Abstract
The stabilization of long-term memories requires de novo protein synthesis. How can proteins, synthesized in the soma, act on specific synapses that participate in a given memory? We studied the dynamics of newly synthesized AMPA-type glutamate receptors (AMPARs) induced with learning using transgenic mice expressing the GluR1 subunit fused to green fluorescent protein (GFP-GluR1) under control of the c-fos promoter. We found learning-associated recruitment of newly synthesized GFP-GluR1 selectively to mushroom-type spines in adult hippocampal CA1 neurons 24 hours after fear conditioning. Our results are consistent with a “synaptic tagging” model to allow activated synapses to subsequently capture newly synthesized receptor and also demonstrate a critical functional distinction in the mushroom spines with learning.
AMPARs are the primary mediators of fast excitatory transmission in the mammalian brain, and play a key role in long-term potentiation (LTP) (1). There is experience-dependent synaptic trafficking of GFP-GluR1 homomers in barrel cortex and lateral amygdala of juvenile rats (12−25 days old) (2, 3). These results suggest that changes in the strength of excitatory synapses contribute to learning, possibly through rapid synaptic insertion of pre-existing GluR1-containing AMPARs, although it is unclear whether similar mechanisms are present in the adult (4-6). Short-term memories are lost if new protein synthesis is inhibited, indicating a requirement for de novo protein synthesis to consolidate the memories into a long-lasting form (7). This has raised a fundamental question: How do new proteins, synthesized in the soma, exert their effect on specific synapses involved in synaptic or behavioral plasticity? It has been suggested that stimulation produces a “tag” at the activated synapses to allow the capture of newly synthesized plasticity-related proteins at later time points to maintain the increased synaptic strength (8). Synthesis of AMPARs is increased after LTP induction (9), suggesting a possible role in this process. To test this might be operating in the adult brain in vivo, we developed transgenic mice to monitor the trafficking and turnover of newly synthesized AMPARs induced at the time of learning in a fear conditioning paradigm.
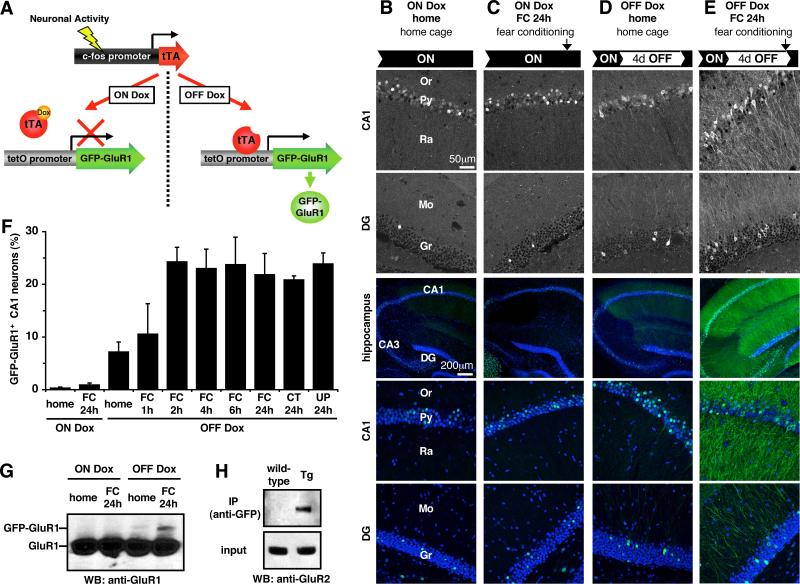
The transgenic mice express a GFP-GluR1 fusion protein in a doxycycline (Dox) regulated (10) and neuronal activity-dependent manner (referred to as GFP-GluR1c-fos Tg mice) (Fig. 1A). We used the activity-dependent c-fos promoter (11, 12) to induce a rapid and transient expression of GFP-GluR1 in response to environmental stimuli and to focus on the molecular and cellular events specifically in those neurons activated by the behaviorally relevant events. Fear conditioning (13) was used in adult animals to evaluate regulated expression in the dorsal hippocampus. GFP fluorescence, immunohistochemical, and immunoblot analysis revealed that GFP-GluR1 expression was negligible in the presence of Dox, even in fear conditioned mice (Fig. 1B, C, F, G), showing successful transgene suppression by Dox. The GFP expression seen in some of the cell nuclei is derived from a cfos-nlsGFP transgene, which is expressed independently of the Dox-regulated system. To test the inducibility of the GFP- GluR1c-fos Tg by behavioral training, mice were removed from Dox for 4 days and were either fear conditioned or allowed to remain in the homecage. At 24 hours after conditioning, prominent GFP-GluR1 expression was detected in the fear conditioned animals relative to homecage controls (Fig. 1D-G), showing activity-dependent expression of GFP-GluR1 in the absence of Dox. The induced somatic GFP-GluR1 was detected in approximately 25% of CA1 pyramidal neurons within 2 hours of fear conditioning and was maintained for more than 24 hours (Fig. 1F, and fig. S1). One concern is the potential for overexpressed receptors to function aberrantly. In many previous studies of recombinant GluR1 trafficking, the receptor formed exclusively homo-oligomers (2, 3, 14, 15), while the endogenous receptor complex is primarily a heteromer (16). The induced expression of the GFP-GluR1 was approximately 3 % of endogenous GluR1 levels (Fig. 1G). GFP-GluR1 was co-immunoprecipitated with the endogenous GluR2 subunit (Fig. 1H), demonstrating the formation of the natural heteromeric forms. The dendritic transport rate and half-life of GFP-GluR1 were consistent with that determined previously for endogenous receptor in vitro (17-19) (fig. S1, S2).
