Abstract
Arterial stiffness is a prominent feature of vascular aging and is strongly related to cardiovascular disease (CVD). Oxidized low-density lipoprotein (ox-LDL), a key player in the pathogenesis of atherosclerosis, may also play a role in arterial stiffening, but this relationship has not been well studied. Thus, we examined the cross-sectional association between ox-LDL and aortic pulse wave velocity (aPWV), a marker of arterial stiffness, in community-dwelling older adults. Plasma ox-LDL levels and aPWV were measured in 2,295 participants (mean age, 74 yrs; 52% female; 40% black) from the Health, Aging and Body Composition study. Mean aPWV significantly increased across tertiles of ox-LDL (tertile 1, 869 ± 376 cm/s; tertile 2, 901 ± 394 cm/s; tertile 3, 938 ± 415 cm/s; p=0.002). In multivariate analyses, ox-LDL remained associated with aPWV after adjustment for demographics and traditional CVD risk factors (p=0.008). After further adjustment for hemoglobin A1c, abdominal visceral fat, anti-hypertensive and antilipemic medications, and CRP the association with ox-LDL was attenuated, but remained significant (p=0.01). Results were similar when ox-LDL was expressed in absolute (mg/dL) or relative amounts (percent of LDL). Moreover, individuals in the highest ox-LDL tertile were 30-55% more likely to have high arterial stiffness, defined as aPWV > 75th percentile (p≤0.02). In conclusion, we found that among elderly persons, elevated plasma ox-LDL levels are associated with higher arterial stiffness, independent of CVD risk factors. These data suggest that ox-LDL may be related to the pathogenesis of arterial stiffness.
Keywords: aging, epidemiology, aortic stiffness, pulse wave velocity, oxidative stress
Introduction
Arterial stiffening, one of the most significant manifestations of vascular aging, is a complex process involving extracellular matrix proteins and smooth muscle cells.1, 2 Arterial stiffness has traditionally been linked to structural alterations in the vascular wall, including fragmentation and degeneration of elastin, increases in collagen content, arterial wall thickening, and luminal dilation. These age-related changes have adverse effects on cardiovascular health, as arterial stiffness increases systolic and pulse pressures, promotes left ventricular hypertrophy and dysfunction, and impairs coronary blood flow.3, 4 Moreover, increased arterial stiffness is associated with increased risk for cardiovascular disease (CVD) and its sequelae, including heart failure, stroke, atherosclerosis, myocardial infarction, and mortality.1, 3, 5-10
Although the exact mechanisms underlying arterial stiffening are not well understood, the imbalance in oxidants and antioxidants, in favor of the former (i.e. oxidative stress) may play an important role. Oxidative stress contributes to vascular dysfunction and has been implicated in the pathogenesis of aging, atherosclerosis, diabetes, hypertension, and hypercholesterolemia.11 The oxidative modification of low-density lipoprotein (LDL) is recognized as a key step in the initiation and progression of atherosclerosis,12 and elevated circulating levels of oxidized LDL (ox-LDL, a marker of oxidative stress) are associated with CVD risk factors, such as blood pressure, fasting glucose, and lipid levels, as well as both clinical and subclinical CVD.13-15 Thus, ox-LDL may be a marker of pathological processes occurring in the vascular wall.
Limited evidence suggests that ox-LDL is associated with the development of arterial stiffness. Ox-LDL stimulates collagen synthesis in arterial smooth muscle cells,16 promote intimal thickening,17 and impair nitric oxide bioactivity,18-20 all of which may contribute to arterial stiffness. In addition, circulating ox-LDL levels are inversely associated with arterial elasticity in young men21 and aortic stiffness in middle-aged men.22 However, it is not clear whether the association holds true in elderly men and women who likely have significant increases in both ox-LDL and arterial stiffness, as well as multiple comorbidites. Thus, the purpose of this study was to examine the association between plasma ox-LDL levels and arterial stiffness in a community-based sample of older adults.
