Abstract
We demonstrate for the first time that a stable, micron-scale segregation of focal enrichments of sterols exists at physiological temperature in the plasma membrane of live murine and human sperm. These enrichments of sterols represent microheterogeneities within this membrane domain overlying the acrosome. Previously, we showed that cholera toxin subunit B (CTB), which binds the glycosphingolipid, GM1, localizes to this same domain in live sperm. Interestingly, the GM1 undergoes an unexplained redistribution upon cell death. We now demonstrate that GM1 is also enriched in the acrosome, an exocytotic vesicle. Transfer of lipids between this and the plasma membrane occurs at cell death, increasing GM1 in the plasma membrane without apparent release of acrosomal contents. This finding provides corroborative support for an emerging model of regulated exocytosis in which membrane communications might occur without triggering the “acrosome reaction.” Comparison of the dynamics of CTB-bound endogenous GM1 and exogenous BODIPY-GM1 in live murine sperm demonstrate that the sub-acrosomal ring functions as a specialized diffusion barrier segregating specific lipids within the sperm head plasma membrane. Our data show significant differences between endogenous lipids and exogenous lipid probes in terms of lateral diffusion. Based on these studies, we propose a hierarchical model to explain the segregation of this sterol- and GM1-enriched domain in live sperm, which is positioned to regulate sperm fertilization competence and mediate interactions with the oocyte. Moreover, our data suggest potential origins of sub-types of membrane raft microdomains enriched in sterols and/or GM1 that can be separated biochemically.
Keywords: Sperm membrane, Ganglioside GM1, Sterols, Diffusion barrier
INTRODUCTION
Vigorous debate regarding the existence of “membrane rafts” has led membrane biophysicists and cell biologists to a working definition that is consistent with current observations. Membrane rafts are now recognized as small, heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains. Debate was largely focused on artifacts that could result from use of detergents to isolate these domains, or fixatives or cross-linking agents (polyvalent probes and antibodies) used to visualize them. Several review articles have explored the “raft hypothesis” by examining what is known from biological systems and by extending observations from model membranes (Anderson and Jacobson, 2002; Kusumi and Suzuki, 2005; London, 2005; Mayor and Rao, 2004; Pike, 2006; Simons and Ikonen, 1997). However, evidence for the existence of membrane rafts in live cell membranes is still considered inconclusive by some.
One problem recognized by all is the difficulty of direct visualization of these membrane domains in live cells. Lack of visualization with standard light microscopy has led to an appreciation of the small size of these domains in cells, although larger-scale domains can be observed in artificial membrane systems. A recent study utilizing giant plasma membrane vesicles showed that micrometer-scale fluid/fluid phase separations could be resolved in complex biological membranes, although this was performed at lower than physiological temperatures (Baumgart et al., 2007). Membrane rafts likely vary in internal composition and the extent to which they comprise the membranes of different types of cells. For example, an examination of GPI-anchored proteins in live CHO cells showed that they mostly existed as monomers with only 20-40% in small clusters of at most 4 proteins (Sharma et al., 2004), whereas 10-15% of macrophage membranes was shown to exist as liquid-ordered regions through the use of the exogenous probe, laurdan (Gaus et al., 2003). An explanation for the absence of large-scale phase separations in live cells has been suggested to be due to the maintenance of membrane asymmetry, complex membrane dynamics including intracellular trafficking, and tethered protein obstacles (Jacobson et al., 2007; Kusumi and Suzuki, 2005). Therefore, complex biological membranes have been classified as unusual liquid-like structures (Jacobson et al., 2007). The nanometer-scale size of membrane rafts combined with limitations in the temporal and spatial resolution of physical tools used to study them have made rafts elusive in live cells (Munro, 2003).
Biological membranes contain an extensive diversity of lipids and proteins, and mammalian sperm are no exception. Sperm are terminally differentiated, highly polarized cells. They have a distinct flagellum and head, the latter of which contains the sperm’s one intracellular vesicle, the acrosome (Fig. 1). The existence of membrane trafficking in sperm is thought not to occur, although recent reports suggest that there might be communications between the plasma membrane and acrosomal membranes that do not result in full exocytosis of the acrosomal contents (Kim and Gerton, 2003).
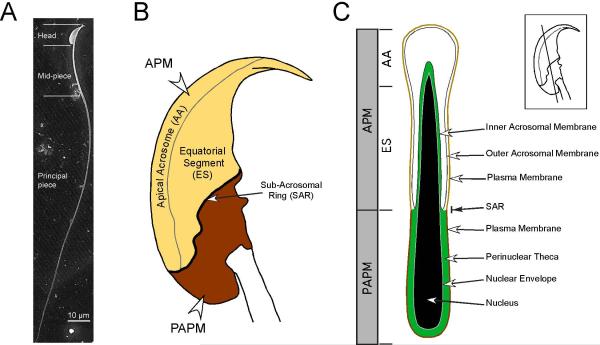
Fig. 1.
Organization of the membranes and structures of the murine sperm head. (A) Scanning electron micrograph showing the entire length of a murine sperm, including the falciform head and the flagellum. The flagellum is again divided into the midpiece, the principal piece, and a small terminal endpiece. (B) Schematic showing a lateral view of a murine sperm head. The plasma membrane of the sperm head is divided into two major regions, the APM and the PAPM, based on morphology and differences in membrane protein and lipid compositions. The APM and PAPM are delimited by a topographical feature known as the sub-acrosomal ring (SAR). On the basis of structure and function the APM is itself divided into the apical acrosome (AA), the region where membrane fusion will take place between the plasma membrane and the acrosomal vesicle, and the equatorial segment (ES). The hook-like structure is called the perforatorium. (C) Schematic showing a longitudinal section through the murine sperm head. Inset lateral view schematic of the sperm head shows the orientation of this longitudinal section. Areas corresponding to the APM (AA and ES) and PAPM are indicated. The nucleus (black) and nuclear membrane (grey) are surrounded by a cytoskeletal meshwork called the peri-nuclear theca (green). The acrosome (white) is seated over the apical region of the nucleus. The section of acrosomal membrane closest to the nucleus is the inner acrosomal membrane (IAM) and the acrosomal membrane apposing the plasma membrane is the outer acrosomal membrane (OAM). The plasma membrane is tightly wrapped around the structures of the sperm head and is closely associated with the OAM in the region forming the APM and the peri-nuclear theca in the PAPM.
Several studies have suggested that the plasma membrane overlying the acrosome (APM) is unusual in composition. We previously showed in live sperm at physiological temperatures that cholera toxin subunit B (CTB), which binds to the ganglioside GM1, localizes to the APM (Selvaraj et al., 2006). Freeze-fracture studies utilizing filipin to complex with membrane sterols in fixed cells have shown that this domain is also enriched in sterols (Friend, 1982; Lin and Kan, 1996; Pelletier and Friend, 1983; Suzuki, 1988). We have shown that several differences in CTB-bound GM1 localization occur between live and dead or weakly fixed cells, with CTB-bound GM1 redistributing to the post acrosomal plasma membrane (PAPM) in dead or weakly fixed cells. We also showed that this redistribution is associated with a disproportionate increase in CTB fluorescence which was not due to the change in fluorescent property or quenching effects of the fluorophore used (Selvaraj et al., 2006). The dramatic changes in CTB-bound GM1 labeling, the potential for binding of CTB to multiple GM1 molecules, and the fact that all studies localizing sterols to the APM domain have been performed in fixed cells combine to limit the conclusions one can draw regarding the membrane properties of the sperm head. Yet, the tremendous importance of this membrane region in fertilization make it essential that we understand the native properties of this domain in live sperm. Should it be true in live sperm, the physiological significance of sterol segregation to this domain could be linked to the functional requirement for sperm sterol efflux to occur within the female tract, rendering them fertilization competent through a process called “capacitation” (Austin, 1952; Chang, 1951; Davis, 1976; Visconti et al., 1999). Upon appropriate stimulation, the plasma membrane of capacitated sperm can fuse at the region of the apical acrosome (AA; Fig. 1B) with the underlying outer acrosomal membrane (OAM; Fig. 1C), resulting in exocytosis.
Previous studies on sperm sterol localization have utilized fixed cells (Friend, 1982; Lin and Kan, 1996; Pelletier and Friend, 1983; Suzuki, 1988), and studies on lipid diffusion have largely used synthetic non-natural exogenous lipid probes (James et al., 2004; Ladha et al., 1997; Wolf et al., 1990; Wolf and Voglmayr, 1984; Wolfe et al., 1998). Studies regarding the diffusion of different GPI-anchored and transmembrane proteins during sperm development have shown restricted lateral diffusion of these molecules across different regions in the sperm plasma membrane (Cowan et al., 1987; Cowan et al., 1997; Myles et al., 1984; Phelps et al., 1988). Although regional differences in diffusion rates exist in sperm for exogenous lipids (Wolfe et al., 1998), several studies examining the diffusion of such probes suggest that there is no restriction to lateral diffusion for lipid molecules in the sperm plasma membrane (James et al., 2004; Mackie et al., 2001). In the present study, we localize focal enrichments of endogenous membrane sterols in live sperm for the first time. We also build on our previous work on GM1, now studying the dynamics of endogenous versus exogenous GM1, and demonstrate that a dense cytoskeletal structure, the sub-acrosomal ring (SAR; Fig. 1B), functions as a specialized diffusion barrier restricting GM1 to the APM domain. Our results show that exogenous GM1 molecules introduced into the plasma membrane bilayer do not behave like their endogenous counterparts. Based on these studies, we propose a hierarchical model to explain the mechanisms behind sterol- and GM1-segregation to the APM domain in sperm. Furthermore, our localization data complement our current non-detergent based membrane fractionation experiments, suggesting the sub-cellular origins of membrane domains contributing to the various fractions (Asano et al., in revision).
