Abstract
For almost two decades, the Th1/Th2 paradigm has offered a productive conceptual framework for investigating the pathogenesis of periodontitis. However, as with many other inflammatory diseases, the observed role of T-cell-mediated immunity in periodontitis did not readily fit this model. A new subset of CD4+ T-cells was recently discovered that explains many of the discrepancies in the classic Th1/Th2 model, and has been termed “Th17” based on its secretion of the novel pro-inflammatory cytokine IL-17. The identification of Th17 cells as a novel effector T-cell population compels re-examination of periodontitis in the context of the new subset and its signature cytokines. This review aims to offer a clarifying insight into periodontal pathogenesis under the extended Th1/Th2/Th17 paradigm, and is predicated on the principle that periodontal disease activity is determined by a complex interplay between the immune system and periodontal pathogens. The re-examination of existing periodontal literature and further studies in the light of these new discoveries may help explain how the inflammatory response results in damage to the periodontium while generally failing to control the pathogens. This knowledge is essential for the development of immunomodulatory intervention strategies for fine-tuning the host response to maximize the protective and minimize the destructive aspects of the periodontal host response. Moreover, with the advent of anti-cytokine biologic drugs that target the Th1 and Th17 pathways in autoimmunity, the potential consequences to periodontal disease susceptibility in humans need to be understood.
Keywords: cytokines, Th1/Th2/Th17, IL-17, periodontal disease
INTRODUCTION
Over two decades ago, Mosmann and Coffman proposed a model wherein T helper (Th) cells are divided into functional subsets on the basis of cytokine secretion, termed Th1 and Th2 (Mosmann et al., 1986). Th1 cells secrete IFNγ, and thereby activate macrophages, NK cells, and CD8+ T-cells (collectively termed “cell-mediated immunity”, or CMI). Th1 cytokines also influence B-cells to secrete opsonizing antibody isotypes that further enhance antigen uptake and presentation to T-cells. Until recently, Th1 cells were also thought to be the major effectors of immunity to extracellular pathogens and autoimmunity. Th2 cells, in contrast, mediate humoral immunity, including production of IgE and activate mast cells, which drive immune responses to helminths. A side-effect of Th2 activation is allergic-type responses, including airway hyper-reactivity. The Th1/Th2 paradigm elegantly explained much of T-cell-mediated immunity; indeed, for 20 years, nearly all diseases were pigeonholed into one or the other category, in many cases regardless of how poorly they actually fit these models (Gor et al., 2003). However, it has long been recognized that there are nagging discrepancies with respect to this model (Gor et al., 2003). In the last few years, the discovery of the new “Th17” subset has resolved many of these controversies, and has simultaneously raised a host of new questions and research directions (reviewed by Steinman, 2007; Weaver et al., 2007; Weaver and Murphy, 2007). The role of Th17 cells and their signature cytokines in periodontal disease is only beginning to be addressed, and the role of Th cells and their specific cytokines in this complex disease will be reviewed here.
REVISING THE Th1/Th2 PARADIGM
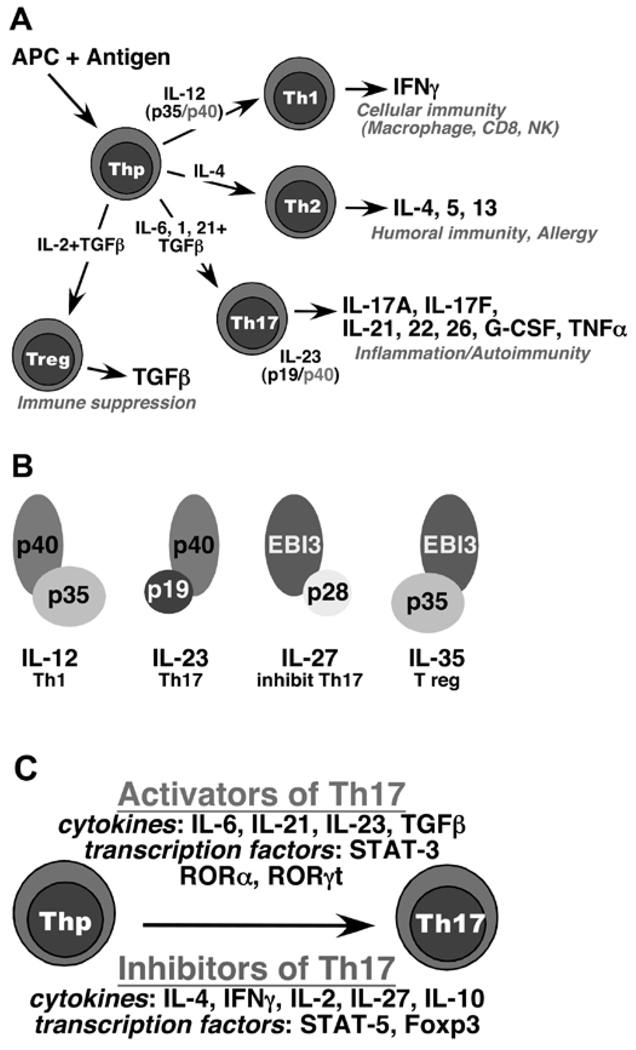
The Th cell differentiation model posits that, while naïve CD4+ T helper cells are committed to a particular antigen by virtue of their specific T-cell receptor (TCR), they are not committed to a particular cytokine-secretion profile. Rather, the invading microbe to which the T-cell is specific, in combination with the antigen-presenting cell, dictates the differentiation of the Th cell into an appropriate mature effector cell, which presumably then mediates an optimal immune response (see “Role of Bacteria as Modulators…”, below). When a naïve CD4+ T-cell is exposed to antigen in the context of IL-12 derived from macrophages or dendritic cells, it is driven to develop into a Th1 phenotype, characterized by the secretion of IFNγ. Other cytokines, such as IL-2, lymphotoxin (TNFβ), and IL-10, have also been called Th1 cytokines, but none defines this lineage as clearly (O’Garra and Vieira, 2007). The Th1 cell uniquely expresses the IL-12 receptor subunit IL-12Rβ2, which further commits a cell to proceed along this differentiation program. This process is dependent on the transcription factor STAT-4, which is activated by IL-12, and also on T-bet, which is considered the master regulator of the Th1 lineage (Glimcher, 2007). Conversely, when a newly activated Th cell is exposed to IL-4, differentiation to a Th2 phenotype occurs. Th2 cells secrete IL-4, IL-5, and IL-13, which are co-regulated transcriptionally. Th2 development is dependent on the transcription factors STAT-6, GATA-3, and c-maf (Yamashita et al., 2007). Th1 and Th2 development is mutually antagonistic and self-reinforcing, in part because IL-4 and IFNγ antagonize one another at cellular and molecular levels (Fig. 1A).
Figure 1.