Methods
Subjects
The present study uses data from the Health, Aging and Body Composition (Health ABC) study, a prospective cohort study of 3,075 well-functioning black and white men and women aged 70-79 years. Participants were recruited from a random sample of white and all black Medicare beneficiaries from March 1997 to July 1998 at field centers in Memphis, TN and Pittsburgh, PA. Eligible participants reported no difficulty walking one quarter of a mile, climbing 10 steps, or performing basic activities of daily living. Participants were excluded if they were being actively treated for cancer, planned to move from the area within 3 years, or were participating in a trial involving a lifestyle intervention. All participants signed a written informed consent form that was approved by the institutional review boards of the University of Pittsburgh and the University of Tennessee. All procedures followed were in accordance with institutional guidelines. Aortic pulse wave velocity (aPWV) data was missing for 354 participants because of equipment problems and 233 participants had waveforms that were of unacceptable quality or out of range (<300 or >3000 cm/s). Another 23 participants were excluded because of missing data on ox-LDL levels. An additional 170 participants were excluded due to missing data on pertinent covariates. Thus, the present study uses data from the remaining 2,295 participants. The 780 participants who were excluded were more likely to be black (47% vs. 40%) and diabetic (18% vs. 14%), have lower systolic blood pressure (SBP, 134 vs. 136 mmHg), and have higher abdominal visceral fat (153 vs. 140 cm2).
Oxidized LDL
Plasma levels of ox-LDL were measured using a monoclonal antibody (4E6)-based competition enzyme-linked immunosorbent assay (ELISA), as previously described.13 The monoclonal antibody 4E6 is directed against a conformational epitope in the apolipoprotein B-100 moiety of LDL that is generated as a consequence of substitution of at least 60 lysine residues of apolipoprotein B-100 with aldehydes. This number corresponds to the minimal number of substituted lysines required for scavengermediated uptake of ox-LDL. Substituting lysines can be produced by peroxidation of LDL lipids, resulting in the generation of ox-LDL. Aldehydes that are released by endothelial cells under oxidative stress or by activated platelets may also induce the oxidative modification of apolipoprotein B-100 in the absence of the peroxidation of LDL lipids. The high specificity of this assay has been previously described.13 The interassay coefficient of variation for this assay is 12%.
Aortic PWV
aPWV was measured from simultaneous Doppler flow signals obtained from the right carotid and femoral arteries with nondirectional transcutaneous Doppler flow probes (Model 810A, 9.0- to 10-MHz probes, Parks Medical Electronics, Inc). Digitized data were recorded by custom programming for subsequent analysis. A minimum of 10 beats were averaged for each simultaneous recording site using the QRS for synchronization. Three separate runs were recorded for each participant, and all usable runs were averaged. The distance between the carotid and femoral sampling sites was measured above the surface of the body with a metal tape measure in three sections: from the site of the carotid probe to the second intercostal space, from the second intercostal space to the umbilicus, and from the umbilicus to the site of the femoral probe. All three distances were summed and then the distance from the carotid probe to the second intercostal space was subtracted twice. This accounts for the fact that the flow from the heart to the carotid artery is in the opposite direction than the flow from the heart to the femoral artery. The time differentials between the onset of flow at carotid and femoral sites were divided by the associated distance to produce flow velocity. Stiffer vessels are associated with a faster PWV. The National Institute on Aging, Laboratory of Cardiovascular Science, Gerontology Research Center (Baltimore, MD) trained and certified all study personnel before data collection, read the waveforms, and evaluated data quality. Results from all acceptable runs were averaged for the final aPWV measure used in the analyses. Replicate measures of aPWV in 14 subjects revealed intraclass correlations of 0.88 between sonographers and 0.84 between readers.