MATERIALS AND METHODS
Reagents and biological materials
All reagents were purchased from Sigma (St. Louis, MO), unless otherwise noted. CTB conjugated with biotin or AlexaFluor 488 and 555 (Invitrogen, Carlsbad, CA) or conjugated with FITC was used as indicated. For testing acrosomal integrity, AlexaFluor 488 conjugated peanut agglutinin (PNA; Invitrogen) was used to label sperm. For indirect immunofluorescence, a monoclonal antibody against mouse acrosomal protein sp56 (clone 7C5; QED Bioscience Inc., San Diego, CA) was used. Secondary antibody used was AlexaFluor 488- or 555-conjugated goat anti-mouse serum (Invitrogen). An anti-biotin 10 nm gold-conjugated antibody (British BioCell Intl., Redding, CA) was used for electron microscopy. Exogenous BODIPY-GM1 (Invitrogen) was used for membrane labeling. Male CD-1 mice were purchased from Charles River Laboratories (Kingston, NY). Human semen samples were obtained with consent from patients visiting the department of urology at Weill Cornell Medical College, New York City, NY. All collections and experiments were performed with approval and oversight by the appropriate Institutional Animal Care and Use Committee or Institutional Review Board.
Media
For murine sperm, a modified Whitten’s medium (MW; 22 mM HEPES, 1.2 mM MgCl2, 100 mM NaCl, 4.7 mM KCl, 1 mM pyruvic acid, 4.8 mM lactic acid hemi-calcium salt; pH 7.35) supplemented with glucose (5.5 mM) was used (Travis et al., 2001a). For germ cell preparation from murine testes, Kreb’s ringer bicarbonate medium (KRB; 120.1 mM NaCl, 4.8 mM KCl, 25.2 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2 and 11.1 mM glucose, pH 7.35) was used (Bellve et al., 1977a; Bellve et al., 1977b). For human sperm, a modified Human Tubal Fluid medium (mHTF; 97.8 mM NaCl, 4.69 mM KCl, 0.2 mM MgSO4, 0.37 mM KH2PO4, 2.04 mM CaCl2, 4 mM NaHCO3, 21 mM HEPES, 2.78 mM glucose, 0.33 mM sodium pyruvate, 21.4 mM sodium lactate and 10 μg/ml gentamicin sulfate) was used (Quinn et al., 1985).
Sperm collection and handling
Murine sperm were collected from the cauda epididymides of male CD-1 mice by a swim-out procedure as described previously (Travis et al., 2001b). Human ejaculates were collected and allowed to liquefy at 37°C for 30 minutes and then washed three times with mHTF to remove seminal plasma. All steps of collection and washing were performed at 37°C, using large orifice pipette tips when handling sperm to minimize membrane damage. Prior to experimental treatments, motility was assessed for both mouse and human sperm.
Mixed germ cell preparation
Murine testes were decapsulated and incubated in KRB medium supplemented with 0.75 mg/ml collagenase in a shaking water bath at 33°C for 30 minutes. The resultant preparation of seminiferous tubules was then washed to remove collagenase and interstitial cells. The separated tubules were teased apart and minced using needles under a dissection microscope to yield a cell suspension. The suspension was then triturated and filtered through a 70 μm nylon mesh (BD Biosciences, Franklin Lakes, NJ), and the flow-through containing individualized cells was collected.
Localization of sterols using filipin III in fixed cells
For localization of filipin-sterol complexes in sperm membranes, sperm were fixed on coverslips for 15 minutes using 4% paraformaldehyde (PF) in PBS. The cells were then washed with PBS and incubated with filipin III (10 μg/ml) for 10 minutes. After labeling, the sperm were washed with PBS and mounted on slides using ProLong Gold Antifade (Invitrogen). Filipin-sterol complexes in sperm were visualized at 340-380 nm excitation.
Recombinant perfringolysin O
Plasmid DNA coding perfringolysin O (PFO) was provided by Markus Grebe, Swedish University of Agricultural Sciences, Umeå, Sweden. The sterol-binding domain 4 of PFO (PFO-D4), cloned together with an N-terminal EGFP (pEGFP-N1, Clontech, Mountain View, CA), was amplified using PCR and cloned into a GST-containing vector (pET41a, Novagen, Madison, WI) with an engineered HRV 3C protease cleavage site. [Primers: 5′-ATGGTGAGCAAG GGCGAGGA-3′; 5′-TTAATTGTAAGTAATACTAG-3′]. A control peptide with a truncated domain 4 (Δ429) lacking the sterol-binding region (PFO-D4-T) was generated by introducing a stop codon using site directed mutagenesis in the GST-EGFP-D4 construct. [Primers: 5′-ATAAGAATAAAAGCAAGATAgTGTACAGGCCTTGCTTGGGAATGG-3′; 5′-CCAT TCCCAAGCAAGGCCTGTACAcTATCTTGCTTTTATTCTTAT-5′]. Protein expression was induced using 4 mM IPTG in BL21(DE3)pLysS bacteria (Novagen). Cells were pelleted and treated with lysis buffer (50 mM Tris, 120 mM NaCl, 50 mM EDTA, 1% v/v Triton X-100, 3 mg/ml lysozyme) with Complete protease inhibitors (Roche, Switzerland) and soluble proteins were collected by centrifugation. Recombinant PFO-D4 and PFO-D4-T were purified from the soluble lysate using GSTrap FF columns (GE Healthcare, Piscataway, NJ) followed by in-column digestion using a 3C protease (PreScission, GE Healthcare). Column binding, washing and elution steps were carried out using a fully automated liquid chromatography system (ÄKTA Design, GE Healthcare).
Localization of sterols and GM1 in live cells
For visualizing focal enrichments of membrane sterols in live sperm, cells were incubated with PFO-D4 (4 μg/ml) in MW medium for 20 minutes at 37°C; a control using PFO-D4-T (4 μg/ml) was performed concurrently. Similarly, for visualizing GM1, cells were incubated with CTB (AlexaFluor 488; 5 μg/ml) for 10 minutes. For experiments assessing acrosomal status, PNA (5 μg/ml) was used after incubating with CTB as above. For induction of sterol efflux, sperm were incubated in MW medium supplemented with 3 mM 2-hydroxypropyl-β-cyclodextrin (2-OHCD) for 30 minutes. For all the above conditions, samples were not washed, but viewed with the respective reagents in the final medium to avoid damage to membranes.
Localization of GM1 and sp56 in developing male germ cells
For labeling GM1 in developing male germ cells, the cells were spread on coverslips and incubated in KRB in a humidity chamber at 37°C for 10 minutes to allow attachment. The cells were then fixed using 4% PF for 10 minutes, permeabilized using 0.1% Triton X-100 for 1 minute, washed and air-dried. They were then rehydrated with phosphate buffered saline (PBS) and incubated with CTB (5 μg/ml) for 10 minutes, and washed again using PBS. For dual labeling experiments, these cells were first blocked for 30 min in PBS with 1% bovine serum albumin, and then incubated with anti-sp56 (1:50) for 1 hr. The cells were then washed using PBS, incubated with the secondary antibody (1:500) for 30 min, and washed again. In dual labeling experiments, the cells were incubated with CTB (AlexaFluor 488 or 555) as a final step. Coverslips were mounted using a GVA mountant (Invitrogen). A control for non-specific binding of the secondary antibody was performed.
Saturation experiment using labeled CTB
To test whether there was exposure of additional GM1 during or after redistribution from the APM to the PAPM, we performed experiments in which GM1 was saturated in murine sperm before and after weak fixation. As a control to demonstrate our ability to saturate all surface-accessible GM1, live sperm were incubated with CTB conjugated with FITC (250 μg/ml) for 1 minute, while simultaneously allowing attachment to coverslips. This was followed by fixation using 0.004% PF and then a final addition of AlexaFluor 555-conjugated CTB (5 μg/ml). If successful, saturation with a 50-fold higher concentration of FITC-conjugated CTB should prevent AlexaFluor 555-conjugated CTB from binding. To investigate if there was exposure of additional GM1 upon redistribution, live sperm were incubated with CTB conjugated with FITC (250 μg/ml) for 1 minute while simultaneously allowing attachment to coverslips, followed by concurrent addition of AlexaFluor 555-conjugated CTB (5 μg/ml) and 0.004% PF. In this experiment, if new GM1 became exposed on the surface, then AlexaFluor 555-conjugated CTB would compete for any new binding sites as they appeared during redistribution. The concentration of FITC-conjugated CTB was selected empirically so that all surface-accessible GM1 was saturated in both live and fixed sperm.
Sequential labeling after saponin permeabilization
This experiment was performed to test whether there was exposure of additional GM1 in the region of the APM after permeabilization of sperm. Mild permeabilization using saponin has been utilized extensively in the past to introduce proteins into cells and to visualize labeling of intracellular organelles in several cell types (Franek et al., 1994; Koch et al., 1987). For this experiment, sperm were allowed to attach on two cover slips and fixed using 4% PF and 0.1% glutaraldehyde in 2.5 mM CaCl2 in PBS for 10 minutes; one of the coverslips was then subjected to permeabilization using 0.1% saponin in PBS. Sperm on both coverslips were then labeled using AlexaFluor 488-CTB (5 μg/ml final concentration) for 10 minutes, washed using PBS and mounted. Images were captured from both permeabilized and unpermeabilized cells using a constant exposure time. Absolute mean pixel intensity values in the APM were computed from the images by mapping the APM domain and subtracting the mean background intensity using Openlab 3.1 software (Improvision, Lexington, MA). Mean intensity values were plotted and statistically compared using the students t test in Kaleidagraph (Synergy Software, Reading, PA).