Th cell differentiation and the extended IL-12 superfamily. (A) The 4 major T helper cell subsets. Naïve CD4+ T-cells are stimulated by antigen in the context of an antigen-presenting cell (APC) to differentiate into specific effector fates. The classic Th1 and Th2 populations are driven by IL-12 (composed of the IL-12p35 and IL-12/23p40 subunits) and IL-4, respectively. The hallmark Th1 cytokine is IFNγ, which activates macrophages, cytotoxic T-cells, and NK cells, as well as driving anti-viral signals in target cells. In addition, IFNγ provides a positive feedback signal to reinforce Th1 development by up-regulating the IL-12 receptor. Signals from Th1 cytokines also inhibit Th2 and Th17 differentiation. IL-4 is the signature cytokine of the Th2 population, and provides an analogous positive reinforcement signal to drive expansion of this lineage. Th17 cells are driven to differentiate by TGFβ, IL-6, IL-1, and IL-21 (reviewed by Weaver et al., 2007). In addition, IL-23 (composed of IL-12/23p40 and IL-23p19) is a key expansion and pathogenicity factor (Ghilardi and Ouyang, 2007). Th17 cells provide important immunity against extracellular pathogens through activation of innate inflammation events, but are also the key Th cell contributors to autoimmunity in various settings (Kolls and Linden, 2004; Gaffen et al., 2006; Kramer and Gaffen, 2007). The immunosuppressive T regulatory (Treg) cell population is driven to develop in opposition to Th17, since signals from TGFβ in the absence of STAT3 (via IL-6 and/or IL-21) serve to drive this lineage. IL-2 is an important cytokine in expanding this lineage, while simultaneously inhibiting Th17 development (Laurence et al., 2007). (B) The extended IL-12 cytokine family. The various components of IL-12, IL-23, IL-27, and the newly-described IL-35 are depicted (Collison et al., 2007; Ghilardi and Ouyang, 2007). (C) Activators and inhibitors of Th17 differentiation. Various cytokines and transcription factors can either enhance or inhibit Th17 development (Weaver et al., 2007).
Despite its seductive simplicity, the Th1/Th2 model does not adequately explain many findings with respect to T-cell immunity. Many cytokines produced by T-cells do not fit obviously into either category. IL-17, for example, was never found to be expressed by Th2 clones and was found only occasionally in cells that also express IFNγ (Aarvak et al., 1999). Moreover, although it was clear that IL-12 was critical for driving a Th1 phenotype, there were major discrepancies between mice with targeted deletions in the IL-12p40 gene and IFNγ-deficient mice. Among many examples, IFNγ-deficient mice are resistant to oral fungal infections and sensitive to collagen-induced arthritis (CIA) and experimental autoimmune encephalomyelitis (EAE) (animal models for rheumatoid arthritis and multiple sclerosis, respectively). In contrast, IL-12-deficient mice are sensitive to yeast and resistant to CIA (Murphy et al., 2003; Langrish et al., 2005; Farah et al., 2006). The basis for this discrepancy was revealed when it became clear that IL-12 is a heterodimeric cytokine composed of two subunits, IL-12p40 and IL-12p35 (Trinchieri et al., 2003) (Fig. 1B). The original “IL-12-deficient” mouse contains a targeted deletion in the p40 subunit. However, IL-12p40 is also a critical subunit in the cytokine IL-23, which has a unique partner, IL-23p19. Thus, IL-12p40-deficient mice lack not only IL-12 but also IL-23, raising the possibility that IL-23 could skew Th development differentially compared with IL-12 (Trinchieri et al., 2003).
Two reports not immediately recognized for their significance started the avalanche that became the first major overhaul of the Th1/Th2 model in two decades. First, Infante-Duarte et al. demonstrated that lipopeptides from Borrelia burgdorferi trigger T-cells to produce IL-17, TNFα, and GM-CSF, cytokines not associated with either the Th1 or the Th2 lineage (Infante-Duarte et al., 2000). These findings were consistent with reports that IL-17 is not made by Th2 or Th1 cells, despite being CD4+ cell-derived (Fossiez et al., 1996; Aarvak et al., 1999). Second, Aggarwal et al. demonstrated that IL-23 stimulates murine CD4+ T-cells to secrete IL-17 following stimulation of the TCR (Aggarwal et al., 2002), and IL-23-deficient mice showed a phenotype similar to that of IL-17-deficient mice with respect to delayed-type hypersensitivity (Nakae et al., 2002; Ghilardi et al., 2004). The suggestion that IL-17-secreting T-cells arise as a specific lineage began as several groups demonstrated that IL-23p19-deficient mice, but not IL-12p35-deficient mice, are resistant to EAE and CIA (Murphy et al., 2003; Langrish et al., 2005). The lineage separation between Th1 and Th17 cells was solidified with findings that IL-17-secreting CD4+ T-cells arise in the absence of Th1- and Th2-induced transcription factors and cytokines (Harrington et al., 2005; Park et al., 2005).
Thus began an explosion of interest in the generation and function of “Th17” lineage that has not shown signs of abating. Although early studies implicated IL-23 in driving Th17 differentiation, it is now clear that TGFβ, IL-6, IL-1, and IL-21 are the key differentiative cytokines, whereas IL-23 is required for Th17 cell expansion, survival, and pathogenicity (Sutton et al., 2006; Jankovic and Trinchieri, 2007; Stockinger and Veldhoen, 2007). This is consistent with the fact that the IL-23R is undetectable in naïve T-cells, but is induced in the Th17 lineage by TGFβ (Mangan et al., 2006). IL-6 and IL-21 signal through STAT-3, which is essential for Th17 differentiation (Chen et al., 2006; Laurence et al., 2007; Zhou et al., 2007). Importantly, Th17 cells produce several cytokines in addition to IL-17, including IL-17F, IL-22, IL-26 (in humans), and IL-21 (Chung et al., 2006; Liang et al., 2006; Wilson et al., 2007) (Fig. 1A). The latter cytokine acts as a positive feedback factor to further reinforce Th17 development, analogous to IFNγ and IL-4 in the Th1 and Th2 systems, respectively (Korn et al., 2007; Nurieva et al., 2007; Zhou et al., 2007). The positive feedback loop mediated by IL-21 is vital in vivo, since Th17 cells develop poorly in IL-21-deficient mice (Nurieva et al., 2007). The key transcription factors driving Th17 differentiation are of the orphan nuclear receptor ROR (retinoic acid receptor) family, including RORγt and RORα (Ivanov et al., 2006; Yang et al., 2008). Finally, it should be pointed out that a subset of Th17 cells produces IFNγ, which develops in several disease states and may express different cell-surface chemokine receptors (Annunziato et al., 2007; Hirota et al., 2007) (Fig. 1C).