Medical History and Clinical Measurements
Prevalent hypertension, diabetes, and CVD were evaluated by questionnaire and confirmed by use of specific medications or procedures. Prevalent CVD was defined as a history of myocardial infarction, angina, stroke, transient cerebral ischemia, or any vascular surgery, including endarterectomy or angioplasty. Medications taken in the past 2 weeks were brought in, recorded, and coded according to the Iowa Drug Information System. Using this system, participants using anti-lipemic and antihypertensive medications were identified. Blood pressure was measured three times using a conventional mercury sphygmomanometer with participants in the seated position after 5 min of quiet rest. The average of the last two measurements was used for SBP and diastolic blood pressure (DBP). Body mass index (BMI) was calculated from measured weight and height. Abdominal visceral fat was measured using a single 1-cm computed tomography image obtained during suspended respiration between the fourth and fifth lumbar vertebrae. Physical activity in the previous 7 days was assessed using an interviewer-administered questionnaire, and participants were categorized into three groups according to their overall physical activity pattern: inactive (i.e. less than 1,000 kcal/wk of exercise activity and 2,719 kcal/wk or less of total physical activity based on the Surgeon General's recommendation and the 25th percentile for the Health ABC cohort, respectively); lifestyle active (i.e. less than 1,000 kcal/wk of exercise activity and more than 2,719 kcal/wk of total physical activity); and exercise (i.e. 1,000 kcal/wk or more of exercise).23
Laboratory Measures
Total and HDL cholesterol, serum triglyceride, and glucose levels were measured on a Johnson & Johnson Vitros 950 analyzer. HDL was assayed after a magnetic precipitation of LDL, VLDL, and chylomicrons. LDL was estimated with the Friedewald equation.24 Fasting glucose levels were measured using an automated glucose oxidase reaction (YSI 2300 Glucose Analyzer; YSI, Yellow Springs, Ohio). C-reactive protein (CRP) was measured by an enzyme-linked immunosorbent assay based on purified protein and polyclonal anti-CRP antibodies (Calbiochem). Hemoglobin A1c was measured by a fully automated analyzer (Variant; Bio-Rad Laboratories, Inc, Hercules, CA) based on the principle of ion-exchange high-performance liquid chromatography.
Statistical Analysis
aPWV and ox-LDL were not normally distributed and were normalized through a log transformation. Other variables that were log transformed for analysis included HDL cholesterol, triglycerides, fasting glucose, hemoglobin A1c, and CRP. Chi-square tests and analysis of variance were used to evaluate associations between tertiles of ox-LDL and categorical and continuous variables, respectively. Tertile definitions were as follows: tertile 1, 0.12 to 0.91 mg/dL; tertile 2, 0.91 to 1.34 mg/dL; tertile 3, 1.34 to 7.31 mg/dL. Multivariate regression analyses were used to determine the relationship between arterial stiffness and ox-LDL (expressed in absolute amounts as mg/dL or in relative amounts as the percent of LDL). We also examined the association with ox-LDL tertile to aid in the interpretation of the results. Interaction terms were examined to assess whether race or gender modified the association between ox-LDL and aPWV. Since there were no significant interactions, gender and race groups were combined for all analyses. Regression models were first adjusted for age, gender, race, and site. When ox-LDL was expressed in mg/dL, LDL cholesterol was also included. Next, to determine whether the association between ox-LDL and arterial stiffness was independent of traditional CVD risk factors, we further adjusted for smoking, physical activity, history of diabetes and hypertension, BMI, blood pressure, fasting glucose, triglycerides, and HDL cholesterol. In the fully adjusted model, we accounted for other variables that may be related to ox-LDL and/or arterial stiffness. Given that hemoglobin A1c and abdominal visceral fat have previously been shown to be independently associated with aPWV in this cohort,25 we further adjusted for these variables to determine whether they altered the relationship between aPWV and ox-LDL. We also adjusted for anti-hypertensive and anti-lipemic medication use to control for their effects on lipid levels and blood pressure. Finally, we added CRP to the model to determine whether inflammation alters the association between ox-LDL and aPWV. Only those variables that remained significant after controlling for other covariates were included in the final multivariate models. The final models were then run using logistic regression to determine the association of ox-LDL tertile with high arterial stiffness (defined as aPWV >75th percentile). A value of p≤0.05 was considered statistically significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Of the 2,295 participants used in this analysis, 52% were female and 40% were black. The mean (SD) age was 73.7 (2.8) yrs. Ox-LDL values ranged from 0.12 to 7.31 mg/dL, with a mean (SD) of 1.27 (0.69) mg/dL and a median (interquartile range) of 1.10 (0.83 to 1.52) mg/dL. When expressed as a percent of LDL, ox-LDL values ranged from 0.08 to 10.2%, with a mean (SD) of 1.06 (0.51)% and a median (interquartile range) of 0.96 (0.75 to 1.23)%. Other subject characteristics by tertile of ox-LDL (mg/dL) are shown in Table 1. In general, individuals in the highest tertile of ox-LDL were more likely to be female (p=0.05), black (p<0.0001), and have hypertension (p=0.05) and diabetes (p=0.05) and were less likely to be on anti-lipemic medications (p=0.003). Higher plasma ox-LDL levels were also associated with higher total and LDL cholesterol, triglycerides, fasting glucose, hemoglobin A1c, CRP, SBP, DBP, BMI, and abdominal visceral fat, and lower HDL cholesterol (all p≤0.02). There were no differences in age, smoking, physical activity, prevalent CVD, or anti-hypertensive drug use between tertiles. Results were similar when ox-LDL was expressed as a percent of LDL, except that higher relative amounts of ox-LDL were associated with lower amounts of total and LDL cholesterol, and a higher prevalence of anti-hypertensive drug use. Ox-LDL (% of LDL) was not associated with anti-lipemic drug use.