Annexin V labeling in live and dead cells
Labeling was carried out by adding AlexaFluor 488-conjugated annexin V (1:5 according to manufacturers instructions; Invitrogen) to live cells in suspension in MW medium at 37°C. After incubation for 20 minutes at 37°C, live and dead sperm were visualized from the same preparation and images recorded as described above.
Fluorescence microscopy and image collection
For localization experiments in live sperm, a stage-mounted incubation chamber (LiveCell, Neue Product Group, Westminister, MD) was used along with an objective heater (Bioptechs, Butler, PA). Both were maintained at 37°C and cells were viewed with a Nikon Eclipse TE 2000-U microscope (Nikon, Melville, NY) equipped with a Photometrics Coolsnap HQ CCD camera (Roper Scientific, Ottobrunn, Germany), and Openlab 3.1 (Improvision) automation and imaging software. For testing co-localization in dual-labeled germ cells, images were acquired using an Olympus IX70 laser-scanning confocal microscope (Olympus, Melville, NY) and merged. Image deconvolution and 3D rendering were performed using Vector 3 (Improvision). Serial z image stacks from spermatids labeled with CTB and anti-sp56 were acquired using the Nikon Eclipse TE 2000-U epifluorescence microscope with a programmed automation in Openlab at z=0.2 μm for 3 channels, AlexaFluor 488, AlexaFluor 555 and Hoechst 33258 respectively. Images were calibrated and transferred as a series to Vector 3 and deconvoluted using an iterative algorithm with a 98% confidence limit. Reconstructed image compilations were made into QuickTime animations showing rotations on several axes as noted.
Labeling with exogenous BODIPY-GM1
Labeling was carried out by adding BODIPY-GM1 (3 μM final concentration in methanol 0.1% v/v) to live cells in suspension in MW medium at 37°C. After probe dispersion in the medium and incubation for 5 minutes, sperm were visualized by exciting the BODIPY conjugate at 488 nm.
Lateral diffusion of CTB-bound GM1 and BODIPY-GM1 in live cells
Photo-bleaching experiments measuring the mobility of CTB-bound GM1 and BODIPY-GM1 on the sperm plasma membrane were performed using a programmed module of the Zeiss software on a LSM 510 Meta confocal microscope (Zeiss, Germany). An optical slice thickness of 8 μm was used for the imaging and bleaches (thickness of the sperm head ∼1 μm). Fluorescence loss in photo-bleaching (FLIP) (van Drogen and Peter, 2004) experiments were carried out selecting a region (∼1 μm diameter) at different points within the APM and PAPM. For both CTB-bound GM1 and BODIPY-GM1, the selected regions were subjected to a series of 3-iteration (∼20 × 5 ms/iteration) bleach scans with a 5-second recovery after each iteration. For CTB-bound GM1, frames were captured after each series of bleach iterations to examine the fluorescence over the entire sperm head. Controls were performed to test: (a) whether bleaching a region over the PAPM would affect fluorescence over the APM and (b) whether capturing frames after each bleach series would produce a bleach effect of the entire sperm head at the imaging fluorescence intensity. For BODIPY-GM1, frames were captured before and after all the bleach iterations to examine the fluorescence over the entire sperm head. This modification was used to minimize fluorescence quenching effects observed for BODIPY. For both experiments, absolute line intensity profiles were generated utilizing the Zeiss software and graphed.
Scanning electron microscopy
Samples were prepared by first allowing sperm to attach to silicon-chips for 20 minutes. The attached cells were fixed either weakly using 0.004% PF or strongly using 4% PF and 0.1% glutaraldehyde in 2.5 mM CaCl2 in PBS (Selvaraj et al., 2006). These conditions had previously been found to induce or prevent CTB-bound GM1 redistribution, respectively. Cells were then incubated with 25 mM glycine for 15 minutes to quench free aldehyde groups. GM1 was then labeled using biotinylated-CTB (5 μg/ml final concentration in PBS) for 10 minutes. After washing with PBS, cells were incubated in blocking solution (0.2% BSA and 0.2% fish gelatin in PBS) for 15 minutes. The chips were then inverted over drops of 10 nm gold-conjugated anti-biotin antibody (1:8 in blocking solution) and incubated for 1 hour. Chips were then washed 4 times in PBS and once with phosphate buffer and fixed with freshly diluted 2.5% glutaraldehyde in phosphate buffer for 15 minutes. Cells were subsequently washed 5 times with distilled water and snap frozen in liquid nitrogen and freeze dried. Finally, samples were sputter coated with carbon and correlated secondary electron and backscatter images were collected using a Zeiss LEO 1550 field emission SEM with a Gemini column (Carl Zeiss Inc., Oberkochen, Germany).
Transmission electron microscopy
Sperm samples were prepared by fixing sperm in suspension with 2% gluteraldehyde and 4% tannic acid in phosphate buffer (pH 7.0 - 7.5) for 4 to 6 hours at room temperature. Samples were then washed overnight in 0.1 M sodium cacodylate buffer (pH 7.4) with 7% sucrose at room temperature. They were then fixed for 2 hours with 1% OsO4 with 5% sucrose in 0.1 M sodium cacodylate at 4°C. They were then treated with 0.5% uranyl acetate containing 4% sucrose for 1 hour at room temperature. Samples were then quickly dehydrated and embedded in epoxy resin. Thin sections were collected on Formvar-carbon-coated nickel grids and stained with 5% aqueous uranyl acetate followed by alkaline lead. Images were then collected using a transmission electron microscope (Philips Tecnai 12 BioTwin, FEI Company, Hillsboro, OR).
RESULTS
Sterol enrichment in a micron-scale plasma membrane domain in live cells
Previous localizations of sterols in sperm invariably used fixed cells, leaving these findings to be subject to artifacts that can be induced by such treatments. We therefore sought to localize focal enrichments of sterols in live sperm through the use of a recombinant EGFP-conjugated domain 4 of perfringolysin O (PFO-D4; Fig. 2A). This reagent has been demonstrated to bind membrane sterols specifically, but it requires a high local mole percent of sterols to do so (Ohno-Iwashita et al., 2004; Waheed et al., 2001). PFO-D4 localized exclusively to the APM in live swimming sperm indicating the segregation of focal enrichments of sterols to this region (Fig. 2B). PFO-D4 was not detrimental to sperm viability and did not have any effect on motility for the incubation conditions we used. We are careful to use the broad term “sterols” because mature sperm have both cholesterol and desmosterol (Bleau and VandenHeuvel, 1974), and PFO is capable of binding both (Nelson et al., 2008). Although PFO-D4 labeled the entire APM, fluorescence appeared as closely apposed punctate spots rather than a diffuse localization. This is in contrast to the relatively uniform distribution of filipin-sterol complexes in this region [Fig. 2E; (Visconti et al., 1999)].
Fig. 2.
Localization of sterols in murine and human sperm. (A) Schematic showing the recombinant PFO peptides used for localization studies. Constructs were made producing the sterol-binding D4 domain of PFO fused with EGFP (PFO-D4; amino acids 363-472). A non-functional control construct was also made in which the sterol-binding region of the D4 domain was truncated (PFO-D4-T; Δ429). (B) Live murine epididymal sperm labeled with PFO-D4. Panels show an epifluorescence and corresponding DIC image of a live swimming sperm (note the movement of the tail) labeled using PFO-D4. There was clear segregation of the PFO-D4 fluorescence to the APM. There was no change in localization in dead cells. (C) Live murine sperm labeled with PFO-D4-T. Panels show an epifluorescence and corresponding DIC image of a live swimming sperm (again, note the movement of the tail) labeled using PFO-D4-T. There was no binding of this EGFP-conjugated non-functional truncated mutant protein confirming the binding specificity of PFO-D4. (D) Live human ejaculated sperm labeled with PFO-D4. Panels show an epifluorescence and corresponding DIC image of a live swimming sperm. Segregation of the PFO-D4 fluorescence to the APM was conserved in human sperm. (E) Fluorescence localization of filipin-sterol complexes in a fixed murine sperm. Panels show an epifluorescence and corresponding DIC image; there is sterol-enrichment in the APM domain of sperm in agreement with the observation in live cells. All panels show representative data obtained from at least 3 separate trials, with multiple cells observed per trial.
We found that the time taken for PFO-D4 labeling differed between individual cells, but that the pattern of onset of labeling was consistent within cells, with the AA usually being labeled first followed by the membrane in the vicinity of the sub-acrosomal ring (SAR), and then the equatorial segment (ES). Some sperm were identified in each experiment that had PFO-D4 signal restricted to these different stages of labeling. The PAPM and flagellum were not labeled with PFO-D4 in live sperm. A control protein was generated that was predicted to be non-functional by means of removal of the sterol-binding sequences of amino acids. EGFP-conjugated domain 4 of perfringolysin O with a truncation (PFO-D4-T; Fig. 2A), failed to bind the APM showing the specificity of binding in these cells (Fig. 2C). Similarly, in live human sperm, PFO-D4 localized to the APM (Fig. 2D). The restriction of PFO-D4 signal to the APM is in agreement with the segregation of filipin-sterol complexes in fixed murine sperm between the APM and PAPM (Fig. 2E). The connecting piece was not labeled by PFO-D4 in live murine sperm, suggesting that the filipin signal in that region in fixed cells might represent internal membranes, or that the sterols were not organized into focal enrichments, or that the reagent was unable to bind because of a steric hindrance issue due to the size of the PFO-D4 reagent. The localization of PFO-D4 seen in live cells did not change with cell death or fixation (data not shown).
