Abstract
During neonatal development exogenous oxytocin increases ERα immunoreactivity in the hypothalamus of female prairie voles. The purpose of this study was to determine if the increase in ERα is associated with an increase in ERα mRNA expression and to determine if the effect is specific to ER subtype or if oxytocin also influences ERβ mRNA expression. On the day of birth female prairie vole pups were treated with oxytocin, an oxytocin antagonist, or saline. Brains were collected and RT-PCR was used to determine the effect of treatment on ER mRNA production in the hypothalamus, hippocampus, and cortex. Within 2 hours of treatment oxytocin significantly increased ERα mRNA expression in the hypothalamus and hippocampus, but not the cortex, while inhibiting the effects of endogenous oxytocin reduced the expression of ERα mRNA in the hippocampus. Neonatal treatment did not affect the expression of ERβ mRNA. The results demonstrate that the effects of oxytocin treatment are region and ER subtype specific and that during the neonatal period oxytocin can affect the expression of ERα by altering message production. The regional specific changes in ERα mRNA expression in females are consistent with studies examining the behavioral and physiological effects of neonatal manipulation of oxytocin in females.
Keywords: oxytocin, estrogen receptor alpha, estrogen receptor beta, prairie vole, RT-PCR, hypothalamus, hippocampus
Introduction
In adult females many of the effects of oxytocin (OT) are steroid dependent, with estrogen increasing the female’s response to OT (Caldwell et al., 1986; Coirini et al., 1989;Witt et al., 1991; Johnson 1992). However, during neonatal development it appears that OT can affect the subsequent response to estrogen. Neonatal manipulation of OT alters a number of estrogen dependent response in adult females, including fat deposition (Uvnäs-Moberg et al., 1998), onset of first estrus and vaginal opening (Withuhn et al. 2003), and mating patterns (Cushing et al., 2005). The changes in response to estrogen in the adult females that were treated neonatally with OT may be the result of changes in the CNS expression of estrogen receptor α (ERα). Neonatal manipulation of OT altered the expression of ERα, with OT increasing and an OT antagonist (OTA) decreasing ERα-IR, in the neonatal period (Yamamoto et al., 2006). While there are several potential methods of action one possibility is that neonatal OT directly affects the expression of ERα by altering the production of ERα mRNA. This prediction is supported by in vivo studies using MCF7 breast cancer cell lines. OT treatment altered the response of MCF7 cells to estrogen (Cassoni et al., 1997) and this was due to changes in the production of ERα mRNA (Cassoni et al., 2002). Therefore the goal of this study was to test the hypothesis that neonatal manipulation of oxytocin can selectively alter ERα mRNA expression. Specifically we predict that neonatal treatment will increase ERα mRNA in the hypothalamus, while inhibition of endogenous OT will result in a decrease in ERα mRNA in the same region. In addition we will determine if the effect of oxytocin is receptor subtype specific or if neonatal manipulation also effects the expression of the other nuclear estrogen receptor subtype, β.
Methods
Animals used in this study were laboratory-reared prairie voles that originated from wild-stock trapped near Urbana, Illinois, USA. Animals were maintained on a 14:10 light/dark cycle and provided Purina High Fiber Rabbit Chow and water ad libitum. Animals were housed in accordance with the USDA and NIH guidelines and prior to conducting any research all procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee.