Table 1.
Subject Characteristics by ox-LDL tertile (mg/dL)
| Characteristic | Tertile 1 (0.12-0.91) | Tertile 2 (0.91-1.34) | Tertile 3 (1.34-7.31) | P-value |
|---|---|---|---|---|
| N | 761 | 769 | 765 | --- |
| Age, yrs | 73.8±2.9 | 73.7±2.8 | 73.6±2.9 | 0.56 |
| Female | 384 (50.5) | 386 (50.2) | 427 (55.8) | 0.05 |
| Black | 266 (35.0) | 299 (38.9) | 353 (46.1) | <0.0001 |
| Smoking | ||||
| Never | 327 (43.0) | 333 (43.3) | 359 (46.9) | 0.53 |
| Former | 358 (47.0) | 355 (46.2) | 332 (43.4) | |
| Current | 75 (10.0) | 81 (10.5) | 74 (9.7) | |
| Physical Activity | ||||
| Inactive | 152 (20.0) | 166 (21.6) | 172 (22.5) | 0.79 |
| Lifestyle Active | 360 (47.3) | 357 (46.4) | 358 (46.8) | |
| Exercise | 249 (32.7) | 246 (32.0) | 235 (30.7) | |
| Hypertension | 310 (40.7) | 338 (44.0) | 359 (46.9) | 0.05 |
| Diabetes | 90 (11.8) | 124 (16.1) | 113 (14.8) | 0.05 |
| CVD | 173 (22.7) | 207 (26.9) | 178 (23.3) | 0.12 |
| Anti-lipemic drugs | 141 (18.5) | 112 (14.6) | 94 (12.3) | 0.003 |
| Anti-hypertensive drugs | 400 (52.6) | 417 (54.2) | 433 (56.6) | 0.28 |
| Total cholesterol, mg/dL | 183±32 | 201±33 | 226±37 | <0.0001 |
| HDL cholesterol, mg/dL | 58±18 | 55±17 | 52±14 | <0.0001 |
| LDL cholesterol, mg/dL | 101±28 | 120±28 | 144±33 | <0.0001 |
| Triglycerides, mg/dL | 120±54 | 131±62 | 151±70 | <0.0001 |
| Fasting glucose, mg/dL | 99.3±31.0 | 104.9±34.9 | 107.4±34.7 | <0.0001 |
| Hemoglobin A1c, %* | 6.1±1.0 | 6.4±1.2 | 6.5±1.1 | <0.0001 |
| CRP, mg/L | 2.96±5.75 | 2.68±3.76 | 3.26±4.94 | <0.0001 |
| SBP, mmHg | 135.3±20.2 | 135.6±20.3 | 138.1 ±21.6 | 0.02 |
| DBP, mmHg | 71.2±11.8 | 71.6±11.7 | 73.0±10.8 | 0.005 |
| BMI, kg/m2 | 26.5±4.5 | 27.4±4.6 | 28.2±4.8 | <0.0001 |
| Abdominal visceral fat, cm2 | 130±66 | 142±65 | 147±65 | <0.0001 |
| Aortic PWV, cm/s | 869±376 | 901±394 | 938±415 | 0.002 |
Table values are means ± SD or numbers (frequencies).
As shown in Table 2, ox-LDL (mg/dL) was associated with aPWV in univariate analysis (p=0.0003). This association remained significant after adjusting for demographic variables and LDL cholesterol (p<0.0001). To determine whether the association between ox-LDL and aPWV was independent of traditional CVD risk factors, we further adjusted for smoking, physical activity, diabetes, hypertension, BMI, blood pressure, fasting glucose, triglycerides, and HDL cholesterol. After adjustment for these risk factors, the association between ox-LDL and aPWV was attenuated, but remained significant (p=0.008). After additional adjustments for hemoglobin A1c, abdominal visceral fat, anti-hypertensive and anti-lipemic drug use, and CRP the association with ox-LDL was further attenuated (p=0.01). Similar results were found when ox-LDL was expressed as the percent of LDL. When we examined the unadjusted model using ox-LDL tertile, we found that individuals in the highest tertile had a significantly higher aPWV (geometric mean ± SE, 871 ± 1 cm/s) than individuals in the middle (832 ± 1 cm/s, p=0.05) and lowest tertiles (813 ± 1 cm/s, p=0.0005). In the fully adjusted model, aPWV remained significantly higher among individuals in the highest tertile compared to those in the lowest tertile (891 ±1 cm/s vs. 851 ± 1 cm/s, p=0.04).