Differences in GM1 labeling using CTB
Using fluorescence localization, we previously showed that CTB-bound GM1 labels the APM in live cells; however, at the time of cell death, the fluorescence in the APM dramatically redistributed to the PAPM within 10 to 100 seconds (Selvaraj et al., 2006). One reproducible observation during this redistribution was the enhancement of total CTB fluorescence in the sperm head. This relative difference in fluorescence intensity between a live and a dead cell is shown (Fig. 3A), and has previously been quantified (Selvaraj et al., 2006). Cells that underwent sterol efflux induced by 2-OHCD, which is a stimulus for in vitro capacitation (Travis et al., 2004), showed a diffuse pattern of CTB fluorescence in both the APM and PAPM after cell death [Fig. 3B; (Selvaraj et al., 2007)]. These effects of sterol efflux on CTB-bound GM1 have been previously described, and appear to vary between sperm (Selvaraj et al., 2007). Differences among sperm within a single sample are of physiological relevance because they indicate which sperm among a heterogeneous population have responded to sterol efflux up to that time (Selvaraj et al., 2007). We also reported that the use of a strong fixative (4% PF with 0.1% glutaraldehyde in PBS with 2.5 mM CaCl2) could prevent the redistribution of CTB. In this manuscript, we examined CTB-bound GM1 distribution using backscatter SEM with 10 nm gold particles to verify the localization seen with fluorescence images, to observe the distribution in much greater detail, and qualitatively to detect potential differences in abundance of GM1 without the use of fluorophores, whose properties can change depending on alterations in local environments. Backscatter SEM imaging after the strong fixation showed CTB-gold particles segregated in the APM (Fig. 3C). This finding confirms what we had observed in live cells and strongly fixed cells when visualized with CTB or antibodies against GM1 (Selvaraj et al., 2006), unlike previous reports from groups using dead or weakly fixed cells. Cells labeled first before weak fixation using 0.004% PF resulted in a PAPM distribution (Fig. 3D). Consistent with the observation using fluorophore-conjugated CTB, we saw an enrichment of CTB-gold particles at the level of the SAR and the PAPM. Although not strictly quantitative, there was an apparent increase in the number of CTB-gold particles after redistribution in the PAPM (compare Fig. 3C and 3D). A clear enrichment of CTB-gold particles was seen at the SAR in cells under similar treatments that showed an incomplete redistribution, with CTB-gold particles in both the APM and PAPM (Fig. 3E).
Fig. 3.
Differences seen in GM1 localization using CTB in live, dead, strongly fixed and unfixed cells. (A) Panels show an epifluorescence and corresponding DIC image of a live (left) and dead (right) unfixed murine sperm labeled with CTB. Fluorescence is in the APM in live cells and in the PAPM in dead cells. Note the higher intensity of fluorescence in the dead cell compared to the live cell. (B) Panels show epifluorescence and corresponding DIC image of a dead unfixed murine sperm labeled with CTB after efflux of sterols using 2-OHCD. CTB labeled the entire sperm head with a diffuse localization pattern. (C) Panels show backscatter SEM images of a murine sperm head labeled using CTB after strong fixation. For C, D, and E, the boxed regions in the miniatures orient the region shown in the high magnification, secondary electron and backscatter images. In these panels, white arrowheads indicate the SAR. CTB-gold particles were segregated to the APM in this cell similar to that seen in live cells. (D) Panels show SEM images of an unfixed murine sperm head labeled using CTB. CTB-gold particles were enriched in the PAPM and the AA. Note the apparent increased intensity of labeling in this cell after redistribution. (E) Panels show SEM images of an unfixed murine sperm head labeled using CTB. This particular cell shows a partial redistribution. Note the enrichment of CTB-gold at the SAR and along the edge of the AA. Backscatter SEM experiments for each group were carried out 4 times, with at least 30 cells visualized for each replicate.
Redistribution caused an increase in GM1 on the plasma membrane
To address the increase in total CTB fluorescence as a result of redistribution upon cell death, we investigated whether intra-cellular compartments were contributing to an increase in surface GM1. For these experiments, we employed a general strategy of saturating all surface-accessible GM1 with FITC-conjugated CTB (250 μg/ml), and then probing with AlexaFluor 555-conjugated CTB (5 μg/ml) to look for any newly exposed binding sites. First, to verify that saturation could be attained, we incubated live sperm with the FITC-CTB, and then probed with AlexaFluor 555-CTB; no binding by the latter conjugate was seen (data not shown). To confirm that saturation could be attained after redistribution to the PAPM, we incubated live sperm with the FITC-CTB, and then induced redistribution with 0.004% PF, still in the presence of the FITC conjugate. We have shown previously that this concentration of PF does not interfere with CTB binding and that it induces redistribution (Selvaraj et al., 2006). In this way, all surface-accessible GM1 should be bound by FITC-CTB before, during and after redistribution. We then added AlexaFluor 555-conjugated CTB to see if any unbound GM1 remained. No new binding was detected, confirming saturation (Fig. 4A). Next, we repeated the first incubation of live sperm with FITC-CTB. Then we added the AlexaFluor 555-conjugated CTB simultaneously with the 0.004% PF. If new GM1 were exposed on the surface during redistribution, there should be a competition between the FITC-CTB and AlexaFluor 555-CTB for these new sites. Assuming they have equal binding efficiencies and based on relative concentrations, only approximately 2% of newly exposed GM1 should be bound by the AlexaFluor 555-CTB. For technical reasons related to risk of membrane damage and sperm death during washing, we chose this approach over first washing out the unbound FITC-CTB. Nonetheless, when we performed this experiment, a percentage of the sperm corresponding roughly with the initial percentage of motile sperm showed AlexaFluor 555-CTB fluorescence, primarily on the borders of the APM and in the PAPM (Fig. 4B). Another sub-population of sperm showed only FITC-CTB signal. These sperm were probably not viable during the initial incubation and served as an efficient internal control, showing that the initial surface saturation with the FITC-conjugate was thorough.
Fig. 4.
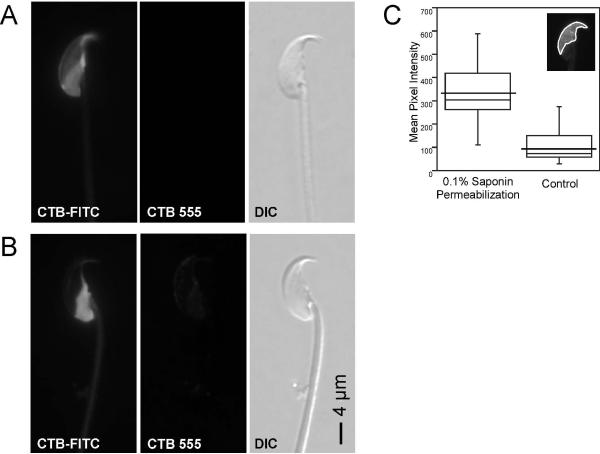
Plasma membrane GM1 dynamics in sperm. We saturated all surface-accessible GM1 using 250 μg/ml FITC-conjugated CTB, and then added 5 μg/ml AlexaFluor 555-conjugated CTB to see if there was exposure of additional GM1 during CTB-induced redistribution to the PAPM. (A) Live sperm were incubated with FITC-CTB, fixed using 0.004% PF to induce redistribution, and then AlexaFluor 555-CTB was added. All sperm had an intense FITC-CTB fluorescence and no detectable AlexaFluor 555-CTB fluorescence, showing that FITC-CTB completely saturated GM1 in murine sperm. (B) Sperm were incubated with FITC-CTB, then simultaneously treated with AlexaFluor 555-CTB and fixed using 0.004% PF to induce redistribution. Some sperm had intense FITC-CTB fluorescence and no detectable AlexaFluor 555-CTB fluorescence [similar to (A); not shown] and others showed AlexaFluor 555-CTB labeling over the APM and PAPM (shown). This finding suggested that additional molecules of GM1 appeared in the plasma membrane during redistribution to the PAPM. The percentages of cells showing the two patterns corresponded with the percentage of dead and live cells, respectively. (C) Fluorescence intensity measurement over the region of the APM with and without saponin permeabilization in fixed cells. Box-whisker plots show mean pixel intensities measured in the region of the APM (inset; region of quantification outlined) in saponin permeabilized and control, unpermeabilized cells. The lower and upper ends of the box mark the 25th and 75th quantiles; the median is represented as a horizontal line within the box, and the mean as a horizontal line through the box. Vertical whiskers extend from the ends of the box to the 10th and 90th quantiles. A student’s t test showed significant differences between the permeabilized and control cells (P<0.0001). This finding provides evidence for an acrosomal pool of GM1 accessible to CTB after saponin permeabilization.
GM1 enrichment in the acrosomal membrane in spermatids and sperm
In light of our previous work (Selvaraj et al., 2006) and the above findings that suggested that redistribution of CTB also involved an increase in GM1 on the sperm surface, we investigated whether an internal membranous compartment might represent a source of additional GM1. Because of its location immediately underneath the APM, and because it is the only intracellular vesicle in the sperm head, we hypothesized that the acrosome was the likely intracellular pool of GM1. We addressed this possibility using two different approaches. First, we labeled sperm after strong fixation with or without saponin permeabilization and compared mean fluorescence intensity in the APM. Our results showed that there was significantly higher mean fluorescence intensity over the APM in permeabilized compared to nonpermeabilized sperm (Fig. 4C; p<0.0001). Note also that saponin did not reveal additional fluorescence in the PAPM, showing that the additional labeling was not originating from some source in that region of the sperm. These data suggested that GM1 was present in the acrosomal vesicle underlying the APM. In mature sperm the APM and OAM are in extremely close apposition, and differential localization to one membrane or the other cannot be distinguished at the level of light or even transmission electron microscopy. Therefore, in the second approach we examined round spermatids for the presence of GM1 in the developing acrosome. In these cells one can more easily distinguish between the acrosomal and plasma membranes because they are separated by cytoplasm. From this experiment, we found that GM1 was strongly enriched in the developing acrosome in round spermatids. In cap phase round spermatids, the GM1 approximately co-localized with the acrosomal matrix component sp56 in the developing acrosome (Fig. 5A). Deconvolution and reconstruction of serial sections taken at higher magnification revealed that the GM1 appeared to surround the acrosomal matrix (Fig. 5B and supplemental movie). These findings showed that GM1 was enriched in the acrosomal membranes of round spermatids and strongly suggested that GM1 could be present in the acrosomal membrane of mature sperm. A more detailed depiction of the localization of GM1 in the acrosome during spermatogenesis can be found in our accompanying manuscript (Asano et al., in revision).