On the day of birth, all pups were removed from the parents and placed on cotton bedding and a lamp was used to maintain temperature. Female pups received one of three treatments. They were given a single intraperitoneal injection (50-μl volume) of isotonic saline (SAL), 3 μg OT/50 μl saline (OT), or 0.3 μg OT antagonist/50 μl saline (OTA, [d(CH2)5, Tyr(Me)2, Orn8]-Vasotocin-Peninsula Laboratories, Belmont, CA), with an n of 5 per treatment. The dosage of OT was approximately 1 μg/g body weight and for OTA, 0.1 μg/g body weight. The dose of OT and OTA were used because there is extensive literature indicating that during the neonatal period these doses can effect a variety of physiological and behavioral responses in both rats and voles (Uvnäs-Moberg et al., 1998; Sohlström et al., 2002; Kramer et al., 2003; Withuhn et al., 2003; Bales et al., 2004; Cushing et al., 2005; Yamamoto et al., 2004), as well as affect neuronal activation in neonates (Cushing et al., 2003). A lower dose of OTA was used because the antagonist binds OT receptors (OTR) more effectively than OT, with an affinity for OTR greater than 10 times that of the natural ligand (Barberis and Tribollet, 1996). Intraperitoneal administration was used because central administration is difficult in newborn pups and i.p. administration has been shown to affect the CNS. The blood-brain barrier is not fully mature in neonates (Johanson, 1980) and evidence suggests small peptides cross the blood-brain barrier (Banks and Kastin, 1985). Neonatal treatment as described here resulted in differential c-Fos expression across treatment groups (Cushing et al., 2003) and subsequent expression of OT neurons (Yamamoto et al., 2004). A single dose was used because it has been demonstrated that a single treatment on the day of birth can affect behavior, physiology, neuroanatomy, and neuronal activation (for review see Carter, 2003). Pups were assigned to treatment groups randomly with the restriction that within each litter there was at least one control (SAL) and one experimental (OT or OTA) pup. No treatment was represented more than once per litter. Pups were returned as a group to the home cage within 10 min of removal. After 1 and 2 hours of injections the pups were removed from parents and were deeply anesthetized using a combination of ketamine and xylazine and then decapitated. The hypothalamus, hippocampus, and cortex were dissected on ice and frozen in liquid nitrogen and then stored at −80°C for RNA extraction.
RNA extraction
Total RNA was extracted from each frozen tissue following (Auffray et al., 1980) with slight modification. Each frozen tissue was homogenized in 5–10 ml LiCl-urea (3M LiCl, 6M urea) per g tissue and incubated overnight at 4°C. Homogenate was then transferred to Corex tubes and centrifuged at 8000 rpm for 25 min. Supernatant was discarded and the walls of the tubes wiped with cotton swabs. Cold LiCl-urea was added to the tubes at half volume and the process repeated. Then the pellet was dissolved in half volume 10mM Tris pH 7.5, 1mM EDTA, 0.5% SDS. An equal volume of phenol:choloroform:isoamyl alcohol (25:24:1) was added and the samples vortexed. Then the samples were centrifuged at 5000 rpm for 15 min and the upper aqueous layer transferred to the new tubes. Next 100% EtOH was added to the each sample at 2x the volume plus 10M NH4OAc at 1/10x the volume and mixed. RNA was precipitated on dry ice for 15 min and then centrifuged at 5000 rpm for 25 min. The pellet was rinsed with 70% ETOH and resuspended in 300μl diethyl pyrocarbonate-treated (DEPC) water. The amount of RNA was estimated by spectrophotometry at 260 nm and the integrity of RNA was verified by agarose gel electrophoresis with ethidium bromide staining.
cDNA synthesis and polymerase chain reaction amplification (RT-PCR)
Total RNA (3 μg) was combined with 25 pmol oligo-d(T) primer, 0.5 μl RNase inhibitor, and the volume brought to 12 μl total with DEPC water. The samples were heated for 10 min at 70°C, placed on ice for 5 min, and then quickly centrifuged. To this mixture, was added 4 μl Superscript 5X first-strand buffer, 2 μl 0.1M DTT, and 1 μl 10mM dNTP mix (Roche #1 969 064). The samples were heated at 37°C for 2 min and 1 μl of Superscript RT added. This mixture was incubated at 42°C for 1 hr and then enzymes denatured by heating for 10 min at 95°C. The RT reaction was diluted 1:10 with DEPC water for PCR reaction. Exactly half of the first strand cDNA synthesis of each sample was used for PCR amplification, using primers designed to span at least one intron (to ensure cDNA and not contaminating genomic DNA is amplified). PCR was performed in a total reaction volume of 100 μl containing oligonucleotide primers (0.2 AM), 1 × PCR buffer (10 mM Tris-HCL, 50 mM KCl, pH 8.3), 2 mM MgCl2, 0.2 m/m dNTPs, and 2.5 units of Taq DNA polymerase. The specific primers used were estrogen receptor alpha (ERα), estrogen receptor beta (ERβ) and β-actin, a ‘house-keeping’ gene used to determine the constitutive level of gene transcription and to control for variations in RNA recoveries.