Fig. 1.
Basic features of GFP-GluR1c-fos Tg mice. (A) Schematic representation of the transgenic system. The c-fos promoter was used to drive rapid and transient expression of the tetracycline-regulated transactivator (tTA) in activated neurons. The tTA in turn activates transcription of a tetO promoter-linked GFP-GluR1 in a Dox-regulated manner. (B-E) Confocal microscopy images of intrinsic GFP fluorescence (upper two panels) and GFP immunoreactivity (lower 3 color panels) in the hippocampal slices. Nuclei were stained with TO-PRO-3 (blue). Or, stratum oriens; Py, stratum pyramidale; Ra, stratum radiatum; Mo, molecular layer; Gr, granule cell layer; DG, dentate gyrus. (F) Proportion of CA1 neurons with cytoplasmic GFP immunoreactive signals. Data in this and all subsequent figures are represented as mean ± s.e.m. ON Dox: n = 5 mice for home and FC24h. OFF Dox: n = 5 for home; n = 4 for FC1h, FC4h, FC24h, CT24h, and UP24h; n = 3 for FC4h and FC6h. (G) Western blot analysis showing the expression of GFP-GluR1 and endogenous GluR1 in the hippocampus. (H) Co-immunoprecipitation of GFP-GluR1 and endogenous GluR2 in the hippocampus.
In contextual fear conditioning, animals learn the association between a specific environment (context) and an aversive stimulus such as a footshock. Brief training produces a stable and long-lasting memory that requires the hippocampus (13). We used fear conditioning in the GFP-GluR1c-fos Tg mice to test for learning-associated synaptic trafficking of newly synthesized AMPARs in the adult hippocampus. Mice were removed from Dox, fear conditioned to elicit a contextual long-term memory, and to induce synthesis of GFP-GluR1 in the activated neurons (FC24h; Fig. 2A). Control mice received either context exposure without foot-shocks (CT24h; Fig. 2A) or unpaired context-shock training, which failed to produce a contextual fear memory (UP24h; Fig. 2A and fig. S3). Mice were returned to their homecage and treated with high Dox (6g/kg mouse chow) to rapidly suppress further expression of GFP-GluR1. Twenty-four hours after training, brain slices were fixed and stained with anti-GFP antibody, without permeabilization, to detect surface GFP-GluR1 expression (fig. S4). A subset of neurons in the CA1 pyramidal cell layer of the dorsal hippocampus was stained with the lipophilic fluorescent dye, DiI, to visualize the entire dendritic arbor and spine distribution on individual neurons (Fig. 2B-D). Consistent with previous studies examining endogenous c-Fos induction (20), all group of mice showed a similar number of GFP-GluR1+ CA1 neurons (Fig. 1F). Levels of GFP-GluR1 expression were also similar in the fear-conditioned and context-exposure group (Fig. 2E). This design allows us to induce the receptor at the time of learning, and to follow its distribution to synapses at later time points specifically in behaviorally activated neurons.
Fig. 2.
Preferential recruitment of newly synthesized GFP-GluR1 to mushroom spines 24 hours after fear-conditioned learning. (A) Experimental design. (B) Confocal image of a hippocampal slice labeled with anti-GFP antibody (green) and DiI (red). (C) Confocal image showing surface GFP-GluR1 localization on apical dendrite after fear conditioning. Some spines were GFP positive (arrow heads) but some were negative, showing an uneven synaptic trafficking of newly synthesized GFP-GluR1. (D) Representative images of spine morphology and surface GFP-GluR1 localization. Scale bar, 1μm. (E) Relative immunoreactivity from western blot analysis (CT24h: n = 4, FC24h: n = 3). (F) The percentage of GFP-GluR1+ mushroom spines was significantly higher (apical: F2,16 = 8.86, p = 0.0026, basal: F2,16 = 15.43, p = 0.0002) in FC24h group (apical: n = 7 mice, 1927 spines, basal: n = 7 mice, 1295 spines) as compared to the CT24h group (apical: n = 6 mice, 1626 spines, basal: n = 6 mice, 1292 spines) and UP24h group (apical: n = 6 mice, 2240 spines, basal: n = 6 mice, 1974 spines). The proportion was significantly decreased (apical: p = 0.0056, basal: p = 0.0010, t-test) at 72 hours after conditioning (apical: n = 6 mice, 1233 spines, basal: n = 6 mice, 1138 spines). *p < 0.01.
Dendritic spines are morphologically diverse but typically categorized into thin, stubby, and mushroom type (21, 22) (Fig. 2D). We counted the proportion of surface GFP immuno-positive spines (GFP-GluR1+ spines) on apical and basal dendrites in the trained (FC24h) and two control groups (CT24h and UP24h), and analyzed the data separately for each spine type (Fig. 2F). There were no significant differences in GFP-GluR1+ thin or stubby spines between groups. In contrast, for mushroom spines, we found a significant increase in the proportion of GFP-GluR1+ spines in the fear-conditioned group compared to the context-only and unpaired-shock group. The increased labeling returned to control levels by 72 hours post training (FC72h). While GFP-GluR1 is delivered to approximately 50% of all spines, independent of behavioral contingencies or spine type, the presence of the shock reinforcer in the fear-conditioned group allows increased capture of receptor specifically in the mushroom-type spines.