Numerous mechanisms are in place to constrain Th17 activity, suggesting its potential for pathogenicity. A new IL-12-family cytokine IL-27 inhibits Th17 development (Stumhofer et al., 2006), and Th1 and Th2 cytokines as well as IL-2/STAT-5 also limit this process (Harrington et al., 2005; Park et al., 2005; Laurence et al., 2007) (Fig. 1C). Another check on Th17 pathogenicity is the production of IL-10, which is negatively controlled by IL-23. Interestingly, Th17 cells generated in vitro in the absence of IL-23 (i.e., with TGFβ and IL-6 alone) fail to mediate pathology in EAE, despite expressing high levels of IL-17 (Jankovic and Trinchieri, 2007; McGeachy et al., 2007). Another potential limit on IL-17-induced pathology relates to IL-17F, an IL-17-family cytokine that is co-expressed in Th17 cells (Akimzhanov et al., 2007). IL-17F binds to the same receptor subunits and mediates qualitatively similar signals as IL-17 (Hymowitz et al., 2001; Kuestner et al., 2007), but its affinity and signaling potency are far weaker. IL-17 and IL-17F form homodimers, and it was recently shown that they also heterodimerize. Not surprisingly, the IL-17A/F heterodimer signals with intermediate potency (Chang and Dong, 2007; Wright et al., 2007). IL-17F is expressed at higher levels than IL-17 (Harrington et al., 2005), and it has been suggested that the dominant form of the cytokine in vivo is the heterodimer (Liang et al., 2007; Wright et al., 2007), although this is not universally accepted. Still, it is possible that expression of IL-17F is another means of mitigating the signaling potency of IL-17 by driving IL-17A/F heterodimers with reduced inflammatory activity.
Curiously, despite these layers of regulation, the activity of IL-17 in vitro is relatively poor compared with strong inflammatory agonists such as TNFα. Many groups have shown only weak activation of NF-κB or NF-κB-dependent genes compared with IL-1β or TNFα (Ruddy et al., 2004b). This paradox may be partly explained by the observation that IL-17 exhibits potent synergy with TNFα and, to a lesser extent, with IL-1 and TLR ligands (Shimada et al., 2002; Granet and Miossec, 2004; Ruddy et al., 2004a,b; Shen et al., 2005, 2006; Maitra et al., 2007). The mechanisms for this are not well-understood and have been reviewed elsewhere (Gaffen et al., 2006; Shen and Gaffen, 2008). Nonetheless, the potent activities of IL-17 in vivo may reflect the enhanced activity of its synergy with partners such as TNFα, rather than the isolated signaling capacity of IL-17 by itself.
Th17 CELLS IN EXPERIMENTAL ANIMAL DISEASE AND IN HUMANS
Even before the discovery of the Th17 lineage, it was evident that IL-17 is an important player in the inflammatory processes that lead to both autoimmunity and host defense (Yu and Gaffen, 2008). IL-17RA-deficient mice are susceptible to a host of infectious diseases, including bacterial, fungal, and parasitic organisms (Kolls and Linden, 2004; reviewed by Gaffen et al., 2006). Mechanistically, this occurs through regulation of neutrophils by IL-17. CXC chemokines such as IL-8, Groα, and GCP-2, as well as ICAM-1 and G-CSF, are major gene targets of IL-17 signaling (Gaffen et al., 2006; Shen and Gaffen, 2008), and, consequently, IL-17RA-deficient mice show deficits in neutrophil migration, expansion, and recruitment (Ye et al., 2001; Yu et al., 2007). Although neutrophils express high levels of the IL-17RA subunit, there is no evidence that IL-17 contributes to neutrophil function directly. IL-17RA-deficient neutrophils migrate normally in transwell assays, and express normal levels of myeloperoxidase and microbial killing (Schwarzenberger et al., 1998; Kelly et al., 2005). The IL-23/IL-17 axis also regulates neutrophil homeostasis in mice, further highlighting the key role for IL-17 in controlling PMN activity (Stark et al., 2005). In fact, recent evidence suggests important roles for PMN and IL-17 in periodontal disease (Oda et al., 2003; Yu et al., 2007), which are discussed below (“Key Cytokines in Periodontal Disease…”) in the context of other important cytokines implicated in periodontitis.
The Th17 paradigm in humans is less well-characterized than in mice, and there is controversy about the precise nature of the Th17 population in humans (Acosta-Rodriguez et al., 2007; Laurence and O’Shea, 2007; Chen and O’Shea, 2008). Thus, a better understanding of the nature and regulation of this T-cell subset is essential to the elucidation of its role in human disease. Current evidence indicates that there are some important differences between the mouse and human systems, since TGFβ drives Th17 differentiation in mice, whereas IL-1 (particularly IL-1α) drives human Th17 differentiation (van Beelen et al., 2007). This may be especially relevant to periodontal disease, since IL-1 is highly elevated in periodontal inflammation (Gemmell et al., 2002). The role of IL-17 in human oral disease has only begun to be investigated, and initial reports indicate that this cytokine is elevated in periodontitis and chronic periapical lesions (Johnson et al., 2004; Takahashi et al., 2005; Vernal et al., 2005; Colic et al., 2007). Moreover, increased IL-17 levels have been detected in individuals with a variety of autoimmune diseases (Kotake et al., 1999; Wong et al., 2000), and a polymorphism in the IL-23 receptor, uniquely expressed on IL-17-secreting T-cells, has been linked to inflammatory bowel disease (Duerr et al., 2006). Consistently, an antibody to IL-12/23p40 is effective in treating Crohn’s disease in humans (Kolls and Zhang, 2005; Zhang et al., 2007). Efforts are under way to test drugs that target the Th17 pathway in humans, which is likely to shed important light on this issue (Kikly et al., 2006; McInnes and Schett, 2007).
PERIODONTAL DISEASE AND CELL-MEDIATED IMMUNITY: AN UNRESOLVED CONTROVERSY
Historical View
Periodontitis is an infection-driven chronic inflammatory disease affecting the integrity of tooth-supporting tissues (Pihlstrom et al., 2005). Subgingival bacterial pathogens are essential for the initiation and progression of the disease, although it is the resulting host reaction that primarily mediates tissue damage (Baker, 2000; Taubman et al., 2005). The periodontal host response is highly complex; it contains both protective and destructive elements, and may be proactively modified by immune-subverting pathogens (Gemmell et al., 2007; Kinane et al., 2007). Despite almost four decades of intensive research, the precise role of the host response in periodontitis and how it can be harnessed therapeutically are far from resolved. In one of the first reports addressing a role for CMI in periodontitis, the authors concluded that “the cell-mediated immune response to some oral micro-organisms may play a protective or aggressive part in the pathogenesis of periodontal disease” (Ivanyi and Lehner, 1970). As is often the case with inflammatory diseases, CMI would be expected to play a pathological role in periodontitis. However, the authors left the door open for a protective function, because suppressed CMI was observed in individuals with advanced periodontitis compared with those with mild disease (Ivanyi and Lehner, 1970). This statement is still relevant, despite a flurry of discoveries and advancements in our understanding of T-cell subsets and their cytokines, as well as the innate immune mechanisms involved in their regulation.