Primers for ERα (cDNA 548-bp) were as follows, [sense 5′-CGCTAACCATGACCATGACCC-3′, antisense 5′-GAGTCTCCTTGACAGACTCCATG-3′], for ERβ (cDNA 565-bp) were as follows, [sense 5′-GGCATTCTACAGTCCTGCTG-3′, antisense 5′-TCTGCATAGAGAAGCGATGA-3′] and for β-actin (cDNA 321-bp), [sense 5′-CACCCACACTGTGCCCATCTA-3′, antisense 5′-CCCTGGAGAAGAGCTATGAGC-3′]. The reaction mixture was incubated in the thermal cycler for 35 cycles as follows: at 94°C for 60 s, at 56°C for 60 s and at 72°C for 60 s. The cycles were terminated on the phase during which there was exponential generation of PCR product before reaching a plateau. When the cycles were completed, the tubes were maintained at 72°C for 5 min. Negation of contaminated genomic DNA was performed by analyzing the absence of templates in control samples without reverse transcriptase. Sequence analysis was performed to confirm the identity of the amplified cDNA.
Quantification of RT-PCR products
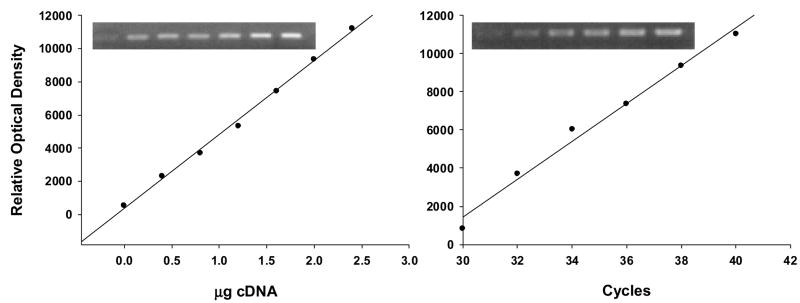
The RT-PCR method used in these assays is semi-quantitative because absolute values of mRNA levels could not be determined since internal controls for the specific mRNAs were not used. To validate this RT-PCR assay as a tool for the semi-quantitative measurement of mRNA, dose–response curves were established for different amounts of total RNA extracted from the hippocampus (Fig 1), and the samples were quantified in the curvilinear phase of PCR amplification. The expression levels were normalized against β-actin. No difference was observed in β-actin levels at any stage. 10 μl of the PCR product was analyzed on 0.8% agarose gel with ethidium bromide. RT-PCR amplification of ERα and ERβ in the hippocampus, hypothalamus, and cortex revealed PCR products of the expected 548-bp and 565-bp, respectively. The quantification of RT-PCR products was performed by densitometric analysis of photographic negatives of agarose gels using NIH Image and then the ratios of ERα and ERβ/β-actin were calculated.
Figure 1.
The figure shows an example of optimized RT-PCR conditions for ERα mRNA using control hippocampus. Note the linear and parallel increases in the optical density of ERα bands with increasing amount of complementary DNA (a) or PCR cycles (b). Optical density is expressed in arbitrary units. Images represent increasing concentration (a) or cycles (b) from left to right. The increase in the ODs of ERα was linear between 0.5 to 1.2 μg cDNA at 36 cycles (Fig. 1a). In addition, there was strong linearity for ERα mRNAs in a range between 32 and 40 cycles (Fig. 1b). A similar linearity was established for ERβ.
Statistical Analysis
For each animal and region the ratios of ERα and ERβ/β-actin were used to determine the effect of treatment on ERα and ERβ mRNA expression. The effect of treatment by region was compared using a one-way ANOVA for each ER subtype. If the results were significant (P < 0.05) then Fisher’s PLSD was used to perform post-hoc pair-wise comparisons.