Fear memory can be attenuated by extinction training, repeated exposure to an unreinforced conditioned stimulus. Extinction is thought to involve new learning rather than loss of the original memory, although the underlying mechanism remains elusive (23). We tested whether memory extinction alters the distribution of pre-existing GFP-GluR1 in neurons that were activated during the original fear conditioning. GFP-GluR1c-fos Tg mice were withdrawn from Dox, fear conditioned, and returned to their homecage for 2 days on a high Dox diet. Conditioned mice received either 3 trials of extinction training (FC72h+EXT; Fig. 3A) or were left undisturbed in the homecage (FC72h; Fig. 3A). We confirmed a markedly reduced freezing response after extinction training (Fig. 3B). At 72 hours after fear conditioning, brain slices were prepared and the distribution of GFP-GluR1 was analyzed (Fig. 3C). For thin and stubby spines, extinction training did not affect the proportion of GFP-GluR1+ spines, while the proportion of GFP-GluR1+ mushroom spines was significantly higher in the extinction group than in the control group. A western blot revealed that extinction training on Dox did not induce additional GFP-GluR1 expression (Fig. 3D).
Fig. 3.
Distribution of pre-existing GFP-GluR1 following memory extinction training. (A) Experimental design. (B) The percentage of time spent freezing before fear conditioning (basal), after fear conditioning (after FC), and during three trials of extinction training (EXT1∼3). n = 7. (C) The proportion of GFP-GluR1+ mushroom spines was significantly higher (apical: p = 0.045, basal: p = 0.033, t-test) in the extinction trained group (apical: n = 7 mice, 1870 spines, basal: n = 6 mice, 1845 spines) compared to the control group (FC72h, the same data as in Fig. 2F). (D) Relative immunoreactivity from western blot analysis (n = 3 for each condition).
We also tested whether fear conditioning induced changes in spine density, as is seen in some strong learning paradigms (24, 25). We analyzed the proportion of each spine type and total spine density in behaviorally activated neurons. There were no significant changes in total spine density (Fig. 4A) or in spine-type distribution (Fig. 4B) in any group.
Fig. 4.
Spine remodeling following behavioral trainings. (A) No significant changes were observed in spine density (apical: F4,27 = 0.72, p = 0.58, basal: F4,27 = 0.88, p = 0.49). n = 6 mice for UP24h, CT24h, and FC72h. n = 7 for FC24h and FC72h+EXT. (B) No significant changes were observed in spine-type distribution. n = 6 mice for UP24h, CT24h, and FC72h. n = 7 for FC24h and FC72h+EXT.
Our results demonstrate that fear conditioning alters trafficking of newly synthesized AMPARs in the adult hippocampus and that the change is spine-type specific. One of the most remarkable features of dendritic spines is their morphological diversity, with the mushroom spines considered to represent the most mature and stable spine morphology (22, 26). At 24-hours following fear conditioning, these spines show an increased ability to incorporate and/or retain newly synthesized AMPARs. The change itself is not persistent and returns to baseline untrained levels by 72 hours. This may represent a subsequent change in receptor composition, for example replacement of GluR1-containing receptors with GluR2/GluR3 receptors (27, 28), or it may indicate a time-limited role for alterations of receptor composition in memory. Surprisingly, extinction training leads to a prolonged elevation in the proportion of GFP-GluR1+ mushroom spines, even though it also leads to a reduction in the behavioral expression of the fear memory response. This could represent a redistribution of AMPARs to an alternate subset of synapses associated with extinction learning. Alternately, this could result from the stabilization of GFP-GluR1 in the synapses labeled with fear conditioning. While little is known about the functional importance in the morphologically distinct spine classes in vivo (22, 26), the current results demonstrate a unique role for the mushroom spines in learning-associated changes in receptor trafficking and suggest they may be critical sites for behaviorally relevant plasticity. The learning-associated increase in GFP-GluR1 recruitment was detected in ∼23% of mushroom spines, representing ∼3% of all spines. Such changes are consistent with a sparse subset of synapses contributing to any given memory trace.
What role this receptor trafficking plays in the fear-conditioning paradigm is unclear. The trafficking changes require the same temporal pairing of context with shock required to produce the behavioral memory and likely represent an associative synaptic event. In fear conditioning, the hippocampus is generally thought to encode a representation of the context with the associative changes for fear occurring in the amygdala (13). However, electrophysiological studies indicate that the shock causes a partial remapping of the hippocampal representation (29) and the receptor trafficking changes may alter those synapses that participate in this incorporation of the unconditioned stimulus signal into the hippocampal representation.
LTP-induction is known to increase AMPAR synthesis (9). By coupling expression of GFP-GluR1 to neuronal activity, we specifically examined this pool of newly synthesized receptor. In this paradigm, the earliest possible time point for the delivery of GFP-GluR1 to spines is offset from the behavioral training by several hours (see fig. S1). This suggests that at the time of learning there are changes in some spines that allow the capture of newly synthesized AMPARs at later time points. This type of “synaptic tagging” has been demonstrated to play a role in the maintenance of LTP (8). The current experiments demonstrate a similar mechanism operating during behavioral learning in the adult brain, which may contribute to the stabilization of long-term memory. They also implicate GluR1-containing AMPARs as one of the cargo molecules selectively delivered to tagged synapse.