Fig. 5.
Localization of GM1 and sp56 in murine male germ cells. (A) Panels show confocal images of CTB (green) and sp56 (red) localization in round spermatids connected by intercellular bridges. Merged image (yellow) from the two channels and the corresponding Nomarski DIC image are also shown. CTB approximately co-localized with the acrosomal matrix protein sp56 during development of the acrosome. (B) 3D-rendered image compilation of GM1 and sp56 localization in a murine round spermatid. Serial z stacks were deconvoluted and reconstructed in 3 dimensions as described. Each row represents frames from the supplemental movie file and show rotation along the vertical axis. CTB (green) and sp56 (red) are seen localized to regions of the developing acrosomal vesicle. The nucleus was stained using Hoechst (blue). These frames (and also the Supplemental Material movie) show that GM1 largely enveloped the sp56 fluorescence of the acrosomal matrix. This suggested that GM1 was associated with the acrosomal membranes and was not localized within the acrosomal matrix. (C) Correlated secondary electron and backscatter SEMs of an acrosome-disrupted murine sperm head labeled with CTB-gold. (I) Shows the entire sperm head. The boxed area indicates the region of plasma membrane and acrosomal disruption. (II) Shows a higher magnification image of the disrupted region. Black arrows indicate the SAR and white arrows point to the fringes of the plasma membrane. (III) Backscatter image showing the localization of CTB using 10 nm gold particles. Gold labeling was seen within the region of the disrupted acrosome visible between the ragged edges of the broken plasma membrane. These observations were consistent with CTB localization in the acrosomal membranes in mature sperm as well as during male germ cell development.
Although strongly suggestive, these results did not rule out that the localization of GM1 changed during epididymal maturation. Therefore, we designed a strategy to first gently disrupt the plasma membrane and the acrosomal vesicle of the sperm head, label them using CTB and perform backscatter SEM imaging for associated gold particles. After gentle disruption of membranes, we found that CTB labeling was seen in regions corresponding to acrosomal membranes in mature sperm (Fig. 5C). These findings suggested that redistribution involved interactions between the APM and the GM1-rich acrosomal membranes, which resulted in the observed increase in fluorescence intensity.
Redistribution did not result in, nor was caused by, the loss of acrosomal integrity
Experimental evidence clearly suggested that the acrosome could be acting as an intracellular pool of GM1, some of which moved to the plasma membrane at cell death. In sperm, any communication that might occur between the plasma membrane and the acrosomal membranes could mimic or induce the process of acrosomal exocytosis. Under normal physiological conditions, this exocytotic reaction in murine sperm results in the vesiculation and loss of the fused plasma membrane and OAM as hybrid vesicles. These fusions take place in the AA, and ultimately result in release of acrosomal matrix components and exposure of the inner acrosomal membrane (IAM) (Yanagimachi, 1994). To examine whether there was exposure of acrosomal membranes after the redistribution of CTB we labeled sperm with peanut agglutinin (PNA), which binds to glycoconjugates associated with the acrosomal membranes of murine sperm (Tao et al., 1993). We found that similar to live intact sperm (Fig. 6B), PNA did not label sperm after CTB redistribution (Fig. 6A). PNA patterns seen after partial spontaneous acrosomal exocytosis (Fig. 6C) and in an acrosome-damaged cell (Fig. 6D) are shown from the same experiment, and served as internal controls. These findings suggest that despite the communication between the acrosomal membranes and the plasma membrane, there was not a full exocytosis, nor focal exposure of the glycoconjugates.
Fig. 6.
Redistribution of CTB-bound GM1 was not correlated with full acrosomal exocytosis. Dual labeling experiments using CTB (AlexaFluor 555-conjugate) and PNA (AlexaFluor 488-conjugate) were performed in unfixed cells. (A) Two dead cells showing CTB redistributed to the PAPM region and no PNA labeling indicating intact acrosomal vesicles. (B) A control live cell with an intact acrosomal vesicle. CTB is in the APM and there is no PNA labeling. (C) A dead sperm cell with acrosomal membranes exposed in the region of the AA had a diffuse CTB labeling with increased intensity in the AA and SAR/PAPM. PNA labeled the AA, revealing a partial acrosomal exocytosis. (D) A damaged cell in which parts of the APM and the OAM were lost, had a loss of CTB labeling and exposure of the acrosomal membranes in the ES. All panels show representative and consistent data obtained from at least 4 separate trials, with multiple cells (>100) observed per trial.
Annexin V localization in live and dead cells
To understand further the nature of the sperm membranes and their changes upon cell death, we investigated potential loss of membrane asymmetry and exposure of phosphatidyl serine (PS) by using annexin V to label PS on the outer leaflet. We did not detect annexin V labeling in live sperm (Fig. 7A). In dead cells, there was a regional loss of membrane asymmetry primarily in the PAPM indicated by annexin V labeling in this region (Fig. 7B). This regional loss of membrane asymmetry might explain or contribute to some of the membrane property changes and lipid redistributions seen upon sperm death, and will be discussed further below.
Fig. 7.
Annexin V labeling in live and dead sperm. Panels show epifluorescence and corresponding DIC images. (A) Annexin V labeling was not seen in live cells. (B) Annexin V labeling was seen only in the PAPM in dead cells. This finding showed that there was a regional loss of membrane asymmetry upon cell death that selectively excluded the APM domain of the sperm head. Several hundred cells were examined over three trials with uniformly consistent results (representative images shown).
CTB-bound endogenous GM1 was mobile in live cells but its movement was restricted to the APM
Fluorescence loss in photobleaching (FLIP) experiments on the APM in live cells established that CTB-bound GM1 was mobile within the APM domain except for a small region over the perforatorium (Fig. 8A). As a control, an area of the same dimension was bleached in the PAPM. The failure of bleaching this region to have any impact on fluorescence in the APM showed that GM1 in the APM was completely segregated from the PAPM in live cells (Fig. 8B).
Fig. 8.
Lateral diffusion of CTB bound to GM1 in live cells. (A) Fluorescence loss in photobleaching (FLIP) on the APM domain in CTB-labeled live sperm. Multiple cells were examined in 4 independent experiments and representative data are shown. (I) Shows the CTB-labeled APM domain of a live sperm; the red circle on the APM and SAR indicates the region of bleach in this experiment and the red arrow shows the location and direction used for generating the line intensity profile. (II) Panels 1, 2 and 3 represent frames captured after each bleach series of the same sperm. Note that there was still residual signal over the perforatorium (white arrowhead) suggesting that CTB-bound GM1 in this region had reduced mobility compared to the AA and ES. (III) Line intensity traces from frames 1, 2 and 3 showing loss of fluorescence intensity over the entire APM (AA and ES) as a result of repeated bleaching of the region indicated. These data demonstrate that CTB-bound GM1 is mobile within the APM domain. (B) Control for FLIP on the APM domain in CTB-labeled live cells. Multiple cells were examined in 4 independent experiments and representative data are shown. (I) Shows the CTB-labeled APM of a live sperm; the red circle on the PAPM indicates the region of bleach in this experiment and the red arrow shows the location and direction used for generating the line intensity profile. (II) Panels 1, 2 and 3 represent frames captured after each bleach series of the same sperm. (III) Line intensity traces from frames 1, 2 and 3 showed no loss of fluorescence intensity over the entire APM domain (AA and ES) as a result of repeated bleaching of the region indicated on the PAPM. These data demonstrated that CTB-bound GM1 in the APM domain was stably segregated from the PAPM, and that capturing frames did not produce a bleaching effect in these experiments.
Exogenous BODIPY-GM1 did not share characteristics of endogenous GM1
To examine the insertion and behavior of exogenous GM1 molecules in the plasma membrane of live sperm, we used a BODIPY-conjugated fluorescent GM1. In live sperm, BODIPY-GM1 did not show a preferential insertion into the APM domain. Rather, it was inserted throughout the sperm plasma membrane showing a diffuse labeling pattern across the APM, PAPM and flagellum alike in live cells (Fig. 9A). FLIP experiments using BODIPY-GM1 showed that exogenous GM1 molecules were not segregated in the sperm head and traversed the SAR in both directions (From the APM to the PAPM and vice versa; Fig. 9B and C). However, signal remained in the flagellum.
Fig. 9.