Early immunohistological studies in humans established that the early periodontal lesion (gingivitis) is characterized by increased numbers of T-cells and macrophages, whereas the progressive lesion (leading to periodontitis) is associated with increased infiltration by B-cells and plasma cells (Page and Schroeder, 1976; reviewed by Gemmell et al., 2007). Moreover, the suppressed potential for the induction of CMI in advanced periodontitis is reversed after periodontal treatment (Evans et al., 1989). It was thus proposed that periodontal bacteria play an active role in suppressing CMI, thereby inducing the transition from an early/stable lesion to a progressive/advanced lesion.
Interplay between Host Defenses and Periodontal Organisms
Nevertheless, numerous subsequent investigations have described both protective and destructive effects mediated by CMI in periodontal disease (reviewed by Gemmell et al., 2002). It is not clear whether these findings represent a genuine controversy or whether CMI displays disparate roles depending on context. This contextual contingency may be related both to the genetic background of the host as well as the type of colonizing bacteria. It is also likely that, given recent discoveries of pattern-recognition receptors (PRR) and the Th17 subset, our incomplete understanding of basic host immune mechanisms created an apparent controversy that can be explained in light of new insights.
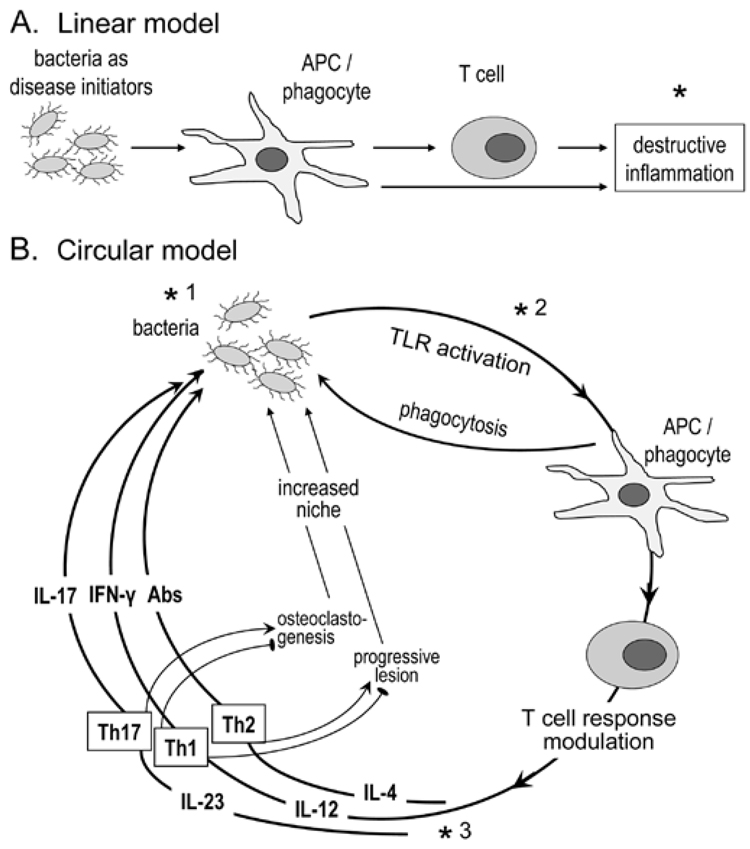
There is only limited consensus regarding the nature of Th cells that predominate in inflamed gingival tissue, a fundamental issue if we are to understand the pathogenesis of periodontal disease and develop rational therapeutics. Specifically, the helper functions of T-cells predominating in early periodontal lesions may determine how the disease develops—i.e., whether it would remain stable or progress to advanced stages clinically manifested as periodontitis. This review aims to offer a clarifying insight into these important questions, and is predicated on the principle that periodontal disease activity is determined by a complex interplay between the immune system and periodontal pathogens. Placing disproportionate emphasis on either ‘player’ (i.e., that periodontitis is simply an aberrant inflammatory response, or that tissue damage is caused exclusively by virulence factors of invading pathogens) would lead to biased and probably erroneous conclusions. In this regard, the periodontal host-pathogen relationship is best described by a circular rather than a linear model (Fig. 2). The latter would be appropriate if bacteria were simply to initiate disease and then have no further role to play, allowing the host inflammatory response to act independently in causing tissue damage. According to the circular model, disease progression is determined by continuous cross-talk between pathogens and the host. The skewing and magnitude of the host response are shaped by periodontal pathogens, which trigger a non-resolving inflammation that is ineffective in controlling the infection. This inflammation in turn provides the bacteria with nutrients and new niches for colonization through the generation of deep periodontal pockets. We maintain that the key to understanding the periodontal immunopathological processes is to explain how host immune responses result in damage to the periodontium while failing to eliminate or appropriately suppress periodontal pathogens.
Figure 2.
Concepts in periodontal disease pathogenesis. In the linear model (A), the bacteria are seen as initiators of the inflammatory process. Disease progression depends exclusively on the host response, which offers a target (*) for therapeutic intervention (e.g., anti-inflammatory agents) (Salvi and Lang, 2005). The circular model (B) contends that bacteria are necessary for both initiation and progression of the disease, by constantly shaping the T-cell response through differential TLR-mediated activation of APC and secreted cytokines. The innate and adaptive host response, in turn, determines the fate of the infection, and consequently whether inflammation will progress to disease or be controlled. Although the role of the individual effector Th subsets is debatable, Th2 cells have been associated with non-protective antibody responses and progressive periodontal lesions, and Th1 with stable lesions (indicated by an inhibitory sign against disease progression;  ) (Gemmell et al., 2002). Th1 cells are thought to protect through IL-12/IFNγ-stimulated cell-mediated immunity (Alayan et al., 2007b; Hajishengallis et al., 2007) and by inhibition of osteoclastogenesis (Gowen et al., 1986; Horwood et al., 2001). In contrast, Th17 cells have been implicated as a specialized osteoclastogenic subset that links T-cell activation to bone loss (Sato et al., 2006). Contrary to this potentially destructive role, IL-17 produced by this subset has been associated with neutrophil-mediated control of the bacterial challenge (Yu et al., 2007). Periodontal bone loss and soft-tissue alterations offer new niches for colonization (generation of deep periodontal pockets) and thus facilitate bacterial overgrowth; this presents increased challenge and reinforces the circular process or, metaphorically, the ongoing vicious cycle. This model offers three types of targets for therapeutic intervention: antimicrobial (*1), TLR-based modulation of APC responses (*2), and cytokine-based control of T-cell activation (*3).