Supplementary Material
Supporting Online Material
Materials and Methods
Generation of transgenic mice. For construction of the fos-tTA transgene (S1), the tTA gene was inserted into a plasmid carrying artificial introns and a polyadenylation signal from SV40 (S2) and then subcloned into the AccI site of the first exon of the mouse c-fos gene (S3). To generate the tetO-GFP-GluR1 transgene, EGFP-GluR1 was similarly flanked by introns and an SV40 polyadenylation signal and placed downstream of the tetO promoter element of plasmid pMM400 (S2). The transgenes were excised from vectors and were purified by agarose gel electroelution and Elutip minicolumn. Purified DNA fragments were microinjected (2ng/μl) into BALB/cByJ and C57BL/6J F2 hybrid embryos and implanted into pseudopregnant females. The fos-tTA construct was co-injected with a fos-nlsGFP transgene (c-fos promoter driven EGFP with nuclear localizing signals) and segregated as a single transgene. Genotypes were determined by PCR with genomic DNA isolated from tails using the following primers: htTaF23 (5’-ACCTGGACATGCTGTGATAA-3’), htTaB22 (5’-TGCTCCCATTCATCAGTTCC-3’), MM400F1 (5’-ATAGAAGACACCGGGACCGAT-3’), and GFPR2 (5’-TTCAGCTCGATGCGGTTCAC-3’). The PCR reaction was run at 94 °C for 30sec, 55 °C for 30sec, and 72 °C for 1min for 35 cycles. All transgenic mice were maintained as heterozygotes and were backcrossed to C57BL/6J mice. All mice were kept on a Dox diet (40mg/kg) beginning from the time of weaning (3∼4 weeks old) and used for experiments at 11∼14 weeks old. Only males were used for experiments.
Fear conditioning. Mice were individually housed for 1 week prior to the experiments. For fear conditioning, mice were placed in a novel rectangle chamber with a grid-floor. After a 3 min baseline period, 4 tone-shock pairings were presented. Each pairing consisted of a 20 sec 85 dB 2800 Hz tone simultaneously ending with a 2 sec 0.75 mA shock. There was a 1 min interval between each pairing. The mice remained in the chamber for 1 min after the last shock before being returned to the home cage. The total duration of training was 500 sec. Freezing behavior was scored and analyzed automatically by a video-based system (S1). For the “context-only” condition, the mice were placed in the same fear conditioning chamber for 500 sec and received the same tones but did not receive foot shock.
Unpaired shock training. An immediate shock alone does not induce c-fos expression in the hippocampus, even if mice are exposed to a new context for the same amount of time as fear conditioning training (data not shown, S4, S5). In order to induce expression of c-fos promoter driven GFP-GluR1 in the hippocampus, mice were first placed in a novel chamber (context A: square plastic chamber surrounded with white walls, plastic floor with sani-chips) for 500 sec without foot-shocks. After the context A exposure, they were returned to their home cages. Fifteen min later, they received 4 shocks (2 sec 0.75 mA/shock) immediately after being placed in the conditioning chamber (context B: rectangle chamber, plexiglass front and back with black-and-white cross-stripes pattern and aluminium side walls, grid-floor). There was a 4 sec interval between shocks. After the final shock, they were returned to their home cages.
Contextual memory extinction training. Mice received 3 extinction trials in a day. Mice were exposed to the chamber where fear conditioning was conducted for 30 min for each trial without shocks. After each training, they were returned to their home cages. There was a 2 hour interval between trials.
Immunoblot. Hippocampal lysates (15μg of total protein) were separated by SDS–PAGE and transferred onto Hybond-P membrane. Membranes were blocked with 5% skim milk in TBST for 1 hour and probed with rabbit anti-GluR1 polyclonal antibody or mouse anti-GluR2 monoclonal antibody, followed by peroxidase-conjugated AffiniPure goat anti-rabbit IgG or peroxidase-conjugated AffiniPure donkey anti-mouse IgG. Signals were developed using the ECL PLUS system and exposed to Hyperfilm ECL. Immunoreactive intensities were analyzed with ImageJ software.
Immunoprecipitation. Hippocampi of the GFP-GluR1c-fos Tg mice were prepared 24 hours after fear conditioning. Hippocampi of wild-type and Tg mice were homogenized in lysis buffer (20mM HEPES, pH 7.5, 150mM NaCl, 1% Triton X-100, protease inhibitors cocktail) and incubated for 1 hour at 4 °C. The homogenate was cleared by centrifugation for 10 min at 15,000 g. The supernatant was pre-cleared with protein A agarose, then incubated with rabbit anti-GFP antibody attached to protein A agarose for 2 hours at 4 °C. The precipitated agarose was washed with lysis buffer 5 times and processed for immunoblotting with mouse anti-GluR2 monoclonal antibody.
Immunohistochemistry. Sagittal brain slices were cut at a thickness of 100 μm using a vibratome and fixed with cold 4% paraformaldehyde in PBS for 1 hour. Free-floating slices were permeabilized with 0.15% of TritonX-100 in 5% BSA/PBS at room temperature for 30 min, then incubated with rabbit anti-GFP antibody at 4 °C overnight, rinsed with PBS, and thereafter incubated with goat anti-rabbit Alexa 488 secondary antibody at 4 °C overnight. For visualization of nuclei, slices were counterstained with TO-PRO-3 and mounted in Slowfade Light antifade mounting medium. For surface GFP-GluR1 staining, slices were not permeabilized.
DiI labeling. Micropipettes were coated with DiI (1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate) dissolved in ethanol at 10 mg/ml. The tip of DiI-coated micropipettes was inserted at several positions in the CA1 pyramidal cell layer of fixed slices using a micromanipulator. Slices were then placed in PBS for 3∼4 days at 4 °C to allow the DiI to spread throughout the dendritic arbor.