Membrane insertion and lateral diffusion of exogenous BODIPY-GM1 in live sperm. (A) Fluorescence localization of BODIPY-GM1 in the sperm plasma membrane. BODIPY-GM1 inserted throughout the sperm plasma membrane showing a diffuse labeling pattern across the APM, PAPM and flagellum alike in live cells. (B) FLIP on the APM domain in BODIPY-GM1 labeled live sperm. Multiple cells were examined in 3 independent experiments and representative data are shown. (I) Shows a BODIPY-GM1 labeled live sperm; the white circle on the APM indicates the region of bleach in this experiment and the white arrow over the PAPM shows the location and direction used for generating the line intensity profile. (II) Panels 1 and 2 represent frames captured before and after the bleach of the same sperm. (III) Line intensity traces from frames 1 and 2 show loss of fluorescence intensity over the entire sperm head including the PAPM. (C) FLIP on the PAPM in BODIPY-GM1 labeled live sperm. Multiple cells were examined in 3 independent experiments and representative data are shown. (I) Shows a BODIPY-GM1 labeled live sperm; the white circle on the PAPM indicates the region of bleach in this experiment and the white arrow over the APM shows the location and direction used for generating the line intensity profile. (II) Panels 1 and 2 represent frames captured before and after the bleach of the same sperm. (III) Line intensity traces from frames 1 and 2 show loss of fluorescence intensity over the entire sperm head including the APM. Taken together, data from (B) and (C) show that exogenous BODIPY-GM1 molecules were capable of diffusion across the SAR in both directions.
A dense cytoskeletal meshwork underlies the SAR
Transmission electron micrographs showing the region of the SAR revealed the presence of a localized electron-dense meshwork, which based on previous experiments (Selvaraj et al., 2006), would be consistent with underlying proteinaceous elements that could constitute a “fence” or boundary between the APM and PAPM (Fig. 10). The SAR has been noted previously as a topographically distinct structure on SEM of both freeze-dried unfixed sperm, as well as demembranated fixed sperm (Selvaraj et al., 2006).
Fig. 10.
Transmission electron micrographs showing the SAR. Panel shows low and high magnification electron micrographs of an oblique cross section through the murine sperm head showing the nucleus (N), acrosomal vesicle (A) and the different regions of the plasma membrane [plasma membrane overlying the acrosome, APM domain; post-acrosomal plasma membrane, PAPM; sub-acrosomal ring, SAR]. Note the electron-dense material underlying the plasma membrane at the SAR.
DISCUSSION
Existence of a micron-scale membrane domain enriched in sterols in live sperm
Several laboratories including ours have previously demonstrated that in fixed sperm there is an enrichment of sterols in the APM. This property appears to be conserved among mammals (Friend, 1982; Lin and Kan, 1996; Pelletier and Friend, 1983; Selvaraj et al., 2006; Suzuki, 1988). Standing in contrast to these reports on sterols and our description of the segregation of GM1 in live sperm (Selvaraj et al., 2006), are studies that have suggested that there is no barrier to lateral diffusion of lipids in live sperm (James et al., 2004; Mackie et al., 2001). In combination with the justified controversy regarding the potential for raft-like domains to be induced by chemical fixation, and our observation that CTB-bound GM1 redistributed dramatically at cell death (Selvaraj et al., 2006), these reports called the localization of sterols in live sperm into question.
Using PFO-D4, we showed in both live murine and human sperm that the APM was enriched in sterols. PFO-D4 was first introduced as a tool to study membrane sterols by Fujimoto et al. (Fujimoto et al., 1997; Ohno-Iwashita et al., 2004). Since then, several studies have utilized this toxin to detect cholesterol-rich membrane microdomains in live cells (Hayashi et al., 2006; Koseki et al., 2007). One significant feature of this reagent is that it requires high local sterol content to bind (Ohno-Iwashita et al., 1992). It has been shown recently that its binding is dependent upon a number of factors including the nature of the sterol, the local pH, and the local phospholipid microenvironment (Nelson et al., 2008). Despite the potential impact of these variables and recent findings about how this toxin interacts with membrane sterols (Soltani et al., 2007), PFO-D4 remains a highly valuable tool because it can convey information in live cells on both the localization of sterols and their focal abundance. In our experiments, PFO-D4 labeling was not homogeneous over the APM, but rather appeared as punctate spots of fluorescence, suggesting the existence of microheterogeneities within the APM domain. This pattern is in stark contrast to the relatively uniform distribution of sterols when visualized with filipin in fixed cells. The data from both reagents could be physiologically correct: sterols could be spread throughout the APM and focal microdomain enrichments could exist within this broader domain. Alternatively, the relatively uniform pattern of filipin fluorescence could be an artifact caused by fixation or membrane perturbation (i.e. deformation required for the globular filipin-sterol complexes could necessitate a minimum radius for their spacing). It is also conceivable that PFO-D4 might be revealing focal variations in sterol distribution between the inner and outer leaflet, which is currently a topic of debate in membrane biology. Alternatively, exposure of sterols to PFO-D4 might depend on local variations in the lipid microenvironment in terms of pH or exposure (e.g. the “phospholipid umbrella” model) (Huang and Feigenson, 1999). Regardless, the data remain clear that enrichments of sterols and GM1 are segregated to the APM versus the PAPM in live sperm, and all interpretations described above require microheterogeneities to exist within the APM.
The demonstration of APM microdomains varying in sterol abundance or accessibility, and our finding that the membranes of the acrosome are enriched in GM1 but not sterols, are both supported by data from our recent biochemical results which show that 3 sub-types of membrane raft domains can be reproducibly separated based on their buoyancy in the absence of any detergent (Asano et al, in revision). One of these raft sub-types is enriched in both sterols and GM1, another just sterols, and the third is greatly enriched in GM1, but to a much lower extent in sterols (Asano et al., in revision). Comparative proteomic data also confirm that the APM contributes to these fractions (Asano et al., in revision).
Membrane interactions between plasma and acrosomal membranes at the time of cell death
One question that was unexplained from our previous study on CTB-bound GM1 was the increase we quantified in CTB fluorescence after redistribution from the APM to the PAPM (Selvaraj et al., 2006). In the present study, results from both surface saturation experiments as well as backscatter SEM showed an increase in plasma membrane GM1 at the time of cell death, suggesting movement from an internal membrane compartment. Saponin permeabilization experiments suggested that the acrosomal membranes formed an intracellular membrane pool enriched in GM1. This finding was confirmed by localization of GM1 to the acrosomal membranes in both spermatids and mature sperm (see also Asano et al., in revision). This localization has potential functional significance because the acrosome has also been shown to be a calcium store (Herrick et al., 2005), and GM1 has been shown to regulate calcium channels in a number of different cell systems (Buckley et al., 1995; Carlson et al., 1994). The impact of GM1 on calcium flux in sperm is a current topic of investigation in our lab (Buttke and Travis, manuscript in preparation). Our present results suggest that interactions between the APM and OAM occur at the time of cell death resulting in more GM1 becoming available for CTB binding in the plasma membrane. This inference is consistent with early morphological observations made on changes in the acrosomal vesicle during sperm death (Austin and Bishop, 1958; Bedford, 1963; Hancock, 1952). Also, we find that these interactions do not lead to a complete exocytosis, but rather might represent a loss of regulation of point-fusion events now proposed to occur over time during the capacitation process (Kim and Gerton, 2003). This model is of great current interest in the field, because it challenges a simplified view of acrosomal exocytosis in which such fusions would result in calcium influx and membrane potential and pH changes, all resulting in quick release of acrosomal contents (i.e. the “acrosome reaction,” akin to a water balloon bursting).
Although our data provide insight into changes in the APM, from a membrane biophysical perspective much information is still needed to understand the redistribution/migration of CTB-bound GM1 to the PAPM. A similar lateral redistribution of CTB-bound GM1 has been previously reported in lymphocyte membranes (Revesz and Greaves, 1975; Spiegel et al., 1984) and in several other cell types (Hansson et al., 1977). All these studies speculate that the cross-linking effect of CTB brings about this lateral redistribution of bound GM1 molecules. This explanation also supports our previous results showing that GM1 redistribution was not induced when GM1 was localized using an antibody; redistribution was rather a unique outcome requiring CTB binding (Selvaraj et al., 2006).
Previously, we showed that sterol efflux resulted in a diffuse distribution of CTB-bound GM1 over the entire sperm head consistent with an incomplete migration. In combination with our new findings, those data provide complementary information that the lipid content of the APM is also involved in driving redistribution. We now also show the somewhat surprising finding that loss of membrane asymmetry (indicated by exposure of PS on the outer leaflet) after cell death occurs only in the PAPM of the sperm head. Thus large-scale membrane changes in the PAPM might facilitate entry of CTB-bound GM1. This might act in concert with the sterol-rich APM providing an energetically unfavorable environment for cross-linked GM1 to remain. Thus, a positive driving force out of the APM might exist as well as changes in the PAPM that allow CTB-GM1 to enter. Changes at cell death might of course also happen in the SAR, which in live cells separates the APM from the PAPM.
The SAR acts as a specialized diffusion barrier
In somatic cells, several models have been put forth to explain how membrane domains might be segregated. In our previous study, we tested some of these models in sperm and found that protein disulfide bonds were critical for the segregation of GM1 to the APM (Selvaraj et al., 2006). The physical structure of the SAR, herein shown to be composed of an electron-dense matrix, forms the line of demarcation between the APM and PAPM. In somatic cells, membrane-associated cytoskeletal elements have been implicated in organizing membrane domains by providing certain lipid and protein tethers within the membrane. The plasma membrane of some cells is divided into distinct compartments, with the diffusion rate of lipids being determined by the size of the compartment and the frequency of jumps between compartments (Sako and Kusumi, 1994; Sako and Kusumi, 1995). Conversely, in other cells, small regions (200 to 300 nm) called transient confinement zones have been identified in which a traveling molecule can be trapped for several seconds (Dietrich et al., 2002; Simson et al., 1995). Consistent with both of these potential models, the existence of transmembrane proteins acting as a “picket fence” might restrict lipid diffusion (Sheets et al., 1997), between or within compartments.
