Fitzpatrick et al., 2002). However, in additional experiments the bandwidth was extended to 0.3 kHz with the same result (H. Heffner, personal communication). Using 300 μs as an estimate of the physiological range, we would expect an ITD discriminibility of 73 μs (300 μs/90° × 22°). The minimum ITD thresholds of 50–60 μs for reference ITDs of ±0–200 μs measured here are within a factor of 1.2–1.5 of this value. The difference is likely to be due to technical considerations. The basic paradigm of conditioned avoidance was the same in the sound localization (Gandy et al., 1995) and in the current study. However, in the current study the major aim was to detect the best performance for ultimate comparsion with neural results in the same species. Consequently, we performed the data analysis to minimize the inclusion of less than optimal data, as described above. In contrast, the aim of the study on sound localization was comparative, to identify the range of sound localization acuity across species and identify physical, behavioral, and anatomical correlates of different capabilities in different species. The need to achieve maximal performance in a species like the rabbit was perhaps less important than maintaining a standardization of technique such that results from different species were obtained in a comparable fashion. Thus, it may be that technical factors contribute to the different value for ITD sensitivity expected based on sound localization acuity and that demonstrated in this study. If so, the sound localization acuity that could be expected based on a 40 μs ITD discrimination threshold is 15° (90°/300 μs × 50 μs).
In species that have a greater sound localization acuity the ITD discrimination threshold is better as well. In humans and macaques, the sound localization thresholds are only a few degrees (Mills, 1958; Brown et al., 1980; Heffner and Heffner, 1986) and the ITD discrimination is 10–20 μs (Klumpp, 1953; Zwislocki and Feldman, 1956; Scott et al., 2007). In the cat, the sound localization acuity is 3–6° (Heffner and Heffner, 1988; Tollin et al., 2005) and the ITD discrimination is ~ 30°s (Wakeford and Robinson, 1974). The results in cats are especially pertinant because the cat and rabbit have similar physiological ranges of about 300 μs. The physiological range in the cat is based on its ITD for a sound source at 90° (Roth et al., 1980), as it has yet to be determined behaviorally. Thus, in animals of similar head size, there are differences in both sound localization acuity and ITD discrimination. It has previously been shown across a wide range of species that sound localization acuity is porportional to the size of the region of increased ganglion cell densitiy in the retina (Heffner, 1997). This result indicates that the precision of localization is determined by the need to bring the most sensitive part of the eye to bear on objects of interest. Thus, animals with a fovea (humans and macaques) or area centrailis (cat) have better sound localization acuity than species with a visual streak, such as rabbits. Our results of increased ITD thresholds in rabbits compared to humans and cats support a similar trend across species for ITD discrimination.
Comparison with neural thresholds
It is not yet clear from a neural perspective why the acuity should be different in different species. The distributions of best ITD in each species and at different brain levels is similar, and the tuning to ITDs is broad (Ivarsson et al., 1981; Yin and Kuwada, 1983; Palmer et al., 1990; Yin and Chan, 1990; Stanford et al., 1992; Spitzer and Semple, 1995; Fitzpatrick et al., 1997; Fitzpatrick et al., 2000). Although the tuning widths are broad, the slopes of the ITD function across the physiological range can be fairly steep, with a minimum detectable change in the ITD equal to or even better than the behavioral acuity (Skottun et al., 2001; Shackleton et al., 2003). A possible explanation for the behavioral difference across species could be the the population code available. For animals with small heads, the modulation of the response occurs over a wide range of ITDs. The non-uniform distribution of best ITDs (or of horizonatal spatial location in free-field studies) indicates that most neurons are tuned to a similar range of ITDs, so this broad range of response modulation can provide a rate code for spatial location. The slopes of the response modulation go in opposite ways in the two hemispheres, so changes in rate due to common factors such as sound level can be accounted for by comparaison across hemispheres (McAlpine et al., 2001; Harper and McAlpine, 2004; Stecker et al., 2005). In contrast, in animals where the slopes and widths occur over a smaller fraction of the total range, a place code