Acquisition and analysis of spine images. Dendrites in stratum oriens (basal dendrites) and stratum radiatum (apical dendrites) were randomly selected for imaging if they were strongly double-labeled with GFP immunoreactive signal and DiI fluorescence. Primary dendrites were excluded from the analysis. Selected dendrites were imaged using a confocal microscope by sequential illumination with Argon (488 nm) and HeNe (543 nm) laser. Under the condition of laser power and PMT we used, intrinsic GFP fluorescence was too dim to detect. Images were acquired as a series of optical z-sections at 0.4 μm increments using an oil immersion 63× objective (NA 1.4) with a zoom of 2. Each optical section was scanned 3 times and Kalman filtering was employed to reduce noise. The stack images were converted to merged TIFF images of green channel (GFP immunoreactivity) and red channel (DiI fluorescence) for every focal plane using ImageJ software. They were exported to Adobe Photoshop for analysis. GFP-positive spines were defined as the existence of overlapping GFP signals on spines, as marked by yellow in the confocal images. GFP signals were often observed at the head of spines as a punctate signal. Spine morphology and density was analyzed on two-dimensional projection images constructed using a maximum intensity method. Spines were classified according to previously described criteria (S6): the “thin” type has a long neck and a small head; the “stubby” type has a large head but does not have a neck; the “mushroom” type has a large head with a neck. For spine density measurement, all protrusions along 30 μm dendritic segments were counted as spines (For apical dendrites, 58, 53, 55, 56, and 61 segments were examined for UP24h, CT24h, FC24h, FC72h, and FC72h+EXT, respectively. For basal, 55, 53, 52, 51, and 68 segments were examined for UP24h, CT24h, FC24h, FC72h, and FC72h+EXT, respectively). Analysis was performed blind with respect to the experimental conditions.
fig. S1. Induction and trafficking of newly synthesized GFP-GluR1 in the hippocampus of GFP-GluR1c-fos Tg mice. Mice were removed from Dox for 4 days and fear conditioned to examine the earliest time point for GFP-GluR1 induction in the soma and the trafficking rate of induced GFP-GluR1 in dendrites of the CA1 field of the dorsal hippocampus. Mice were examined at 1, 2, and 6 hours following training. Brain slices were fixed, pearmeabilized with 0.15% TritonX-100, then stained with anti-GFP antibody (green) and TO-PRO-3 nuclear marker (blue). Images were acquired using a confocal microscope. At 1 hour after stimulus (FC 1h), GFP-GluR1 expression was slight in the soma and dendritic layer, which is similar to home cage controls (home). At 2 hours (FC 2h), prominent GFP-GluR1 expression was detected in the soma but the dendritic expression was slight and limited to the proximal region. At 6 hours (FC 6h), GFP-GluR1 expression was detected in the soma and throughout the dendritic layer. These results suggest that the GFP-GluR1 is expressed by 2 hours in the soma in response to behavioral stimuli and transported to the distal dendrites within at least 6 hours in CA1 hippocampal neurons of GFP-GluR1c-fos Tg mice.
fig. S2. Metabolic stability of GFP-GluR1 in the hippocampus of GFP-GluR1CaMKIIα Tg mice. (A) Mice in which GFP-GluR1 expression was driven broadly in the hippocampus by a CaMKIIα-tTA transgene (S2) were fed a regular diet without Dox for more than 2 months to induce maximal expression of GFP-GluR1. They were then switched to a Dox diet (6g/kg chow for the first 2 days and then to 40mg/kg) for the period of days indicated to suppress new expression of GFP-GluR1. Sagittal brain slices were cut at a thickness of 100μm and fixed with cold 4% paraformaldehyde in PBS for 1 hour. Images of GFP fluorescence in the dorsal hippocampus were acquired using a fluorescent microscope with the same exposure time. (B) Relative GFP fluorescent intensities (%) in the hippocampus over time after Dox treatment. Data are represented as mean ± s.e.m. (n = 2 for each condition).
fig. S3. Contextual memory retention test after fear conditioning and unpaired-shock training. (A) Behavioral protocol. GFP-GluR1c-fos Tg animals used in the memory test are different animals that are analyzed for GFP-GluR1+ spines in Fig.2 because of the absence of memory test in the experiments of Fig. 2. Mice (n = 7 females, 20∼27 weeks old) were trained with fear conditioning in context B (rectangle chamber, plexiglass front and back with black-and-white cross-stripes pattern and aluminium side walls, grid-floor), then returned to the context B for 3 min for a contextual memory test at 24 hours after training (FC). For unpaired shock training (UP), mice (n = 7 females, 20∼27 weeks old) were first exposed to context A (square plastic chamber surrounded with white walls, plastic floor with sani-chips) for 500 sec without shocks, then received immediate shocks in context B. Twenty-four hours after training, they were returned to the context A, then B for 3 min for memory tests. (B) Percentage of time spent freezing. The UP group showed low levels of freezing for both context A and B, indicating that the unpaired-shock training caused less long-term fear memory associated with context B where shocks were given and context A where c-fos promoter driven GFP-GluR1 expression was induced. Data are represented as mean ± s.e.m.