Results
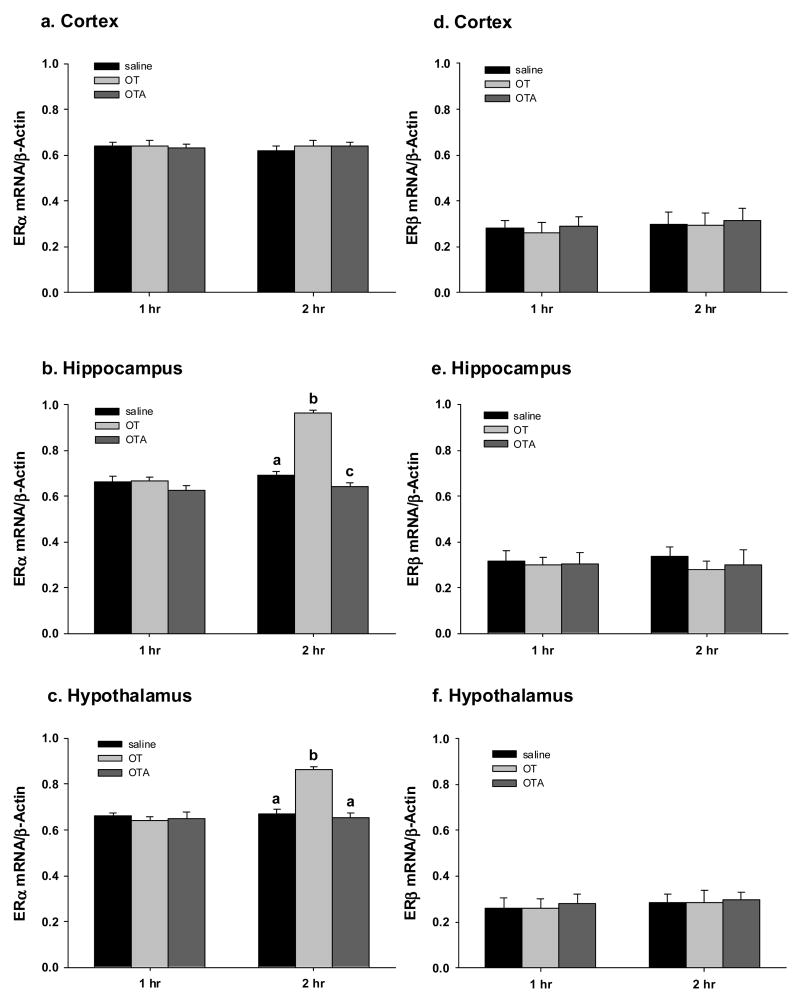
One hour after treatment there were no changes in the levels of ERα mRNA in any region of the brain (Fig 2). After 2 hours there was a significant treatment effect on the expression of ERα in the hypothalamus (one-way ANOVA: F(2,12)=42; P <.0001) and the hippocampus (one-way ANOVA: F(2,12)=116; P < 0.0001), but not the cortex. In both the hypothalamus and the hippocampus OT treatment produced a significant increased in ERα mRNA compared with both saline and OTA treated females (Fig 2). In the hippocampus OTA treatment significantly reduced ERα mRNA compared with saline treated females (Fig 2). There was no treatment on the expression of ERβ mRNA expression either 1 or 2 hr after treatment (Fig 2).
Figure 2.
Shows RT-PCR results by region, treatment, and time for ERα mRNA (a–c) and ERβ mRNA (d–f). 2 hr oxytocin (OT) treatment significantly increased ERα mRNA expression in the hypothalamus (b) and the hippocampus (c), but not the cortex (a). Additionally treatment with an oxytocin antagonist (OTA) decreased ERα mRNA expression in the hippocampus. There were no treatment effects on ERβ mRNA expression. Different letters indicate a significant difference between treatments (P < 0.05).