can be based on the peak or the edge of a population response (Harper and McAlpine, 2004). Examples of species that could take advantage of a place code are humans, by virtue of their large head, and barn owls, because of the high (up to 10 kHz) frequencies used (Knudsen and Konishi, 1978; Koppl, 1997). Of most importance to this study, both cats and rabbits have similar physiological ranges and neural tuning to ITDs, yet have different behavioral acuity to sound localization and ITD discrimination. It may be that the neural capability is optimized for different behavioral capabilities in different species. In animals where sound localization is critical and available from the neural circuits, it can be used in that way. If not, the general neural architecture still supports adequate sensitivity to sound location, and in addition provides for increased separation of signals from noise and a sensitivity to auditory “spaciousness” through variations in the responses to interaural correlation (Yin et al., 1987; Shackleton et al., 2005; Coffey et al., 2006)
In humans using broadband stimuli, there is a gradual increase in thresholds within the physiological range as the sound becomes increasingly lateralized towards the ear. Measurements of this increase range from about a factor of two with clicks (Hafter et al., 1975) up to a factor of about four with noise (Klumpp and Eady, 1957; Mossop and Culling, 1998). A similar increase has been observed in a model based on the neural responses to ITDs in cats and guinea pigs (Hancock and Delgutte, 2004). In the model, the property of the elements that gave rise to the increase in ITD threshold was a relatively constant sensitivity to phase across elements with different CFs. This constancy in phase rather than time means that when information is pooled across neurons with different CFs the slope of the average rate vs. ITD function decreases as the ITD increases. In rabbits, the evidence for higher behavioral thresholds as the reference ITD increases is slight. Overall, the trend was from about 50 μs at 0 μs to 59 μs at 200 μs. This trend was small enough and the variability in the measurements high enough that it did not reach statistical significance in any animal or across animals for points within the physiological range. Thus, from the current data we cannot conclude that there is a comparable increase in thresholds to ITDs across the physiological range in rabbits as there is in humans.
However, there is a possible neural explanation for the increase in threshold when the physiological range is exceeded, at least in rabbits. At the peaks of the ITD curves the response variability is greater and the change to a small change in ITD is less than on the steepest slope (also see Bala et al., 2003; Shackleton et al., 2003). Most neural tuning curves in the rabbit show a peak in the range of 100–300 μs (Kuwada et al., 1987). Thus, for reference ITDs of 0–200 μs most neurons are responding on the slope, where discrimination is best, while for a reference ITD of 300 μs most neurons are forced to detect changes near their peaks, where discrimination is less optimal. It would be interesting to test the ITD discrimination in a species with a head even smaller than the rabbit, such as a gerbil, where the physiological window is expected to be about 120 μs (Maki and Furukawa, 2005). Because the ITD tuning of neurons is similar between rabbits and gerbils (Spitzer and Semple, 1995) this might be a case where the decrease in ITD discrimination does not match the physiological window.
Acknowledgments
We thank Dr. Henry Heffner for teaching us the method of conditioned avoidance. We thank Stephen Pulver for technical assistance, Xiaohu Wan for computer programming, and Dr. Emily Buss for help with the psychometric functions. This work was supported by the NIH (DC-04398 and DC-005360), the Deafness Research Foundation, and the Distinguished Medical Scholars Program at the University of North Carolina School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bala AD, Spitzer MW, Takahashi TT. Prediction of auditory spatial acuity from neural images on the owl’s auditory space map. Nature. 2003;424:771–774. doi: 10.1038/nature01835. [DOI] [PubMed] [Google Scholar]
- Bargones JY, Werner LA, Marean GC. Infant psychometric functions for detection: mechanisms of immature sensitivity. J Acoust Soc Am. 1995;98:99–111. doi: 10.1121/1.414446. [DOI] [PubMed] [Google Scholar]
- Borg E, Engstrom B. Hearing thresholds in the rabbit. A behavioral and electrophysiological study. Acta Otolaryngol. 1983;95:19–26. doi: 10.3109/00016488309130911. [DOI] [PubMed] [Google Scholar]
- Brown CH, Beecher MD, Moody DB, Stebbins WC. Localization of noise bands by Old World monkeys. J Acoust Soc Am. 1980;68:127–132. doi: 10.1121/1.384638. [DOI] [PubMed] [Google Scholar]