fig. S4. Visualization of surface GFP-GluR1 in the hippocampal slice. (A) Predicted membrane topology of GFP-GluR1. (B) For surface GFP-GluR1 labeling, sagittal brain slices of GFP-GluR1CaMKIIα Tg mice were fixed with 4% PFA on ice for 1 hour. They were then incubated with rabbit anti-GFP polyclonal antibody, rinsed with PBS, and thereafter incubated with anti-rabbit Alexa594 antibody (red). To detect total GFP-GluR1, the slices were subsequently permeabilized with 0.15% Triton X-100 for 30min, and labeled with rabbit anti-GFP polyclonal antibody and anti-rabbit Alexa488 antibody (green). Confocal hippocampal images were acquired using a confocal microscope. Note that the lack of cytoplasmic labeling in the CA1 pyramidal cells before permeabilization (red), indicating the method labels the plasma membrane surface GFP-GluR1 but not internal receptor. Scale bar, 30μm. (C) To verify the ability of fixed tissue to exclude antiserum until permeabilized, brain slices of GFP-GluR1c-fos Tg mice (FC 24h) were stained with anti-GluR1 polyclonal antibody to a C-terminal epitope with and without permeabilization (0.15% Triton X-100 for 30min). Confocal hippocampal images were acquired with identical condition. Scale bar, 200μm.
References
S1. L. G. Reijmers, B. L. Perkins, N. Matsuo, M. Mayford, Science 317, 1230−1233 (2007).
S2. M. Mayford et al., Science 274, 1678−1683 (1996).
S3. K. Schilling, D. Luk, J. I. Morgan, T. Curran, Proc Natl Acad Sci U S A 88, 5665−5669 (1991).
S4. S. Milanovic et al., Brain Res 784, 37−47 (1998).
S5. N. C. Huff et al., J Neurosci 26, 1616−1623 (2006).
S6. K. M. Harris, F. E. Jensen, B. Tsao, J Neurosci 12, 2685−2705 (1992).
Footnotes
References and Notes
- 1.Malinow R, Malenka RC. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Svoboda K, Malinow R. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- 3.Rumpel S, LeDoux J, Zador A, Malinow R. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 4.Heynen AJ, Quinlan EM, Bae DC, Bear MF. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 5.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 7.Dudai Y. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 8.Frey U, Morris RG. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 9.Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Nature. 1998;394:680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- 10.Mayford M, et al. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 11.Smeyne RJ, et al. Neuron. 1992;8:13–23. doi: 10.1016/0896-6273(92)90105-m. [DOI] [PubMed] [Google Scholar]
- 12.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 13.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Shi SH, et al. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, et al. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 16.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammen AL, Huganir RL, O'Brien RJ. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archibald K, Perry MJ, Molnar E, Henley JM. Neuropharmacology. 1998;37:1345–1353. doi: 10.1016/s0028-3908(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 19.Adesnik H, Nicoll RA, England PM. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Milanovic S, et al. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 21.Harris KM, Jensen FE, Tsao B. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimchinsky EA, Sabatini BL, Svoboda K. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 23.Myers KM, Davis M. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 24.Leuner B, Falduto J, Shors TJ. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knafo S, Ariav G, Barkai E, Libersat F. Hippocampus. 2004;14:819–825. doi: 10.1002/hipo.10219. [DOI] [PubMed] [Google Scholar]
- 26.Bourne J, Harris KM. Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Hayashi Y, Esteban JA, Malinow R. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 28.McCormack SG, Stornetta RL, Zhu JJ. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. J Neurosci. 2004;24:7015–7023. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.We thank Leon Reijmers for helpful comments. This work was supported by NIH grant MH57368 and MH070020 (to M.M.). N.M. was supported by postdoctoral fellowship from the Uehara Memorial Foundation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Online Material
Materials and Methods
Generation of transgenic mice. For construction of the fos-tTA transgene (S1), the tTA gene was inserted into a plasmid carrying artificial introns and a polyadenylation signal from SV40 (S2) and then subcloned into the AccI site of the first exon of the mouse c-fos gene (S3). To generate the tetO-GFP-GluR1 transgene, EGFP-GluR1 was similarly flanked by introns and an SV40 polyadenylation signal and placed downstream of the tetO promoter element of plasmid pMM400 (S2). The transgenes were excised from vectors and were purified by agarose gel electroelution and Elutip minicolumn. Purified DNA fragments were microinjected (2ng/μl) into BALB/cByJ and C57BL/6J F2 hybrid embryos and implanted into pseudopregnant females. The fos-tTA construct was co-injected with a fos-nlsGFP transgene (c-fos promoter driven EGFP with nuclear localizing signals) and segregated as a single transgene. Genotypes were determined by PCR with genomic DNA isolated from tails using the following primers: htTaF23 (5’-ACCTGGACATGCTGTGATAA-3’), htTaB22 (5’-TGCTCCCATTCATCAGTTCC-3’), MM400F1 (5’-ATAGAAGACACCGGGACCGAT-3’), and GFPR2 (5’-TTCAGCTCGATGCGGTTCAC-3’). The PCR reaction was run at 94 °C for 30sec, 55 °C for 30sec, and 72 °C for 1min for 35 cycles. All transgenic mice were maintained as heterozygotes and were backcrossed to C57BL/6J mice. All mice were kept on a Dox diet (40mg/kg) beginning from the time of weaning (3∼4 weeks old) and used for experiments at 11∼14 weeks old. Only males were used for experiments.