Table 2.
Relationship between log (ox-LDL) and log (aPWV)
| Model | Log Ox-LDL (mg/dL) |
Log Ox-LDL (% of LDL) |
||
|---|---|---|---|---|
| β ± SE | P-value | β ± SE | P-value | |
| Model 1 | 0.063±0.017 | 0.0003 | 0.092±0.020 | <0.0001 |
| Model 2 | 0.085±0.020 | <0.0001 | 0.085±0.020 | <0.0001 |
| Model 3 | 0.053±0.020 | 0.008 | 0.053±0.019 | 0.006 |
| Model 4 | 0.049±0.019 | 0.01 | 0.052±0.019 | 0.008 |
Table values are standardized regression coefficients for ox-LDL. Model 1: Unadjusted; Model 2: Adjusted for age, gender, race, clinical site, and LDL cholesterol (when expressed as mg/dL only); Model 3: Adjusted for variables in Model 2 plus smoking, physical activity, HDL cholesterol, triglycerides, fasting glucose, BMI, DBP, SBP, diabetes, and hypertension; Model 4: Adjusted for variables in Model 3 plus hemoglobin A1c, abdominal visceral fat, anti-lipemic and anti-hypertensive drug use, and CRP
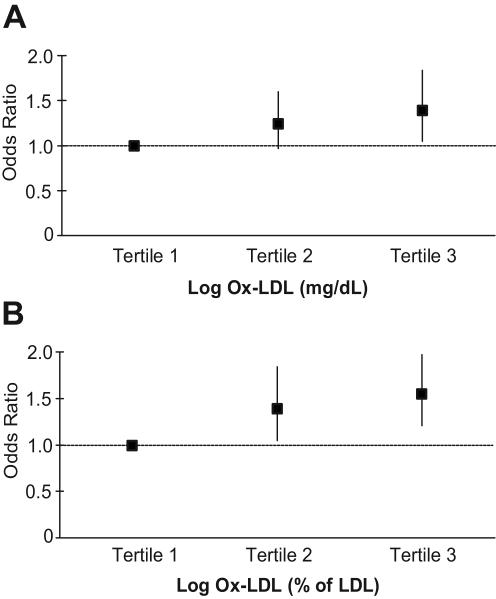
Logistic regression was performed to study the relation of high arterial stiffness, defined as having aPWV values >75th percentile (>1054 cm/s), with ox-LDL. As shown in panel A of the Figure, individuals in the middle ox-LDL tertile (mg/dL) had a similar odds of having a high aPWV compared to individuals in the lowest tertile after adjusting for age, gender, race, site, smoking, LDL cholesterol, SBP, and abdominal visceral fat (OR, 1.24, 95% CI, 0.97-1.60, p=0.09). However, individuals in the highest ox-LDL tertile were ~40% more likely to have a high aPWV (OR, 1.39, 95% CI, 1.05-1.84, p=0.02). When expressed as a percent of LDL (panel B), individuals in the middle and highest tertiles of ox-LDL were 33% (OR, 1.33, 95% CI, 1.04-1.70, p=0.03) and 55% (OR, 1.55, 95% CI, 1.21-1.97, p=0.0005) more likely, respectively, to have a high aPWV compared to individuals in the lowest tertile of ox-LDL.
Figure.
Odds ratios and 95% confidence intervals for the association of a high aPWV with ox-LDL expressed as A) mg/dL and B) percent of LDL. Odds ratios are adjusted for age, gender, race, site, smoking, LDL cholesterol (when expressed as mg/dL), SBP, and abdominal visceral fat.