) (Gemmell et al., 2002). Th1 cells are thought to protect through IL-12/IFNγ-stimulated cell-mediated immunity (Alayan et al., 2007b; Hajishengallis et al., 2007) and by inhibition of osteoclastogenesis (Gowen et al., 1986; Horwood et al., 2001). In contrast, Th17 cells have been implicated as a specialized osteoclastogenic subset that links T-cell activation to bone loss (Sato et al., 2006). Contrary to this potentially destructive role, IL-17 produced by this subset has been associated with neutrophil-mediated control of the bacterial challenge (Yu et al., 2007). Periodontal bone loss and soft-tissue alterations offer new niches for colonization (generation of deep periodontal pockets) and thus facilitate bacterial overgrowth; this presents increased challenge and reinforces the circular process or, metaphorically, the ongoing vicious cycle. This model offers three types of targets for therapeutic intervention: antimicrobial (*1), TLR-based modulation of APC responses (*2), and cytokine-based control of T-cell activation (*3).
Limitations of the Th1/Th2 Paradigm for Explaining Periodontal Disease Pathogenesis
With regard to potentially harmful responses, Th1 cells have often been implicated in inflammatory diseases, whereas Th2 cells have been implicated in allergic reactions (Lucey et al., 1996). However, following the discovery of the Th17 subset, the role of Th1 in destructive inflammation has been questioned in at least some diseases (Cua et al., 2003; Sato et al., 2006). A plethora of human studies supports the notion that Th1 cells and their cytokines predominate in early/stable periodontal lesions, while Th2 cells are associated with disease progression, consistent with the B-cell nature of the progressive lesion stage (Manhart et al., 1994; Aoyagi et al., 1995; Tokoro et al., 1997; Sigusch et al., 1998; Bartova et al., 2000; Lappin et al., 2001). A synthesis of these studies resulted in a hypothesis according to which a predominant Th2-type response in the periodontium predisposes to susceptibility to disease progression (Gemmell et al., 2002). This is based partly on the inability of Th2 cells to support IFNγ-mediated innate immunity for effective control of the infection. Moreover, Th2 cell responses provide the cytokines required for B-cell proliferation and polyclonal B-cell activation (facilitated by the presence of bacterial LPS), leading to elevated levels of low-affinity, non-protective antibodies and the ongoing production of IL-1β that contributes to bone resorption (Gemmell et al., 2002). However, the “protective Th1/destructive Th2” model is disputed by some studies undertaken to determine the Th1 vs. Th2 role in human periodontitis. Some studies found that the expression of Th1-type cytokines predominates over that of Th2-type cytokines in diseased periodontal tissue, indirectly suggesting Th1 involvement in the disease (Takeichi et al., 2000; Ukai et al., 2001). According to other studies, lesions in advanced periodontitis seem to be characterized by a comparable presence of both Th1 and Th2 cytokines (Fujihashi et al., 1996; Prabhu et al., 1996; Berglundh et al., 2002).
To clarify the role of Th1 vs. Th2, it is vital that one consider data from animal model research, which is a valid and powerful tool for testing mechanistic hypotheses that cannot be addressed in humans (reviewed by Graves et al., 2008). Although early studies in the mouse oral gavage model of periodontitis did not specifically examine Th1 vs. Th2 roles, those findings suggested a prominent participation of lymphocytes in periodontal tissue destruction. Severe combined immunodeficient (SCID) mice, which lack both T- and B-cells, are substantially more resistant to P. gingivalis-induced periodontal bone loss than are immunocompetent mice (Baker et al., 1994). Mice deficient in MHC class II-restricted CD4+ T-cells also display greater resistance to P. gingivalis-induced bone loss than do normal mice or mice deficient in MHC class I-restricted CD8+ T-cells (Baker et al., 1999). Furthermore, adoptive transfer of A. actinomycetemcomitans-specific B-cells into rats infected with the same pathogen leads to increased periodontal bone resorption (Harada et al., 2006).
More recent studies have identified a mixed Th1/Th2 response pattern in diseased periodontal tissues of mice infected with human periodontal pathogens (Teng, 2002; Garlet et al., 2006). Intriguingly, kinetic analysis showed an initial predominance of Th1-type cytokines (IFNγ, IL-12), followed by their decline and a rise in Th2-type cytokines (IL-4) at later stages of infection (Garlet et al., 2006). These mouse model findings are reminiscent of the Th1-to-Th2 transition described by the “Seymour hypothesis” in human periodontitis (Gemmell et al., 2002) and are in line with findings that Th1 signature cytokines (IFNγ and IL-12) are negatively correlated with human periodontal disease activity (Johnson and Serio, 2005). It thus appears that there is supportive, but not conclusive, evidence that inability to sustain a Th1 response may lead to disease progression (Fig. 2B).
In general, the Th1/Th2 paradigm has offered a productive conceptual framework for investigating the pathogenesis of periodontitis. However, the discovery of the Th17 population compels re-examination of periodontal disease in this context (see also “Key Cytokines in Periodontal Disease…”, below). Interestingly, cytokines characteristic of this subset have been found in inflamed periodontal tissue, suggesting a potential role in pathogenesis (Johnson et al., 2004; Takahashi et al., 2005; Vernal et al., 2005; Lester et al., 2007). IL-17 regulates matrix metalloproteinases and inflammatory cytokines in gingival fibroblasts (Beklen et al., 2007), and P. gingivalis can stimulate IL-17 production from T-cells in vitro (Oda et al., 2003). Moreover, Th17 cell development is inhibited by Th1 and Th2 cytokines, but is promoted by TGFβ along with IL-6 and IL-21 (Fig. 1). TGFβ mediates the suppressive activity of Tregs against both Th1 and Th2 cells (Bettelli et al., 2006; Romagnani, 2006), whereas IL-6 contributes to the alveolar bone loss induced by P. gingivalis (Baker et al., 1999). Strikingly, IL-17R-deficient mice are more susceptible to alveolar bone loss induced by P. gingivalis (Yu et al., 2007), suggesting a protective role for this cytokine. However, in many cases, inflammatory cytokines may “switch sides”, depending on the state of disease (e.g., acute vs. chronic; see below) (O’Shea et al., 2002), which may be the case for IL-17. The question as to the influence of the local periodontal milieu on the differentiation and regulation of the three T-cell effector subsets may be challenging to dissect, but is likely to yield insightful information (see “Role of Bacteria as Modulators…”, below).
Intriguingly, prostaglandin E2 (PGE2), which has been strongly associated with periodontal tissue destruction (Heasman et al., 1993; Roberts et al., 2004), inhibits IL-12p35, but enhances IL-23 expression and hence may contribute to Th17 development (Sheibanie et al., 2004). Whether the destructive effects of PGE2 in periodontitis are mediated through Th17 cells is uncertain, but remains a distinct possibility, since IL-17 regulates COX-2 and PGE2 production (Jovanovic et al., 2001; LeGrand et al., 2001). Alternatively or in addition, the destructive effects of PGE2 could be attributed to its ability to inhibit Th1, but favor Th2 development and responses (Harris et al., 2002), consistent with the Seymour model (Gemmell et al., 2002). Therefore, there is compelling evidence, from the above-summarized studies, that the Th1/Th2 paradigm cannot accurately describe periodontal disease independent of the involvement of the novel Th17 subset.