Discussion
Neonatal manipulation of OT alters the subsequent expression of ERα-IR in female prairie voles (Yamamoto et al., 2006), with OT increasing and OTA decreasing ERα-IR in selective nuclei within the limbic system. Results from the current study suggest that OT may increase the expression of ERα-IR by increasing ERα mRNA, with OT treatment producing an increase in ERα mRNA in the hypothalamus and hippocampus within 2 hr. The effect of neonatal OT was site/regionally specific as OT treatment did not affect ERα mRNA production in the cortex.
While the directional effect of OT, increasing, and OTA, decreasing, ERα mRNA was consistent with the effect of OT and OTA on ERα-IR reported in pups on postnatal day 21 (Yamamoto et al., 2006), there were some regional differences in the expression of these effects. In both the current study and Yamamoto et al, (2006) exogenous OT increased ERα mRNA and ERα-IR in the hypothalamus. In contrast, while inhibiting the endogenous OT decreased ERα the specific regions affected differed between the two studies. Neonatal OTA significantly reduced ERα-IR in the medial preoptic area (Yamamoto et al., 2006), while in the current study the difference between OTA and saline was not significant in the hypothalamus, but instead in the hippocampus. The lack of a significant reduction in the hypothalamus suggests several possibilities. The findings of a reduction in ERα-IR in the MPOA (Yamamoto et al., 2006) could be the result of a different mechanism. For example, in the neonatal ovary, a sight of estrogen production, OT affects apoptosis (Marzona et al., 2003). Perhaps in the MPOA inhibiting endogenous OT increases apoptosis in neurons that contain ERα. It is also possible that treatment with OTA did reduce ERα mRNA in the MPOA, but that our assay was not sensitive enough to detect the effect. The effect of inhibition of OTR could be more nuclei-specific than OT, which could mean the effects of OTA were washed out by regional analysis. Although Yamamoto et al., (2006) only reported a significant increase in ERα-IR in the ventromedial hypothalamus of OT treated females the mean number of ERα-IR cells was highest in OT treated females in all regions of the hypothalamus compared to controls thereby increasing the probability that analysis of the hypothalamus would indicate an overall increase in ERα mRNA expression. In contrast, OTA significantly decreased ERα-IR in the MPOA, but the relative effect of OTA compared to saline varied in the other regions (Yamamoto et al., 2006). Therefore it is possible that the site-specific effect could have been overwhelmed by the fact that mRNA was analyzed through out the hypothalamus.
The results of this study also suggest that the effects of OT on the expression of ERα mRNA maybe tissue specific. While OT also affected the production of ERα in MCF7 breast cancer cells the direction of the effect was the opposite of that observed in the present study. Breast cancer cells are highly mitotic with estrogen stimulating mitosis. Treatment with OT inhibited mitosis by reducing the effects of estrogen (Cassoni et al., 1997), through the reduction of ERα by inhibiting ERα mRNA expression (Cassoni et al., 2002). In contrast to cancer cells neurons are non-mitotic and therefore increasing ERα has a very different effect on the cellular processes of neurons in response to estrogen compared to breast cells, instead stimulating neuronal activation.
While this study was to designed to determine the early potential effects of oxytocin on the expression of ERα in neonates the results presented here are potentially relevant to behavioral studies examining the neonatal effects of oxytocin. A single pharmacological treatment with oxytocin or blocking the effects of endogenous oxytocin on the day of birth can have significant effects on the subsequent expression of behavior even into adulthood (for review see Carter 2003), and the results from this study suggest one possible mechanism of the effects of oxytocin during the neonatal period. Finally, the results from this study suggest that, at least within the first two hours, that the neonatal effects of oxytocin are ER subtype specific, altering the expression of ERα but not ERβ mRNA.
In conclusion the results from this study provide a mechanism through which OT during the neonatal period could have an organizational effect on ERα expression and subsequently alter estrogen dependent behavioral and physiological responses. The region specific effects of OT on ERα mRNA are consistent with findings from behavioral studies on the effects of neonatal manipulation of OT. Neonatal manipulation of OT altered female sexual and sociosexual behavior (Uvnäs-Moberg et al., 1998; Sohlström et al., 2002; Cushing et al., 2005;Kramer et al., 2006), responses that are regulated by the hypothalamus. While not yet studied the results from this study also suggest that neonatal OT could have a significant effect on memory, learning and other possible cognitive effects that are regulated by estrogen in the hippocampus.