- Coffey CS, Ebert CS, Jr, Marshall AF, Skaggs JD, Falk SE, Crocker WD, Pearson JM, Fitzpatrick DC. Detection of interaural correlation by neurons in the superior olivary complex, inferior colliculus and auditory cortex of the unanesthetized rabbit. Hear Res. 2006;221:1–16. doi: 10.1016/j.heares.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Early SJ, Mason CR, Zheng L, Evilsizer M, Idrobo F, Harrison JM, Carney LH. Studies of binaural detection in the rabbit (Oryctolagus cuniculus) with Pavlovian conditioning. Behav Neurosci. 2001;115:650–660. doi: 10.1037//0735-7044.115.3.650. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Batra R. Neural sensitivity to interaural time differences: beyond the Jeffress model. J Neurosci. 2000;20:1605–1615. doi: 10.1523/JNEUROSCI.20-04-01605.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada SK, Batra R. Transformations in processing interaural time differences between the superior olivary complex and inferior colliculus: Beyond the Jeffress Model. Hear Res. 2002;168:79–89. doi: 10.1016/s0378-5955(02)00359-3. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Batra R, Stanford TR, Kuwada S. A neuronal population code for sound localization. Nature. 1997;388:871–874. doi: 10.1038/42246. [DOI] [PubMed] [Google Scholar]
- Gandy RA, Heffner RS, Heffner HE. Sound localization in domestic rabbits: Left/right, front/back, and vertical. Assoc Res Otolaryngol Abs. 1995:64. [Google Scholar]
- Hafter ER, De Maio J, Hellman WS. Difference thresholds for interaural delay. J Acoust Soc Am. 1975;57:181–187. doi: 10.1121/1.380412. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Delgutte B. A physiologically based model of interaural time difference discrimination. J Neurosci. 2004;24:7110–7117. doi: 10.1523/JNEUROSCI.0762-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature. 2004;430:682–686. doi: 10.1038/nature02768. [DOI] [PubMed] [Google Scholar]
- Heffner H, Masterton B. Hearing in Glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. [Google Scholar]
- Heffner HE, Heffner RS. Hearing loss in Japanese macaques following bilateral auditory cortex lesions. J Neurophysiol. 1986;55:256–271. doi: 10.1152/jn.1986.55.2.256. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Conditioned avoidance. In: Klump GM, Dooling RJ, Fay RR, Stebbins WG, editors. Methods in Comparative Psychoacoustics. Basel: Birkhauser Verlag; 1995. pp. 79–93. [Google Scholar]
- Heffner RS. Comparative study of sound localization and its anatomical correlates in mammals. Acta Otolaryngol Suppl. 1997;532:46–53. doi: 10.3109/00016489709126144. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization acuity in the cat: effect of azimuth, signal duration, and test procedure. Hear Res. 1988;36:221–232. doi: 10.1016/0378-5955(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Ivarsson C, de Ribaupierre Y, Baroffio A, de Ribaupierre F. Interaural delay sensitive units in the MGB of the cat. In: Syka J, Aitkin L, editors. Neuronal Mechanisms of Hearing. New York: Plenum; 1981. pp. 245–249. [Google Scholar]
- Klein SA. Measuring, estimating, and understanding the psychometric function: a commentary. Percept Psychophys. 2001;63:1421–1455. doi: 10.3758/bf03194552. [DOI] [PubMed] [Google Scholar]
- Klumpp RG. Discriminability of interaural time difference. J Acoust Soc Am. 1953;25:823. [Google Scholar]
- Klumpp RG, Eady HR. Some measurements of interaural time difference thresholds. J Acoust Soc Am. 1957;28:859–860. [Google Scholar]
- Knudsen EI, Konishi M. Space and frequency are represented separately in auditory midbrain of the owl. J Neurophysiol. 1978;41:870–884. doi: 10.1152/jn.1978.41.4.870. [DOI] [PubMed] [Google Scholar]
- Koppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci. 1997;17:3312–3321. doi: 10.1523/JNEUROSCI.17-09-03312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CF. Model for the interaural time differences in the azimuthal plane. J Acoust Soc Am. 1977;62:157–167. [Google Scholar]
- Kuwada S, Stanford TR, Batra R. Interaural phase sensitive units in the inferior colliculus of the unanesthetized rabbit. Effects of changing frequency. J Neurophysiol. 1987;57:1338–1360. doi: 10.1152/jn.1987.57.5.1338. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467+. [PubMed] [Google Scholar]
- Maki K, Furukawa S. Acoustical cues for sound localization by the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Am. 2005;118:872–886. doi: 10.1121/1.1944647. [DOI] [PubMed] [Google Scholar]
- Martin GK, Lonsbury-Martin BL, Kimm J. A rabbit preparation for neuro-behavioral auditory research. Hear Res. 1980;2:65–78. doi: 10.1016/0378-5955(80)90017-9. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat Neurosci. 2001;4:396–401. doi: 10.1038/86049. [DOI] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Am. 1958;65:991–1000. [Google Scholar]