Fear conditioning. Mice were individually housed for 1 week prior to the experiments. For fear conditioning, mice were placed in a novel rectangle chamber with a grid-floor. After a 3 min baseline period, 4 tone-shock pairings were presented. Each pairing consisted of a 20 sec 85 dB 2800 Hz tone simultaneously ending with a 2 sec 0.75 mA shock. There was a 1 min interval between each pairing. The mice remained in the chamber for 1 min after the last shock before being returned to the home cage. The total duration of training was 500 sec. Freezing behavior was scored and analyzed automatically by a video-based system (S1). For the “context-only” condition, the mice were placed in the same fear conditioning chamber for 500 sec and received the same tones but did not receive foot shock.
Unpaired shock training. An immediate shock alone does not induce c-fos expression in the hippocampus, even if mice are exposed to a new context for the same amount of time as fear conditioning training (data not shown, S4, S5). In order to induce expression of c-fos promoter driven GFP-GluR1 in the hippocampus, mice were first placed in a novel chamber (context A: square plastic chamber surrounded with white walls, plastic floor with sani-chips) for 500 sec without foot-shocks. After the context A exposure, they were returned to their home cages. Fifteen min later, they received 4 shocks (2 sec 0.75 mA/shock) immediately after being placed in the conditioning chamber (context B: rectangle chamber, plexiglass front and back with black-and-white cross-stripes pattern and aluminium side walls, grid-floor). There was a 4 sec interval between shocks. After the final shock, they were returned to their home cages.
Contextual memory extinction training. Mice received 3 extinction trials in a day. Mice were exposed to the chamber where fear conditioning was conducted for 30 min for each trial without shocks. After each training, they were returned to their home cages. There was a 2 hour interval between trials.
Immunoblot. Hippocampal lysates (15μg of total protein) were separated by SDS–PAGE and transferred onto Hybond-P membrane. Membranes were blocked with 5% skim milk in TBST for 1 hour and probed with rabbit anti-GluR1 polyclonal antibody or mouse anti-GluR2 monoclonal antibody, followed by peroxidase-conjugated AffiniPure goat anti-rabbit IgG or peroxidase-conjugated AffiniPure donkey anti-mouse IgG. Signals were developed using the ECL PLUS system and exposed to Hyperfilm ECL. Immunoreactive intensities were analyzed with ImageJ software.
Immunoprecipitation. Hippocampi of the GFP-GluR1c-fos Tg mice were prepared 24 hours after fear conditioning. Hippocampi of wild-type and Tg mice were homogenized in lysis buffer (20mM HEPES, pH 7.5, 150mM NaCl, 1% Triton X-100, protease inhibitors cocktail) and incubated for 1 hour at 4 °C. The homogenate was cleared by centrifugation for 10 min at 15,000 g. The supernatant was pre-cleared with protein A agarose, then incubated with rabbit anti-GFP antibody attached to protein A agarose for 2 hours at 4 °C. The precipitated agarose was washed with lysis buffer 5 times and processed for immunoblotting with mouse anti-GluR2 monoclonal antibody.
Immunohistochemistry. Sagittal brain slices were cut at a thickness of 100 μm using a vibratome and fixed with cold 4% paraformaldehyde in PBS for 1 hour. Free-floating slices were permeabilized with 0.15% of TritonX-100 in 5% BSA/PBS at room temperature for 30 min, then incubated with rabbit anti-GFP antibody at 4 °C overnight, rinsed with PBS, and thereafter incubated with goat anti-rabbit Alexa 488 secondary antibody at 4 °C overnight. For visualization of nuclei, slices were counterstained with TO-PRO-3 and mounted in Slowfade Light antifade mounting medium. For surface GFP-GluR1 staining, slices were not permeabilized.
DiI labeling. Micropipettes were coated with DiI (1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate) dissolved in ethanol at 10 mg/ml. The tip of DiI-coated micropipettes was inserted at several positions in the CA1 pyramidal cell layer of fixed slices using a micromanipulator. Slices were then placed in PBS for 3∼4 days at 4 °C to allow the DiI to spread throughout the dendritic arbor.
Acquisition and analysis of spine images. Dendrites in stratum oriens (basal dendrites) and stratum radiatum (apical dendrites) were randomly selected for imaging if they were strongly double-labeled with GFP immunoreactive signal and DiI fluorescence. Primary dendrites were excluded from the analysis. Selected dendrites were imaged using a confocal microscope by sequential illumination with Argon (488 nm) and HeNe (543 nm) laser. Under the condition of laser power and PMT we used, intrinsic GFP fluorescence was too dim to detect. Images were acquired as a series of optical z-sections at 0.4 μm increments using an oil immersion 63× objective (NA 1.4) with a zoom of 2. Each optical section was scanned 3 times and Kalman filtering was employed to reduce noise. The stack images were converted to merged TIFF images of green channel (GFP immunoreactivity) and red channel (DiI fluorescence) for every focal plane using ImageJ software. They were exported to Adobe Photoshop for analysis. GFP-positive spines were defined as the existence of overlapping GFP signals on spines, as marked by yellow in the confocal images. GFP signals were often observed at the head of spines as a punctate signal. Spine morphology and density was analyzed on two-dimensional projection images constructed using a maximum intensity method. Spines were classified according to previously described criteria (S6): the “thin” type has a long neck and a small head; the “stubby” type has a large head but does not have a neck; the “mushroom” type has a large head with a neck. For spine density measurement, all protrusions along 30 μm dendritic segments were counted as spines (For apical dendrites, 58, 53, 55, 56, and 61 segments were examined for UP24h, CT24h, FC24h, FC72h, and FC72h+EXT, respectively. For basal, 55, 53, 52, 51, and 68 segments were examined for UP24h, CT24h, FC24h, FC72h, and FC72h+EXT, respectively). Analysis was performed blind with respect to the experimental conditions.
