Abstract
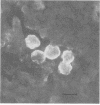
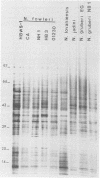
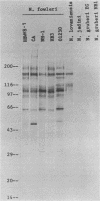
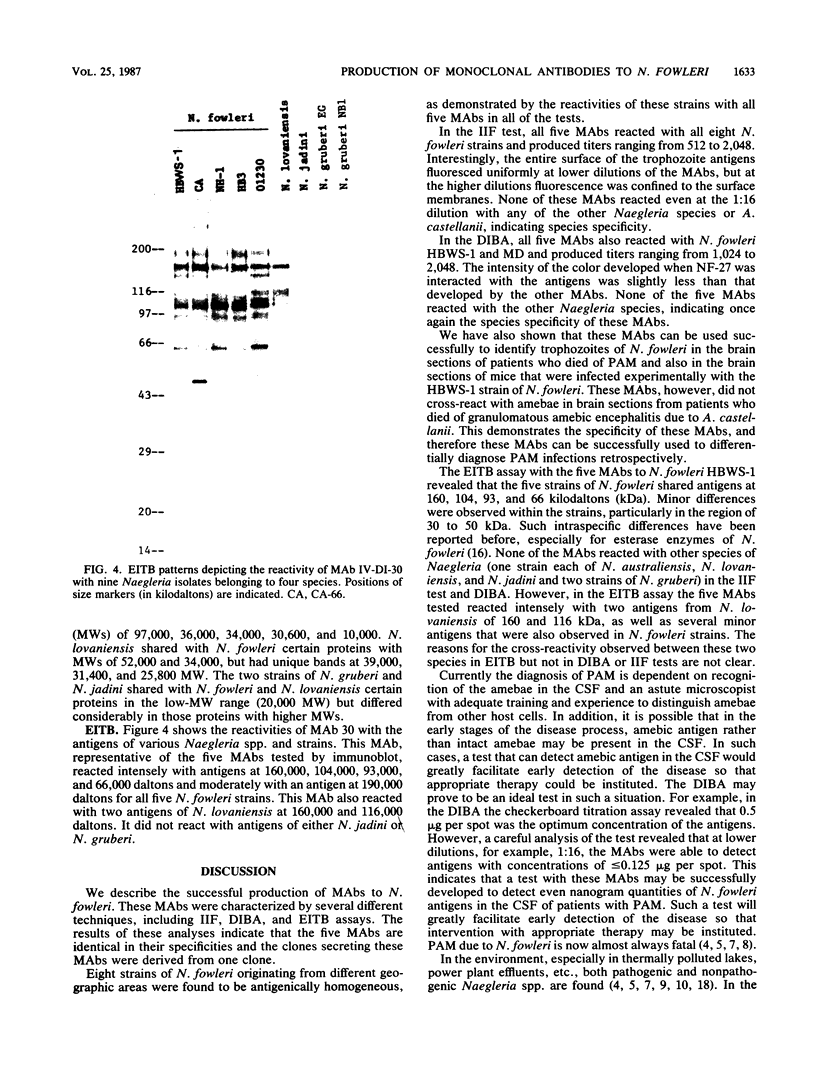
Monoclonal antibodies (MAbs) to Naegleria fowleri, the etiologic agent of primary amebic meningoencephalitis (PAM), have been produced and used as probes to identify N. fowleri amebae in brain sections of patients who died of that disease. These MAbs were characterized for their specificity by the indirect immunofluorescence assay (IIF), dot immunobinding assay (DIBA), and enzyme-linked immunotransfer blot technique (EITB). The MAbs reacted intensely with all strains of N. fowleri tested originating from different geographic areas in the IIF and DIBA tests, but showed no reactivity with four other species of Naegleria, N. gruberi, N. jadini, N. lovaniensis, and N. australiensis, or a strain of Acanthamoeba castellanii. In the EITB assay the MAbs reacted with the antigens of N. fowleri and produced intensely staining bands at the 160-, 104-, 93-, and 66-kilodalton (kDa) regions and several minor bands at the 30- and 50-kDa regions. The MAbs also reacted with the antigens of N. lovaniensis and produced a darkly staining band at 160 kDa and a diffusely staining band at 116 kDa, indicating that these antigens were shared by the two species. The MAbs, however, showed no reactivity with N. jadini and N. gruberi in the EITB assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett F. C., Yeoman L. C. An improved procedure for the 'dot immunobinding' analysis of hybridoma supernatants. J Immunol Methods. 1983 Jul 15;61(2):201–207. doi: 10.1016/0022-1759(83)90163-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gamble H. R. Application of hybridoma technology to the diagnosis of parasitic disease. Vet Parasitol. 1984 Jun;14(3-4):251–261. doi: 10.1016/0304-4017(84)90095-5. [DOI] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- McCool J. A., Spudis E. V., McLean W., White J., Visvesvara G. S. Primary amebic meningoencephalitis diagnosed in the emergency department. Ann Emerg Med. 1983 Jan;12(1):35–37. doi: 10.1016/s0196-0644(83)80132-2. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., De Jonckheere J., Willaert E. Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int J Parasitol. 1980 Feb;10(1):51–64. doi: 10.1016/0020-7519(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Tyndall R. L., Coutant C. C., Willaert E. Isolation of the etiological agent of primary amoebic meningoencephalitis from artifically heated waters. Appl Environ Microbiol. 1977 Dec;34(6):701–705. doi: 10.1128/aem.34.6.701-705.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Balamuth W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J Protozool. 1975 May;22(2):245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Healy G. R. Comparative antigenic analysis of pathogenic and free-living Naegleria species by the gel diffusion and immunoelectrophoresis techniques. Infect Immun. 1975 Jan;11(1):95–108. doi: 10.1128/iai.11.1.95-108.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara G. S., Healy G. R. Disc electrophoretic patterns of esterase isoenzymes of Naegleria fowleri and N. gruberi. Trans R Soc Trop Med Hyg. 1980;74(3):411–412. doi: 10.1016/0035-9203(80)90117-0. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Smith P. D., Healy G. R., Brown W. R. An immunofluorescence test to detect serum antibodies to Giardia lamblia. Ann Intern Med. 1980 Dec;93(6):802–805. doi: 10.7326/0003-4819-93-6-802. [DOI] [PubMed] [Google Scholar]
- Wellings F. M., Amuso P. T., Chang S. L., Lewis A. L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977 Dec;34(6):661–667. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]