Given the dynamic time scale and relatively small size predicted for most rafts, our data on the sperm APM domain might at first seem incongruous. However, our FLIP data on endogenous GM1 and our biochemical data (Asano et al., in revision), suggest the presence of microheterogeneities within the APM. These would be consistent with the microdomains known as rafts in somatic cells. The sperm therefore differ in having a higher order regional segregation between the APM and PAPM. Our FLIP data on endogenous GM1 show that the SAR acts as a diffusion barrier stably segregating GM1 and potentially sterols to the APM domain. However, the SAR did not act as a diffusion barrier for the exogenous BODIPY-GM1. Thus it seems that the endogenous GM1 molecules behave differently than exogenous, added GM1. The difference between behavior of endogenous lipids and exogenously added lipids has also been supported by our findings using NBD-sphingomyelin, which also does not seem to reflect the segregation of endogenous sterols and GM1 (data not shown). Segregation of endogenous GM1, but not BODIPY-GM1 suggests that in nature, GM1 might be associating with another component/composite within the APM domain. This could be essential for creating this domain and/or for maintaining it. Exogenous BODIPY-GM1 molecules in the membrane bilayer that did not or could not integrate with these components/composites were capable of diffusing freely throughout the sperm plasma membrane. Even during the breakdown of segregation of endogenous GM1 that happens upon cell death, our SEM studies showed that CTB-bound GM1 congregated at the SAR. This would be consistent with the SAR offering resistance to endogenous GM1 movement as a linear “picket fence.” It is possible that in live cells, the SAR does not provide a barrier against the diffusion of single molecules, which could move between the pickets. This would again be supported by our FLIP experiments, which showed that BODIPY-GM1 could move in both directions across the SAR. Such a property has been previously demonstrated in boar sperm for another exogenous lipid probe DiIC16 that showed no restriction of movement across the SAR (James et al., 2004).
Together, these data lead to our suggestion that the SAR does not act as a complete barrier to diffusion of all molecules, but is rather a more specialized barrier selective for only specific classes of endogenous lipids or for specific lipid-protein composites. At the time of cell death, there could be a loss of critical interactions needed to maintain the picket fence, or the membrane changes on both sides of the fence and potential cross-linking of GM1 might sum to overcome the barrier. Preliminary attempts to perturb this stable segregation by using known cytoskeletal disruptors to disorganize the SAR have not been successful (Selvaraj et al., 2006). However, it should be noted that in this region sperm have unique classes of basic cytoskeletal elements called calicins and cylicins whose structure and properties have not been completely characterized (Hess et al., 1993; Hess et al., 1995; Longo et al., 1987; von Bulow et al., 1995).
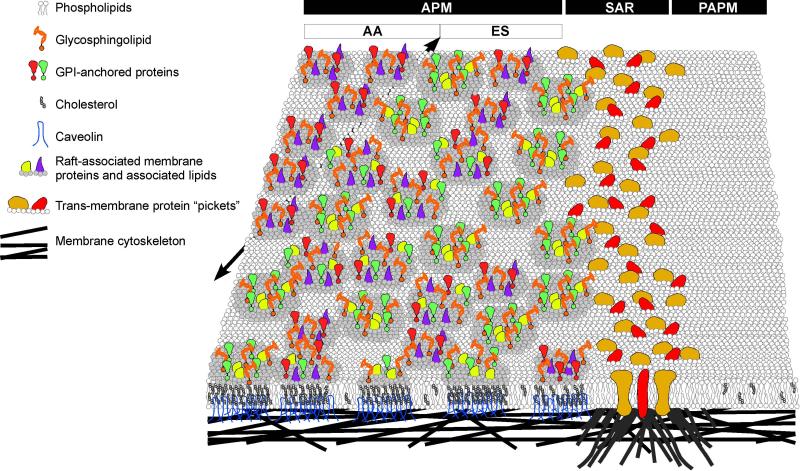
Combining our cell biological and biochemical observations with what is known in the literature, we now propose a hierarchical model involving multiple mechanisms to explain the segregation of sterols and GM1 to the APM domain (Fig. 11). This model accounts for the ability of individual molecules to cross the SAR, but not lipids and proteins that might exist in specific complexes, such as raft micro-domains. These complexes in the APM are heterogeneous, but are kept segregated from the PAPM at least in part by a cytoskeletal boundary at the SAR. Sperm are unique in having such a large and stable regional segregation of membrane complexes that does not require contact with other cells, such as seen in epithelial cells that have distinctions between apical and basolateral domains.
Fig. 11.
Schematic diagram showing a multi-level model for the mechanism of lipid segregation in the APM domain. (A) First level: there are free uni-molecular membrane lipids (e.g. most phospholipids) and proteins that follow the tenets of the Singer-Nicholson fluid-mosaic model (Singer and Nicolson, 1972). These can move across the SAR. (B) Second level: the existence of proteins and lipids in pre-assembled macromolecular composites. These could be heterogeneous or conform to specific compositional subtypes but are stable in not allowing sterol or GM1 exchange across the SAR. A less likely alternative at this level would be that the SAR could selectively prevent single molecules of specific lipid classes and a wide variety of proteins from diffusing across it, while allowing the movements described in the first level. (C) Third level: the existence of cytoskeletal-anchored transmembrane proteins as picket fences at the SAR restricts lateral diffusion of the macromolecular composites. This would require that all segregated molecules be components of such composites. Alternatively, cytoskeletal elements underlying the membrane might interact directly with membrane lipids and remove the necessity for transmembrane protein pickets. In this alternative, the pickets would be composed of the tethered, immobilized lipids. (D) Fourth level: spatial and temporal restrictions of specific sub-types of the macromolecular composites might lead to localized specializations (seen here distinguishing the regions of the AA and ES within the APM domain; a black arrow beneath the bilayer demarcates this region). Unidentified cytoskeletal proteins are shown interacting with the membrane at multiple locations, but in a more concentrated fashion at the SAR. Note: the depictions regarding the diversity of molecules are highly simplified in this schematic.
Our study is the first report showing a micron-scale segregation of focal enrichments of membrane sterols in live sperm. In addition to its size, the stability of the APM membrane domain in live cells is striking. We have examined the dynamics of, and mechanisms behind, sterol and GM1 segregation to this domain and demonstrate that a specialized diffusion barrier is present at the SAR. Furthermore, these data have led to a complex model that involves several complementary mechanisms of lipid segregation acting at different spatial scales. Our model reconciles apparently conflicting observations made with endogenous lipids versus exogenous lipids or probes, and is consistent with our biochemical data, which show the existence of multiple sub-types of membrane raft in sperm. These observations substantially advance our current understanding of sperm membrane properties and function. Testing this model by exploring the specific behaviors and functions of individual lipids and proteins will be a subject of continuing investigations.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant R01-HD-045664 (A.J.T). We also thank Colin Parrish, John Parker and Claudia Fischbach at the Baker Institute, Cornell University, for sharing laboratory resources used in this study; Darius Paduch and Peter Schlegel at the Weill-Cornell Medical College for help obtaining human sperm; Rodney Tweten, University of Oklahoma for help obtaining PFO and Markus Grebe for providing us with a plasmid containing domain 4 of PFO; Rebecca Williams at the Cornell Molecular Imaging Facility for technical advice during the photo-bleaching experiments; Malcolm Thomas with the Keck Integrated Microscopy Facility (NSF-MRSEC program; DMR 0520404) at Cornell University for technical help for the FE-SEM imaging; Shannon Caldwell with the Cornell Integrated Microscopy Facility for technical help for the TEM imaging.
Contract grant sponsor: National Institutes of Health ; Contract grant number: R01-HD-045664.