Discussion
The present study is the first epidemiologic analysis to examine the relationship between ox-LDL and arterial stiffness in older persons. Our results show that elevated plasma ox-LDL levels are associated with higher arterial stiffness, independent of demographics and traditional CVD risk factors. Previously in the Health ABC cohort, subjects with aPWV >641 cm/s (corresponding to the 25th percentile of the aPWV distribution) were found to have a > 2-fold increase in the risk of CVD, a 2- to 3-fold increase in stroke, and a >50% increase in coronary heart disease events compared to those with values below this level.10 In keeping with these previous findings, we performed additional analyses to determine the effect of changing the definition of high arterial stiffness from aPWV values >1054 cm/s (75th percentile) to aPWV values >641 cm/s (25th percentile). We found that the association between ox-LDL and arterial stiffness remained essentially the same, regardless of the definition. Taken together, our data suggest that individuals with ox-LDL levels > 1.34 mg/dL (or 1.2% of LDL) have higher arterial stiffness, putting them at increased risk for future cardiovascular events.
We examined plasma levels of ox-LDL to determine the contribution of oxidative stress to arterial stiffness. Previous data suggest that in healthy middle-aged and older individuals, mean plasma ox-LDL levels range from 0.57 to 1.48 mg/dL (0.48 to 1.25% of LDL).14, 26-28 Similar levels were observed in our study population. The low plasma concentration is maintained, in part, by the presence of scavenger receptors on endothelial cells and specialized macrophages (i.e. Kupffer cells) in the liver that are capable of rapidly clearing ox-LDL from the circulation.29, 30 As such, increased levels of ox-LDL in plasma may reflect an impaired ability to remove modified lipoproteins from the circulation. Alternatively, higher levels may result from a reduced antioxidant capacity or an excessive production of oxidants. Elevated levels of ox-LDL are associated with coronary artery disease, acute coronary syndromes, myocardial infarction, intima-media thickness, plaque occurrence in the carotid and femoral arteries, coronary artery calcification, metabolic abnormalities, and inflammatory cytokines.13-15, 26, 31, 32 Our data demonstrate that elevated ox-LDL levels are also associated with arterial stiffness. In fact, we found that persons with elevated ox-LDL levels were 30-55% more likely to have stiff arteries for their age than persons with low ox-LDL levels. Thus, plasma ox-LDL levels may be a marker of oxidative stress and the associated pathological changes that occur in the vascular wall, independent of the effects of dyslipidemia, hyperglycemia, hypertension, or inflammation.
Arterial stiffness, which affects predominantly the aorta and proximal elastic arteries, is one of the cardinal manifestations of vascular aging.4 Arterial stiffness can impair the ability of the vascular system to distribute blood from the heart as steady flow through the peripheral capillaries, and thus, can have a devastating effect on the heart and microcirculation, especially in the brain and kidneys.4 The two major direct consequences of arterial stiffening are a reduced capacitance and a faster PWV, both of which can modify central hemodynamics.3 Arterial stiffness can increase SBP, and because of the rapid PWV, the reflected wave returns during systole rather than diastole, thereby amplifying SBP even further and imposing an additional workload on the heart.3, 4 Early return of wave reflection can also reduce DBP, thereby limiting coronary blood flow and predisposing to ischemia and angina. Increased arterial stiffness, along with the associated increases in SBP and pulse pressure, has been linked to a higher risk for developing heart failure,3, 6 stroke,1, 8-10 atherosclerosis,1, 7, 9, 10, 33 and myocardial infarction.3 Moreover, arterial stiffness has been associated with increased left ventricular hypertrophy and dysfunction,3, 34-37 as well as increased all-cause and cardiovascular mortality.5, 7, 10
Arterial stiffness increases with age even in healthy individuals without clinical CVD.38 The presence of CVD risk factors, such as obesity or hypertension, may accelerate vascular changes that result in arterial stiffening.38 The underlying mechanisms remain to be elucidated; however, oxidative stress, or the imbalance in oxidants and antioxidants, in favor of the former, may play an important role. Oxidative stress can cause oxidative damage to lipids, proteins, DNA, and other biological molecules that are critical for normal cellular functioning.11, 12 Accordingly, oxidative stress contributes to vascular dysfunction and has been implicated in the pathogenesis of hypercholesterolemia, atherosclerosis, hypertension, diabetes, and heart failure.11, 39