fig. S1. Induction and trafficking of newly synthesized GFP-GluR1 in the hippocampus of GFP-GluR1c-fos Tg mice. Mice were removed from Dox for 4 days and fear conditioned to examine the earliest time point for GFP-GluR1 induction in the soma and the trafficking rate of induced GFP-GluR1 in dendrites of the CA1 field of the dorsal hippocampus. Mice were examined at 1, 2, and 6 hours following training. Brain slices were fixed, pearmeabilized with 0.15% TritonX-100, then stained with anti-GFP antibody (green) and TO-PRO-3 nuclear marker (blue). Images were acquired using a confocal microscope. At 1 hour after stimulus (FC 1h), GFP-GluR1 expression was slight in the soma and dendritic layer, which is similar to home cage controls (home). At 2 hours (FC 2h), prominent GFP-GluR1 expression was detected in the soma but the dendritic expression was slight and limited to the proximal region. At 6 hours (FC 6h), GFP-GluR1 expression was detected in the soma and throughout the dendritic layer. These results suggest that the GFP-GluR1 is expressed by 2 hours in the soma in response to behavioral stimuli and transported to the distal dendrites within at least 6 hours in CA1 hippocampal neurons of GFP-GluR1c-fos Tg mice.
fig. S2. Metabolic stability of GFP-GluR1 in the hippocampus of GFP-GluR1CaMKIIα Tg mice. (A) Mice in which GFP-GluR1 expression was driven broadly in the hippocampus by a CaMKIIα-tTA transgene (S2) were fed a regular diet without Dox for more than 2 months to induce maximal expression of GFP-GluR1. They were then switched to a Dox diet (6g/kg chow for the first 2 days and then to 40mg/kg) for the period of days indicated to suppress new expression of GFP-GluR1. Sagittal brain slices were cut at a thickness of 100μm and fixed with cold 4% paraformaldehyde in PBS for 1 hour. Images of GFP fluorescence in the dorsal hippocampus were acquired using a fluorescent microscope with the same exposure time. (B) Relative GFP fluorescent intensities (%) in the hippocampus over time after Dox treatment. Data are represented as mean ± s.e.m. (n = 2 for each condition).
fig. S3. Contextual memory retention test after fear conditioning and unpaired-shock training. (A) Behavioral protocol. GFP-GluR1c-fos Tg animals used in the memory test are different animals that are analyzed for GFP-GluR1+ spines in Fig.2 because of the absence of memory test in the experiments of Fig. 2. Mice (n = 7 females, 20∼27 weeks old) were trained with fear conditioning in context B (rectangle chamber, plexiglass front and back with black-and-white cross-stripes pattern and aluminium side walls, grid-floor), then returned to the context B for 3 min for a contextual memory test at 24 hours after training (FC). For unpaired shock training (UP), mice (n = 7 females, 20∼27 weeks old) were first exposed to context A (square plastic chamber surrounded with white walls, plastic floor with sani-chips) for 500 sec without shocks, then received immediate shocks in context B. Twenty-four hours after training, they were returned to the context A, then B for 3 min for memory tests. (B) Percentage of time spent freezing. The UP group showed low levels of freezing for both context A and B, indicating that the unpaired-shock training caused less long-term fear memory associated with context B where shocks were given and context A where c-fos promoter driven GFP-GluR1 expression was induced. Data are represented as mean ± s.e.m.
fig. S4. Visualization of surface GFP-GluR1 in the hippocampal slice. (A) Predicted membrane topology of GFP-GluR1. (B) For surface GFP-GluR1 labeling, sagittal brain slices of GFP-GluR1CaMKIIα Tg mice were fixed with 4% PFA on ice for 1 hour. They were then incubated with rabbit anti-GFP polyclonal antibody, rinsed with PBS, and thereafter incubated with anti-rabbit Alexa594 antibody (red). To detect total GFP-GluR1, the slices were subsequently permeabilized with 0.15% Triton X-100 for 30min, and labeled with rabbit anti-GFP polyclonal antibody and anti-rabbit Alexa488 antibody (green). Confocal hippocampal images were acquired using a confocal microscope. Note that the lack of cytoplasmic labeling in the CA1 pyramidal cells before permeabilization (red), indicating the method labels the plasma membrane surface GFP-GluR1 but not internal receptor. Scale bar, 30μm. (C) To verify the ability of fixed tissue to exclude antiserum until permeabilized, brain slices of GFP-GluR1c-fos Tg mice (FC 24h) were stained with anti-GluR1 polyclonal antibody to a C-terminal epitope with and without permeabilization (0.15% Triton X-100 for 30min). Confocal hippocampal images were acquired with identical condition. Scale bar, 200μm.
References
S1. L. G. Reijmers, B. L. Perkins, N. Matsuo, M. Mayford, Science 317, 1230−1233 (2007).
S2. M. Mayford et al., Science 274, 1678−1683 (1996).
S3. K. Schilling, D. Luk, J. I. Morgan, T. Curran, Proc Natl Acad Sci U S A 88, 5665−5669 (1991).
S4. S. Milanovic et al., Brain Res 784, 37−47 (1998).
S5. N. C. Huff et al., J Neurosci 26, 1616−1623 (2006).
S6. K. M. Harris, F. E. Jensen, B. Tsao, J Neurosci 12, 2685−2705 (1992).