- Mossop JE, Culling JF. Lateralization of large interaural delays. J Acoust Soc Am. 1998;104:1574–1579. doi: 10.1121/1.424369. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Rees A, Caird D. Interaural delay sensitivity to tones and broad band signals in the guinea-pig inferior colliculus. Hear Res. 1990;50:71–86. doi: 10.1016/0378-5955(90)90034-m. [DOI] [PubMed] [Google Scholar]
- Roth GL, Kochhar RK, Hind JE. Interaural time differences: implications regarding the neurophysiology of sound localization. J Acoust Soc Am. 1980;68:1643–1651. doi: 10.1121/1.385196. [DOI] [PubMed] [Google Scholar]
- Saberi K. Some considerations on the use of adaptive methods for estimating interaural-delay thresholds. J Acoust Soc Am. 1995;98:1803–1806. doi: 10.1121/1.413379. [DOI] [PubMed] [Google Scholar]
- Saberi K, Green DM. Adaptive psychophysical procedures and imbalance in the psychometric function. J Acoust Soc Am. 1996;100:528–536. doi: 10.1121/1.415865. [DOI] [PubMed] [Google Scholar]
- Scott BH, Malone BJ, Semple MN. Effect of behavioral context on representation of a spatial cue in core auditory cortex of awake macaques. J Neurosci. 2007;27:6489–6499. doi: 10.1523/JNEUROSCI.0016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton TM, Arnott RH, Palmer AR. Sensitivity to Interaural Correlation of Single Neurons in the Inferior Colliculus of Guinea Pigs. J Assoc Res Otolaryngol. 2005:1–16. doi: 10.1007/s10162-005-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton TM, Skottun BC, Arnott RH, Palmer AR. Interaural time difference discrimination thresholds for single neurons in the inferior colliculus of Guinea pigs. J Neurosci. 2003;23:716–724. doi: 10.1523/JNEUROSCI.23-02-00716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottun BC, Shackleton TM, Arnott RH, Palmer AR. The ability of inferior colliculus neurons to signal differences in interaural delay. Proc Natl Acad Sci U S A. 2001;98:14050–14054. doi: 10.1073/pnas.241513998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski WJ, Trahiotis C. Discrimination of interaural temporal disparities by normal-hearing listeners and listeners with high-frequency sensorineural hearing loss. J Acoust Soc Am. 1986;79:1541–1547. doi: 10.1121/1.393680. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Keller CH, Marrocco RT, Takahashi TT. Head-related transfer functions of the Rhesus monkey. Hear Res. 2000;144:73–88. doi: 10.1016/s0378-5955(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Spitzer MW, Semple MN. Neurons sensitive to interaural phase disparity in gerbil superior olive: diverse monaural and temporal response properties. J Neurophysiol. 1995;73:1668–1690. doi: 10.1152/jn.1995.73.4.1668. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Batra R, Kuwada SK. A comparison of the interaural time sensitivity of neurons in the inferior colliculus and thalamus of the unanesthetized rabbit. J Neurosci. 1992;12:3200–3216. doi: 10.1523/JNEUROSCI.12-08-03200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker GC, Harrington IA, Middlebrooks JC. Location coding by opponent neural populations in the auditory cortex. PLoS Biol. 2005;3:e78. doi: 10.1371/journal.pbio.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterbing SJ, Hartung K, Hoffmann KP. Spatial tuning to virtual sounds in the inferior colliculus of the guinea pig. J Neurophysiol. 2003;90:2648–2659. doi: 10.1152/jn.00348.2003. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TC. Sound-localization performance in the cat: the effect of restraining the head. J Neurophysiol. 2005;93:1223–1234. doi: 10.1152/jn.00747.2004. [DOI] [PubMed] [Google Scholar]
- Wakeford OS, Robinson DE. Lateralization of tonal stimuli by the cat. J Acoust Soc Am. 1974;55:649–652. doi: 10.1121/1.1914577. [DOI] [PubMed] [Google Scholar]
- Werner LA, Marean GC. Psychometric functions of human infants for short duration tone bursts. ARO Abstr. 1991;14:105. [Google Scholar]
- Yin TCT, Kuwada S. Binaural interaction in low-frequency neurons in inferior colliculus of the cat. III. Effects of changing frequency. J Neurophysiol. 1983;50:1020–1042. doi: 10.1152/jn.1983.50.4.1020. [DOI] [PubMed] [Google Scholar]
- Yin TCT, Chan JCK. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- Yin TCT, Chan JCK, Carney LH. Effects of interaural time delays of noise stimuli of low-frequency cells in the cat’s inferior colliculus. III. Evidence for cross-correlation. J Neurophysiol. 1987;58:562–583. doi: 10.1152/jn.1987.58.3.562. [DOI] [PubMed] [Google Scholar]
- Zwislocki J, Feldman RS. Just noticeable differences in dichotic phase. J Acoust Soc Am. 1956;28:860–864. [Google Scholar]