Acknowledgments
We wish to thank Adam Perry and Dr. Kristin Krammer for their suggestions and assistance on this study and Nancy Cushing for her suggestions and comments on this manuscript. The research was funded by grants from the National Institute of Mental Health MH 01992 and National Institute of Child Health and Human Development HD 48390.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auffray C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980;107:303–14. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Permeability and vascularity of the blood-brain barrier to neuropeptides: the case for penetration. Psychoneuroendocrinology. 1985;10:385–399. doi: 10.1016/0306-4530(85)90079-4. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Prange AJ, Jr, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Inter J Oncol. 1997;72:340–344. doi: 10.1002/(sici)1097-0215(19970717)72:2<340::aid-ijc23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Catalano MG, Sapino A, Marrocco T, Fazzari A, Bussolati G, Fortunati N. Oxytocin modulates estrogen receptor alpha expression and function in MCF7 human breast cancer cells. Inter J Oncol. 2002;21:375–378. [PubMed] [Google Scholar]
- Coirini H, Johnson AE, McEwen BS. Estradiol modulation of oxytocin binding in the ventromedial hypothalamic nucleus of male and female rats. Neuroendocrinology. 1989;50:193–198. doi: 10.1159/000125221. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Levine K, Cushing NL. Neonatal manipulations of oxytocin affect reproductive behavior and reproductive success of adult female prairie voles (Microtus ochrogaster) Horm Behav. 2005;47:22–28. doi: 10.1016/j.yhbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Carter CS, Hoffman GE. Central c-Fos expression in neonatal male and female prairie voles in response to treatment with oxytocin. Dev Brain Res. 2003;143:129–136. doi: 10.1016/s0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Permeability and vascularity of the developing brain: cerebellum vs cerebral cortex. Brain Res. 1980;190:3–16. doi: 10.1016/0006-8993(80)91155-5. [DOI] [PubMed] [Google Scholar]
- Johnson AE. The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by gonadal steroids. Ann NY Acad Sci. 1992;652:357–373. doi: 10.1111/j.1749-6632.1992.tb34367.x. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS. Developmental effects of oxytocin on stress response: single versus repeated exposure. Physiol Behav. 2003;79:775–782. doi: 10.1016/s0031-9384(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Marzona L, Arletti R, Benelli A, Sena P, DePol A. Effects of estrogens and oxytocin on the development of neonatal mammalian ovary. In vivo. 2003;15:271–279. [PubMed] [Google Scholar]
- Sohlström A, Olausson H, Brismar K, Uvnäs-Moberg K. Oxytocin treatment during early life influences reproductive performance in ad libitum fed and food-restricted female rats. Biol Neonate. 2002;81:132–138. doi: 10.1159/000047198. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Alster P, Petersson M, Sohlström A, Bjorkstrand E. Postnatal oxytocin injections cause sustained weight gain and increased nociceptive thresholds in male and female rats. Ped Res. 1998;43:1–5. doi: 10.1203/00006450-199803000-00006. [DOI] [PubMed] [Google Scholar]
- Withuhn TF, Kramer KM, Cushing BS. Early exposure to oxytocin affects the age of vaginal opening and first estrus in female rats. Physiol Behav. 2003;80:135–138. doi: 10.1016/s0031-9384(03)00222-1. [DOI] [PubMed] [Google Scholar]
- Witt DM. Regulatory mechanisms of oxytocin-mediated sociosexual behavior. Ann NY Acad Sci. 1997;807:287–301. doi: 10.1111/j.1749-6632.1997.tb51927.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson P, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Carter CS, Cushing BS. Neonatal manipulation of oxytocin effects expression of estrogen receptor alpha. Neuroscience. 2006;137:157–164. doi: 10.1016/j.neuroscience.2005.08.065. [DOI] [PubMed] [Google Scholar]