There is strong evidence to support a role for ox-LDL in the pathogenesis of arterial stiffening via changes in both the structure and function of the arterial wall. For example, ox-LDL increases the expression of matrix metalloproteinases in macrophages and endothelial cells, which may promote the breakdown of extracellular matrix components and contribute to vascular remodeling.40, 41 Ox-LDL-mediated vascular remodeling is also characterized by an inflammatory and fibroproliferative response that leads to intimal thickening.17 This occurs, in part, from ox-LDL-induced endothelial cell activation, leukocyte adhesion, smooth muscle cell migration and proliferation, and increased collagen synthesis.16, 42-46 The ox-LDL-induced expression of genes that facilitate vascular calcification may also contribute to stiffer arteries.47, 48 Moreover, the capacitance of the arterial system may become compromised as ox-LDL also induces endothelial dysfunction by impairing endothelium-dependent vasodilation, inhibiting nitric oxide bioavailability, and reducing endothelial nitric oxide synthase expression and activity.18-20
Several limitations of the present study need to be considered. First, because this study was cross-sectional, we cannot determine whether increased ox-LDL is a cause of arterial stiffening or simply a by-product of other disease processes. Second, although there is strong in vitro evidence supporting a role for ox-LDL in the development of vascular changes that promote arterial stiffness, this study cannot determine how or where the oxidation occurred, nor can we identify the mechanisms by which plasma ox-LDL may be related to arterial stiffness. Third, the oxidative modification of LDL leads to a heterogeneous population of ox-LDL particles with a variety of oxidized products and diverse biological properties. Thus, it is difficult to quantify the full range of oxidatively modified LDL particles in plasma. However, the 4E6 antibody used to measure ox-LDL in the present study has been well-characterized and appears to be clinically useful in the assessment of oxidative stress and disease status in various populations.14, 15, 26, 28, 31, 32 Fourth, it is possible that residual confounding from either unmeasured or imperfectly measured variables could explain the observed association.
Perspectives
This study demonstrates for the first time that plasma ox-LDL levels are related to arterial stiffness in elderly men and women. Our data suggest that the oxidative modification of LDL may be associated with changes in the elastic properties of blood vessels. As such, reducing oxidative stress is an attractive therapeutic target in the prevention of age-related changes in arterial structure and function and subsequent disease. While antioxidant supplementation trials have been found to be largely ineffective in preventing cardiovascular outcomes, other interventions including aerobic exercise training and pharmacological treatment with lipid- and blood pressure-lowering medications may have significant antioxidant effects that are related to reductions in CVD risk. In understanding the relationship between oxidative stress and CVD, it is important to remember that free radicals and other oxidants are involved in both normal and pathological processes, and therefore, manipulating cellular redox pathways could potentially cause harm. Thus, more research is needed to determine whether oxidative stress is a cause or a consequence of the disease process. Moreover, it is critical that researchers identify the major oxidants involved, and if and how antioxidant therapies can be used to slow arterial stiffening with age and prevent cardiovascular events.
Acknowledgments
Sources of Funding This research was supported by grants N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, and R01-AG-027529-01A1S1. This research was also supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Disclosures None
Reference List
- (1).Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- (2).Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- (3).Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of agerelated medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- (4).O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- (5).Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- (6).Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- (7).Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- (8).Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- (9).Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- (10).Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- (11).Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195–199. doi: 10.1136/pmj.79.930.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- (13).Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM, Simonsick EM, Colbert LH, Kritchevsky SB. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23:1444–1448. doi: 10.1161/01.ATV.0000080379.05071.22. [DOI] [PubMed] [Google Scholar]
- (14).Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194:245–252. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- (15).Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study) Arterioscler Thromb Vasc Biol. 2002;22:1162–1167. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- (16).Jimi S, Saku K, Uesugi N, Sakata N, Takebayashi S. Oxidized low density lipoprotein stimulates collagen production in cultured arterial smooth muscle cells. Atherosclerosis. 1995;116:15–26. doi: 10.1016/0021-9150(95)05515-x. [DOI] [PubMed] [Google Scholar]