Role of Bacteria as Modulators of the Th1 vs. Th2 vs. Th17 Responses: Pattern Recognition
Antigen-presenting cells, especially dendritic cells (DC), regulate the development of T-cell subsets and thus influence the outcome of T-cell immunity. Inasmuch as Toll-like receptors (TLR) link innate to adaptive immunity, and APCs receive cues from bacterial TLR agonists (Iwasaki and Medzhitov, 2004), it can be reasonably assumed that periodontal bacteria dictate the types of T-cell responses in the periodontium (Fig. 2B).
Experimental evidence indicates that TLR4 agonists (e.g., E. coli LPS) promote the production of Th1-inducing IL-12, in contrast to TLR2 agonists (e.g., bacterial lipopeptides), which instead foster Th2 responses (Re and Strominger, 2001). Indeed, TLR4 activation induces both IL-12/23p40 and IL-12p35 mRNA expression, whereas TLR2 induces IL-12/IL-23p40, but not IL-12p35, mRNA (Goriely et al., 2008). Moreover, unlike TLR4, stimulation of TLR2 does not induce the Th1-recruiting chemokine known as interferon-γ-inducible protein-10 (IP-10) (Re and Strominger, 2001). The notion that TLR4 and TLR2 agonists preferentially favor Th1 and Th2 cell development, respectively, was substantiated by an in vivo study with classic (enterobacterial) LPS and an atypical LPS molecule from P. gingivalis that triggers TLR2 signaling (Pulendran et al., 2001). Whereas E. coli LPS induces a Th1-type response characterized by high levels of IFNγ, but little or no IL-4, IL-13, or IL-5, P. gingivalis LPS induces a predominantly Th2-like response, with abundant IL-13, IL-5, and IL-10, but relatively low IFNγ levels (Pulendran et al., 2001). In fact, P. gingivalis expresses a heterogeneous mixture of LPS molecules, including species that weakly stimulate TLR4, but potently antagonize TLR4 activation by strong agonists (Darveau et al., 2002; Hajishengallis et al., 2002). Another virulence factor of P. gingivalis, its cell-surface fimbriae, selectively inhibits IL-12p70 production by TLR4 agonists through CD11b/CD18-mediated activation of extracellular signal-regulated kinase 1/2 (ERK1/2) (Hajishengallis et al., 2007). Consistently, P. gingivalis cells predominantly activate TLR2 in vitro and in vivo (Yoshimura et al., 2002; Burns et al., 2006; Hajishengallis et al., 2006). Thus, P. gingivalis possesses mechanisms for predominantly inducing TLR2 activation, which in turn may skew the host response toward Th2.
Although it is uncertain whether a Th2 response is beneficial for this pathogen, inhibition of Th1 cytokines (IFNγ and IL-12p70) promotes its in vivo survival and virulence (Hajishengallis et al., 2007). In contrast, Aggregatibacter actinomycetemcomitans expresses a TLR4 agonistic LPS that induces IL-12 and IFNγ, but not IL-4, suggesting a potential for skewing T-cell responses toward Th1 (Kikuchi et al., 2004). On the basis of the above discussion, induction of Th1 responses appears counterproductive for establishing infection. However, unlike P. gingivalis, A. actinomycetemcomitans may overcome CMI through the expression of several toxins (e.g., leukotoxin and cytolethal distending toxin) that target immune cells at the site of infection (Kinane et al., 2007). With regard to Th17, TLR4 also drives Th17 responses in both infectious and autoimmune settings (Happel et al., 2003; Abdollahi-Roodsaz et al., 2008), and P. gingivalis antigens appear to stimulate T-cells preferentially in vitro to produce IL-17 (Oda et al., 2003). Accordingly, contextual stimulation may still be critical, and further work needs to be done to determine how stimulation of pattern-recognition receptors by periodontal pathogens shapes the T-cell response.
Mechanistically, the property of TLR2 agonists to yield a Th2 bias has been attributed to the sustained activation of ERK1/2, which leads to stabilization of the transcription factor c-Fos, which in turn suppresses production of IL-12p70 (Agrawal et al., 2003). That study, however, did not specify if IL-12p70 suppression was due to inhibition of expression of the IL-12p35 or the IL-12/23p40 subunit (or both). Thus, it could not be predicted whether TLR2 agonists could also suppress induction of IL-23. Far from this possibility, however, the TLR2 agonist peptidoglycan readily induces IL-12/23p40 and IL-23p19 expression and, consequently, IL-23 production (Carmody et al., 2007). Paradoxically, another TLR2 agonist, the Pam3CysSerLys4 lipopeptide, is relatively weak in inducing IL-23p19 (Carmody et al., 2007). One possibility is that maximal induction of IL-23p19 requires TLR2/6 cooperative signaling (activated in response to peptidoglycan), rather than TLR2/1 signaling (activated by Pam3CysSerLys4). Alternatively, this distinction may be due to the ability of peptidoglycan to activate cytosolic pattern-recognition receptors, such as nucleotide-binding oligomerization domain-2 (Nod2) (Girardin et al., 2003). Indeed, Nod2 synergizes with TLR2 for inducing IL-23p19 mRNA (van Beelen et al., 2007), and induction of IL-23 is regulated by both TLR2-dependent and TLR2-independent mechanisms (Yang et al., 2006). In fact, IL-23p19 can be induced by other TLRs, such as TLR4 and TLR9, although LPS (TLR4 agonist) and CpG oligonucleotides (TLR9 agonists) are not as potent as peptidoglycan (Carmody et al., 2007).
IL-27 is another heterodimeric cytokine produced by activated macrophages and dendritic cells and belongs to the IL-12 superfamily of cytokines (Fig. 1B). IL-27 is composed of p28 and Epstein-Barr-virus-induced gene 3 (EBI3) subunits, which display homology with IL-12p40 and IL-12p35, respectively (Pflanz et al., 2002) (Fig. 1B). Although TLR2 activation induces EBI3 via MyD88-dependent signaling, it fails to induce p28 that is under the control of the TRIF/IRF3 (TIR-domain-containing adapter/inducing interferon regulatory factor-3) pathway. In contrast, activation of TLR4, which signals through both MyD88 and TRIF, leads to expression of both constituent subunits and production of IL-27 (Goriely et al., 2008). Interestingly, IL-27 appears to regulate the balance between Th1 and Th17, by limiting Th17-cell development in favor of Th1 (Weaver et al., 2007). Recently, a heterodimer of IL-12p35 and EBI3 has also been described that promotes Treg function, further cementing the role of the extended IL-12 cytokine family in controlling the balance between tolerance and inflammation (Collison et al., 2007) (Fig. 1B). Strikingly, although P. gingivalis LPS is considerably weaker than E. coli LPS in the induction of most pro-inflammatory mediators (Hajishengallis et al., 2002), it is significantly more potent in inducing PGE2 (Noguchi et al., 1996), which promotes IL-23, IL-12 and IL-27 production (Sheibanie et al., 2007).