REFERENCES
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170(4321):326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Austin CR, Bishop MWH. Some features of the acrosome and perforatorium in mammalian spermatozoa. Proc Roy Soc B. 1958;149:234–242. doi: 10.1098/rspb.1958.0065. [DOI] [PubMed] [Google Scholar]
- Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104(9):3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JM. Morphological reaction of spermatozoa in the female reproductive tract of the rabbit. J Reprod Fertil. 1963;6:245–255. doi: 10.1530/jrf.0.0060245. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977a;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Millette CF, Bhatnagar YM, O’Brien DA. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977b;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Bleau G, VandenHeuvel WJ. Desmosteryl sulfate and desmosterol in hamster epididymal spermatozoa. Steroids. 1974;24(4):549–556. doi: 10.1016/0039-128x(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Buckley NE, Su Y, Milstien S, Spiegel S. The role of calcium influx in cellular proliferation induced by interaction of endogenous ganglioside GM1 with the B subunit of cholera toxin. Biochim Biophys Acta. 1995;1256(3):275–283. doi: 10.1016/0005-2760(95)00030-g. [DOI] [PubMed] [Google Scholar]
- Carlson RO, Masco D, Brooker G, Spiegel S. Endogenous ganglioside GM1 modulates L-type calcium channel activity in N18 neuroblastoma cells. J Neurosci. 1994;14(4):2272–2281. doi: 10.1523/JNEUROSCI.14-04-02272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168(4277):697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Cowan AE, Myles DG, Koppel DE. Lateral diffusion of the PH-20 protein on guinea pig sperm: evidence that barriers to diffusion maintain plasma membrane domains in mammalian sperm. J Cell Biol. 1987;104(4):917–923. doi: 10.1083/jcb.104.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AE, Nakhimovsky L, Myles DG, Koppel DE. Barriers to diffusion of plasma membrane proteins form early during guinea pig spermiogenesis. Biophys J. 1997;73(1):507–516. doi: 10.1016/S0006-3495(97)78089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK. Inhibitory effect of synthetic phospholipid vesicles containing cholesterol on the fertilizing ability of rabbit spermatozoa. Proc Soc Exp Biol Med. 1976;152(2):257–261. doi: 10.3181/00379727-152-39374. [DOI] [PubMed] [Google Scholar]
- Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82(1 Pt 1):274–284. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franek KJ, Wolcott RM, Chervenak R. Reliable method for the simultaneous detection of cytoplasmic and surface CD3 epsilon expression by murine lymphoid cells. Cytometry. 1994;17(3):224–236. doi: 10.1002/cyto.990170306. [DOI] [PubMed] [Google Scholar]
- Friend DS. Plasma-membrane diversity in a highly polarized cell. J Cell Biol. 1982;93(2):243–249. doi: 10.1083/jcb.93.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Hayashi M, Iwamoto M, Ohno-Iwashita Y. Crosslinked plasmalemmal cholesterol is sequestered to caveolae: analysis with a new cytochemical probe. J Histochem Cytochem. 1997;45(9):1197–1205. doi: 10.1177/002215549704500903. [DOI] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Kable EP, Jones AS, Gelissen I, Kritharides L, Jessup W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100(26):15554–15559. doi: 10.1073/pnas.2534386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JL. The morphology of bull spermatozoa. J Exp Biol. 1952;29:445–450. [Google Scholar]
- Hansson HA, Holmgren J, Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Shimada Y, Inomata M, Ohno-Iwashita Y. Detection of cholesterol-rich microdomains in the inner leaflet of the plasma membrane. Biochem Biophys Res Commun. 2006;351(3):713–718. doi: 10.1016/j.bbrc.2006.10.088. [DOI] [PubMed] [Google Scholar]
- Herrick SB, Schweissinger DL, Kim SW, Bayan KR, Mann S, Cardullo RA. The acrosomal vesicle of mouse sperm is a calcium store. J Cell Physiol. 2005;202(3):663–671. doi: 10.1002/jcp.20172. [DOI] [PubMed] [Google Scholar]
- Hess H, Heid H, Franke WW. Molecular characterization of mammalian cylicin, a basic protein of the sperm head cytoskeleton. J Cell Biol. 1993;122(5):1043–1052. doi: 10.1083/jcb.122.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H, Heid H, Zimbelmann R, Franke WW. The protein complexity of the cytoskeleton of bovine and human sperm heads: the identification and characterization of cylicin II. Exp Cell Res. 1995;218(1):174–182. doi: 10.1006/excr.1995.1145. [DOI] [PubMed] [Google Scholar]
- Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76(4):2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9(1):7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- James PS, Hennessy C, Berge T, Jones R. Compartmentalisation of the sperm plasma membrane: a FRAP, FLIP and SPFI analysis of putative diffusion barriers on the sperm head. J Cell Sci. 2004;117(Pt 26):6485–6495. doi: 10.1242/jcs.01578. [DOI] [PubMed] [Google Scholar]
- Kim KS, Gerton GL. Differential release of soluble and matrix components: evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev Biol. 2003;264(1):141–152. doi: 10.1016/j.ydbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Koch GL, Macer DR, Smith MJ. Visualization of the intact endoplasmic reticulum by immunofluorescence with antibodies to the major ER glycoprotein, endoplasmin. J Cell Sci. 1987;87(Pt 4):535–542. doi: 10.1242/jcs.87.4.535. [DOI] [PubMed] [Google Scholar]
- Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res. 2007;48(2):299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746(3):234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Ladha S, James PS, Clark DC, Howes EA, Jones R. Lateral mobility of plasma membrane lipids in bull spermatozoa: heterogeneity between surface domains and rigidification following cell death. J Cell Sci. 1997;110(Pt 9):1041–1050. doi: 10.1242/jcs.110.9.1041. [DOI] [PubMed] [Google Scholar]
- Lin Y, Kan FW. Regionalization and redistribution of membrane phospholipids and cholesterol in mouse spermatozoa during in vitro capacitation. Biol Reprod. 1996;55(5):1133–1146. doi: 10.1095/biolreprod55.5.1133. [DOI] [PubMed] [Google Scholar]
- London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim Biophys Acta. 2005;1746(3):203–220. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Krohne G, Franke WW. Basic proteins of the perinuclear theca of mammalian spermatozoa and spermatids: a novel class of cytoskeletal elements. J Cell Biol. 1987;105(3):1105–1120. doi: 10.1083/jcb.105.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, James PS, Ladha S, Jones R. Diffusion barriers in ram and boar sperm plasma membranes: directionality of lipid diffusion across the posterior ring. Biol Reprod. 2001;64(1):113–119. doi: 10.1095/biolreprod64.1.113. [DOI] [PubMed] [Google Scholar]
- Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5(4):231–240. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Myles DG, Primakoff P, Koppel DE. A localized surface protein of guinea pig sperm exhibits free diffusion in its domain. J Cell Biol. 1984;98(5):1905–1909. doi: 10.1083/jcb.98.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L, Johnson AE, London E. How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure and physiological low pH: Insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem. 2008 doi: 10.1074/jbc.M709483200. DOI:10.1074(M709483200. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y, Iwamoto M, Ando S, Iwashita S. Effect of lipidic factors on membrane cholesterol topology--mode of binding of theta-toxin to cholesterol in liposomes. Biochim Biophys Acta. 1992;1109(1):81–90. doi: 10.1016/0005-2736(92)90190-w. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y, Shimada Y, Waheed AA, Hayashi M, Inomata M, Nakamura M, Maruya M, Iwashita S. Perfringolysin O, a cholesterol-binding cytolysin, as a probe for lipid rafts. Anaerobe. 2004;10(2):125–134. doi: 10.1016/j.anaerobe.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Friend DS. Development of membrane differentiations in the guinea pig spermatid during spermiogenesis. Am J Anat. 1983;167(1):119–141. doi: 10.1002/aja.1001670110. [DOI] [PubMed] [Google Scholar]
- Phelps BM, Primakoff P, Koppel DE, Low MG, Myles DG. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 1988;240(4860):1780–1782. doi: 10.1126/science.3381102. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47(7):1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril. 1985;44(4):493–498. doi: 10.1016/s0015-0282(16)48918-1. [DOI] [PubMed] [Google Scholar]
- Revesz T, Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- Sako Y, Kusumi A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol. 1994;125(6):1251–1264. doi: 10.1083/jcb.125.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y, Kusumi A. Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J Cell Biol. 1995;129(6):1559–1574. doi: 10.1083/jcb.129.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Asano A, Buttke DE, McElwee JL, Nelson JL, Wolff CA, Merdiushev T, Fornes MW, Cohen AW, Lisanti MP, Rothblat GH, Kopf GS, Travis AJ. Segregation of micron-scale membrane sub-domains in live murine sperm. J Cell Physiol. 2006;206(3):636–646. doi: 10.1002/jcp.20504. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Buttke DE, Asano A, McElwee JL, Wolff CA, Nelson JL, Klaus AV, Hunnicutt GR, Travis AJ. GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J Androl. 2007;28(4):588–599. doi: 10.2164/jandrol.106.002279. [DOI] [PubMed] [Google Scholar]
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116(4):577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- Sheets ED, Lee GM, Simson R, Jacobson K. Transient confinement of a glycosylphosphatidylinositol-anchored protein in the plasma membrane. Biochemistry. 1997;36(41):12449–12458. doi: 10.1021/bi9710939. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simson R, Sheets ED, Jacobson K. Detection of temporary lateral confinement of membrane proteins using single-particle tracking analysis. Biophys J. 1995;69(3):989–993. doi: 10.1016/S0006-3495(95)79972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(23):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci U S A. 2007;104(51):20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Kassis S, Wilchek M, Fishman PH. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J Cell Biol. 1984;99(5):1575–1581. doi: 10.1083/jcb.99.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F. Changes in the distribution of intramembranous particles and filipin-sterol complexes during epididymal maturation of golden hamster spermatozoa. J Ultrastruct Mol Struct Res. 1988;100(1):39–54. doi: 10.1016/0889-1605(88)90057-2. [DOI] [PubMed] [Google Scholar]
- Tao J, Critser ES, Critser JK. Evaluation of mouse sperm acrosomal status and viability by flow cytometry. Mol Reprod Dev. 1993;36(2):183–194. doi: 10.1002/mrd.1080360209. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001a;276(10):7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001b;240(2):599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod. 2004;71(1):139–145. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- van Drogen F, Peter M. Revealing protein dynamics by photobleaching techniques. Methods in molecular biology (Clifton, NJ. 2004;284:287–306. doi: 10.1385/1-59259-816-1:287. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274(5):3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- von Bulow M, Heid H, Hess H, Franke WW. Molecular nature of calicin, a major basic protein of the mammalian sperm head cytoskeleton. Exp Cell Res. 1995;219(2):407–413. doi: 10.1006/excr.1995.1246. [DOI] [PubMed] [Google Scholar]
- Waheed AA, Shimada Y, Heijnen HF, Nakamura M, Inomata M, Hayashi M, Iwashita S, Slot JW, Ohno-Iwashita Y. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts) Proc Natl Acad Sci U S A. 2001;98(9):4926–4931. doi: 10.1073/pnas.091090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DE, Maynard VM, McKinnon CA, Melchior DL. Lipid domains in the ram sperm plasma membrane demonstrated by differential scanning calorimetry. Proc Natl Acad Sci U S A. 1990;87(17):6893–6896. doi: 10.1073/pnas.87.17.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DE, Voglmayr JK. Diffusion and regionalization in membranes of maturing ram spermatozoa. J Cell Biol. 1984;98(5):1678–1684. doi: 10.1083/jcb.98.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CA, James PS, Mackie AR, Ladha S, Jones R. Regionalized lipid diffusion in the plasma membrane of mammalian spermatozoa. Biol Reprod. 1998;59(6):1506–1514. doi: 10.1095/biolreprod59.6.1506. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In: Mammalian fertilization. Knobil E, Neill JD, editors. Raven Press, Ltd; New York: 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.