- (17).Matthys KE, Van Hove CE, Kockx MM, Andries LJ, Van ON, Herman AG, Bult H. Local application of LDL promotes intimal thickening in the collared carotid artery of the rabbit. Arterioscler Thromb Vasc Biol. 1997;17:2423–2429. doi: 10.1161/01.atv.17.11.2423. [DOI] [PubMed] [Google Scholar]
- (18).Fleming I, Mohamed A, Galle J, Turchanowa L, Brandes RP, Fisslthaler B, Busse R. Oxidized low-density lipoprotein increases superoxide production by endothelial nitric oxide synthase by inhibiting PKCalpha. Cardiovasc Res. 2005;65:897–906. doi: 10.1016/j.cardiores.2004.11.003. [DOI] [PubMed] [Google Scholar]
- (19).Hein TW, Liao JC, Kuo L. oxLDL specifically impairs endothelium-dependent, NO-mediated dilation of coronary arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H175–H183. doi: 10.1152/ajpheart.2000.278.1.H175. [DOI] [PubMed] [Google Scholar]
- (20).Thomas SR, Chen K, Keaney JF., Jr. Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redox Signal. 2003;5:181–194. doi: 10.1089/152308603764816541. [DOI] [PubMed] [Google Scholar]
- (21).Toikka JO, Niemi P, Ahotupa M, Niinikoski H, Viikari JS, Ronnemaa T, Hartiala JJ, Raitakari OT. Large-artery elastic properties in young men: relationships to serum lipoproteins and oxidized low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1999;19:436–441. doi: 10.1161/01.atv.19.2.436. [DOI] [PubMed] [Google Scholar]
- (22).Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- (24).Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- (25).Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- (26).Holvoet P, Vanhaecke J, Janssens S, Van de WF, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- (27).Holvoet P, Van CJ, Collen D, Vanhaecke J. Oxidized low density lipoprotein is a prognostic marker of transplant-associated coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:698–702. doi: 10.1161/01.atv.20.3.698. [DOI] [PubMed] [Google Scholar]
- (28).Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, Collen D, Muls E, Van de WF. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:844–848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- (29).Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- (30).De Rijke YB, Biessen EA, Vogelezang CJ, Van Berkel TJ. Binding characteristics of scavenger receptors on liver endothelial and Kupffer cells for modified low-density lipoproteins. Biochem J. 1994;304:69–73. doi: 10.1042/bj3040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- (32).Nordin FG, Hedblad B, Berglund G, Nilsson J. Plasma oxidized LDL: a predictor for acute myocardial infarction? J Intern Med. 2003;253:425–429. doi: 10.1046/j.1365-2796.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- (33).Meaume S, Rudnichi A, Lynch A, Bussy C, Sebban C, Benetos A, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular disease in subjects over 70 years old. J Hypertens. 2001;19:871–877. doi: 10.1097/00004872-200105000-00006. [DOI] [PubMed] [Google Scholar]
- (34).Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O'Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- (35).Pini R, Cavallini MC, Bencini F, Silvestrini G, Tonon E, De AW, Marchionni N, Di BM, Devereux RB, Masotti G, Roman MJ. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol. 2002;40:1283–1289. doi: 10.1016/s0735-1097(02)02159-9. [DOI] [PubMed] [Google Scholar]
- (36).Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- (37).Safar ME, Toto-Moukouo JJ, Bouthier JA, Asmar RE, Levenson JA, Simon AC, London GM. Arterial dynamics, cardiac hypertrophy, and antihypertensive treatment. Circulation. 1987;75:I156–I161. [PubMed] [Google Scholar]
- (38).Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- (39).Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- (40).Huang Y, Mironova M, Lopes-Virella MF. Oxidized LDL stimulates matrix metalloproteinase-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2640–2647. doi: 10.1161/01.atv.19.11.2640. [DOI] [PubMed] [Google Scholar]
- (41).Xu XP, Meisel SR, Ong JM, Kaul S, Cercek B, Rajavashisth TB, Sharifi B, Shah PK. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation. 1999;99:993–998. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]
- (42).Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992;111:143–147. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- (43).Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McMurray HF, Parthasarathy S, Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J Clin Invest. 1993;92:1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Rangaswamy S, Penn MS, Saidel GM, Chisolm GM. Exogenous oxidized low-density lipoprotein injures and alters the barrier function of endothelium in rats in vivo. Circ Res. 1997;80:37–44. doi: 10.1161/01.res.80.1.37. [DOI] [PubMed] [Google Scholar]
- (47).Bear M, Butcher M, Shaughnessy SG. Oxidized low-density lipoprotein acts synergistically with beta-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cells. J Cell Biochem. 2008;101:185–193. doi: 10.1002/jcb.21812. [DOI] [PubMed] [Google Scholar]
- (48).Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 2004;320:424–427. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]