Finally, other types of PRRs, such as dectins, NODs, and mannose receptors, may cooperate with TLRs to influence the overall shape of the host immune response. Dectin-1, for example, has been suggested to drive a predominantly Th17 response following systemic Candida albicans infection in mice (Taylor et al., 2007; Willment and Brown, 2008). In Staphylococcus aureus infection, cooperativity between mannose-binding lectins and TLRs appears to be crucial for an effective response (Ip et al., 2008). Thus, the net response to a particular micro-organism is the combinatorial activation of various innate pattern-recognition receptors, allowing for a nuanced adaptive response.
Key Cytokines in Periodontal Disease: Role of IL-12, IL-10, and IL-17
Although individual PRR ligands in various periodontal pathogens have the potential to modulate the T-cell response, the pathogenesis of periodontitis might be too complex to be resolved in terms of starkly differential roles by distinct T-cell subsets. Since this disease is polymicrobial, pathogens may modulate the T-cell response to promote their own adaptive fitness, and the net immune response is a mélange of immune responses mediated by all the microbes represented in the biofilm. Therefore, it may not be possible to dissect dominant Th1, Th2, or Th17 activity patterns reliably among collected samples of diseased periodontal tissue. Accordingly, it might be simpler and more productive to consider the roles of individual Th1, Th2, or Th17 cytokines in periodontal infection.
IL-12
IL-12p70 plays a key role in mediating bacterial clearance through the induction of IFNγ, which in turn activates the bactericidal function of macrophages (Trinchieri et al., 2003). The importance of IL-12 against infection is attested to by the fact that several pathogens have developed mechanisms for IL-12 suppression (Karp et al., 1996; Marth and Kelsall, 1997; Matsunaga et al., 2003). Several mechanistic animal studies have examined the role of IL-12 in protection against periodontal pathogens. IL-12p40(−/−) mice exhibit a reduced inflammatory cell infiltrate, but increased tissue destruction, upon subcutaneous challenge with P. gingivalis. In contrast, mice deficient in Th2 cytokines, such as IL-4 or IL-10, did not display increased susceptibility to P. gingivalis-induced tissue destruction (Alayan et al., 2007a). Moreover, IL-12p40(−/−) mice are more susceptible to naturally occurring murine periodontitis than are wild-type controls (Alayan et al., 2007b). These findings could be interpreted to mean that IL-12 is important for the immune and inflammatory responses that control P. gingivalis infection. However, since IL-12p40(−/−) mice are also deficient in IL-23, the relative roles of Th1 and Th17 remain unclear from this work. An independent study also implicated IL-12 in clearance of P. gingivalis, in which the protective effect of IL-12 could be reversed by antibodies recognizing the IL-12p35 and IL-12p40 subunits, but not by anti-IL-23p19 antibodies (Hajishengallis et al., 2007). Therefore, there is compelling evidence that control of P. gingivalis infection may be attributed to IL-12 and, presumably, Th1 cells.
Consistent with the above notion, clinical studies indicate an inverse relationship between IL-12p70 and severity of periodontal disease (Ellis et al., 1998; Orozco et al., 2006). Moreover, removal of P. gingivalis by periodontal therapy results in augmentation of monocyte production of IL-12p70 (Fokkema et al., 2003). In another study, IFNγ and IL-12 were negatively correlated with gingival sulcular depth, in contrast to other cytokines (IL-6 and IL-18) that displayed positive correlations and were thus thought to contribute to non-resolving inflammation (Johnson and Serio, 2005). Moreover, it was hypothesized in these studies that periodontal infection and resulting inflammation may persist due to decreased IL-12 levels. In this respect, P. gingivalis possesses immune subversion mechanisms for inhibiting the production of IL-12 and IFNγ in humans and mice (Hajishengallis et al., 2007; Wang et al., 2007) and for degrading human IL-12 (Yun et al., 2001). The potential of P. gingivalis for “stealth assault” is also supported by its ability to induce a more general immunosuppression of CD4+ and CD8+ T-cells in mice (Gemmell et al., 2006).
The role of IL-12 has also been investigated in bacterially induced periapical inflammation in mice (Sasaki et al., 2004a). Although this anaerobic infection involves the dental pulp, it is still an infection-driven inflammatory and immune response that involves the participation of lymphocytes and leads to bone resorption. Both IL-12 and IFNγ are significantly elevated in murine periapical lesions, and it was speculated that these cytokines may play a role in periapical bone destruction (Kawashima and Stashenko, 1999). However, it was later shown that IL-12(−/−) and IFNγ (−/−) mice display infection-induced bone resorption comparable with that of wild-type controls (Sasaki et al., 2004a). Although it was not specified whether the IL-12(−/−) mice used were deficient in p35 or p40, the observation that infusion of mice with recombinant IL-12 resulted in bone resorption similar to that of controls (Sasaki et al., 2004a) supports the concept that IL-12 does not participate in inflammatory bone resorption in that model. In this regard, in contrast to the bone-resorbing effects of IL-1β and TNFα (Graves and Cochran, 2003), IL-12 and IFNγ suppress bone resorption by inhibiting osteoclastogenesis (Gowen and Mundy, 1986; Gowen et al., 1986; Horwood et al., 2001). It is uncertain whether IL-12 or IFNγ had any impact on the bacterial infection per se, since this question was not addressed. The same model was used to determine the role of Th2-type cytokines. It was found that IL-10(−/−) mice, but not IL-4(−/−) mice, exhibit significantly greater infection-induced periapical bone resorption compared with wild-type controls (Sasaki et al., 2000). These very interesting data are more suggestive of an important homeostatic role of IL-10 in destructive inflammation, rather than of a protective role of Th2 cytokines.
IL-10
Similarly to the findings in the periapical bone loss model, IL-10(−/−) mice display increased susceptibility to P. gingivalis-induced periodontal bone loss (Sasaki et al., 2004b), further supporting a role for IL-10 in the control of destructive inflammation. However, mice with an additional deficiency involving IL-12p40, i.e., IL-10(−/−)/IL-12p40(−/−), are rendered resistant to periodontal bone loss in the same experimental model, and so are T-cell/IL-10 double-deficient mice (Sasaki et al., 2008). However, since IL-12p40 is shared between IL-12p70 and IL-23, it is uncertain whether Th1 or Th17 cells mediate periodontal destructive effects in this hyperinflammatory model of IL-10 deficiency. Although IL-17 has been associated with protective effects in experimental periodontitis (Yu et al., 2007), Th17 cells express high levels of additional pro-inflammatory mediators (e.g., TNFα and receptor activator of NF-κB ligand) and function as a specialized osteoclastogenic T-cell subset (Sato et al., 2006) (Fig. 2B). In fact, a role for Th1 cells in inflammatory bone resorption has been ruled out, at least in rheumatoid arthritis, where they actually inhibit osteoclastogenesis (Sato et al., 2006) (Fig. 2B). It seems reasonable to speculate that an optimal balance of pro- and anti-inflammatory cytokines, although hard to define, may be more important for protection against infection-induced destructive inflammation than a strong polarized response on either side. This concept is supported by another study that found that deficiencies in either Th1 (IL-12, IFNγ) or Th2 cytokines (IL-4) resulted in enhanced naturally induced periodontitis (Alayan et al., 2007b). It is probable that periodontal health represents a dynamic state where the activity of pro-inflammatory/antimicrobial cytokines to control infection is optimally balanced by anti-inflammatory mechanisms to prevent unwarranted inflammation. In contrast, when this balance is disrupted, either due to pathogens that dysregulate host defense mechanisms or genetic differences in host immunity, disease progression and tissue destruction may occur. In a recent review, it was hypothesized that the role of T-cells in periodontal disease may not be one of protection or destruction, but rather that T-cells play a homeostatic role in maintaining a balance between host and the biofilm (Gemmell et al., 2007). This concept would include the participation of CD4+CD25+ regulatory T-cells (Tregs), which suppress Th1 and Th2 responses, although their effects on Th17 cells are largely unknown (Romagnani, 2006).
In contrast to a balanced state, a strongly polarized response in any direction may be detrimental to the host. For example, mice pre-treated with IL-12 in alum develop a strong Th1-biased response and exhibit increased periapical bone destruction after P. gingivalis intrapulpal infection compared with controls (Stashenko et al., 2007). Moreover, gingival injection of rats with A. actinomycetemcomitans 29-kDa outer membrane protein and LPS, followed by adoptive transfer of antigen-specific Th1 clone cells, leads to high levels of bone resorption (Kawai et al., 2000). Whereas these elegant studies underscore the potential for destructive inflammation in strongly biased Th1 conditions, they do not necessarily rule out Th1-mediated protection under more balanced, physiological conditions.
IL-17
There is increasing evidence that Th17 cytokines participate in periodontal disease, but whether their dominant role is host-protective or destructive is open for debate (Kramer and Gaffen, 2007). Despite the potential of neutrophils to engage in destructive inflammation (Van Dyke and Serhan, 2003), recent studies support a role for neutrophils as a critical protective component of the periodontal host response. For example, impaired post-phagocytic killing in lysosomal-associated membrane protein-2-deficient neutrophils leads to bacterial overgrowth and dramatic periodontal disease in mice (Beertsen et al., 2008). Similarly, mice deficient in CXCR2, the primary receptor for murine neutrophil-attracting chemokines such as CXCL1 (KC, Groα) and CXCL5 (LIX), show enhanced periodontal bone loss (Yu et al., 2007). In addition, reduced expression of CXCL1 and CXCL5 in IL-17R(−/−) mice correlates with reduced neutrophil recruitment to gingival tissue and increased susceptibility to P. gingivalis-induced alveolar bone loss (Yu et al., 2007). This finding is in contrast to the bone-destructive effects of TNFα and IL-1β, demonstrated by anti-cytokine blockade in a primate model of experimental periodontitis (Assuma et al., 1998). The protective role for IL-17 is consistent with the protective role played by Th17 cells in infectious inflammatory diseases, as opposed to events in sterile inflammatory situations where IL-17 is tissue-destructive (Cua et al., 2003). Indeed, Th17 cells clearly protect against extracellular bacteria and fungi, which are not adequately dealt with by Th1-mediated immunity (Kolls and Linden, 2004). However, since Th17 cells secrete additional cytokines, such as IL-21, IL-22, IL-26 (in humans), and TNFα, their role in periodontitis warrants further investigation.
Conversely, some evidence implicates IL-17 in the destructive phase of periodontal disease (Oda et al., 2003). Another possible destructive role for Th17 cells is supported by recent findings that implicate Th17, rather than Th1, as the specialized osteoclastogenic lymphocyte that links T-cell activation to bone resorption (Sato et al., 2006). Ultimately, studies of periodontitis in humans treated with anti-IL-17 biologics or with IL-23R polymorphisms may provide the final answer as to the role of the “IL-23/IL-17 axis” in humans (Kikly et al., 2006) (see below).
TARGETING Th17 CELLS FOR THERAPY
The increasing success of anti-cytokine biologics has now enabled mechanistic studies of inflammatory cytokines to be undertaken in humans. Drugs targeting TNFα, IL-1β, IL-12p40, and IL-6 were developed before the advent of the Th17 paradigm (Feldmann and Steinman, 2005), and yet they all affect this pathway. Moreover, antibodies or other reagents to neutralize IL-23p19 and IL-17 are in the pipeline (Kikly et al., 2006; Ghilardi and Ouyang, 2007). While these drugs are clearly effective in treating rheumatoid arthritis, Crohn’s disease, and other autoimmune conditions, their impact on common infections such as periodontal disease is poorly defined in humans. However, such studies will shed light on the true nature of the cytokine response in humans as it pertains to these types of infections.
CONCLUDING REMARKS
Several studies in humans and mice support the concept that Th1 cells and their cytokines characterize early/stable periodontal lesions, whereas Th2 cells are associated with disease progression, consistent with the B-ell nature of the progressive lesion. Other studies, however, have attributed destructive effects to Th1 cells. We presented evidence from recent literature indicating that periodontal disease is not adequately describable in simple Th1 vs. Th2 dichotomous terms. In this regard, the discovery of the Th17 subset may lead to a more nuanced understanding of host-pathogen interactions in the periodontium. The implication of Th1 cells in destructive inflammation in various diseases should be re-interpreted, since the presence or involvement of IL-12, a signature Th1 cytokine, was assessed by evaluation of the IL-12/23 common p40 subunit, and hence involvement of the Th17 subset cannot be ruled out. Current approaches in clinical periodontal practice focus primarily on decreasing bacterial challenge rather than modulating the host response. Under the extended Th1/Th2/Th17 paradigm, it may be feasible to elucidate what constitutes protective vs. destructive host response in periodontitis. It would then be possible to develop therapeutic intervention modalities to maximize the protective and minimize the destructive aspects of the periodontal T-cell responses.
ACKNOWLEDGMENTS
SLG is supported by the NIH (AI49329, AR054389) and the Alliance for Lupus Research. GH is supported by the NIH/NIDCR (DE015254, DE017138, and DE018292).
Abbreviations
- CIA
collagen-induced arthritis
- CMI
cell-mediated immunity
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular signal-regulated kinase
- IL-
interleukin
- IRF
interferon regulatory factor
- KO
knockout
- PGE2
prostaglandin E2
- PPR
pattern-recognition receptor
- ROR
retinoic acid receptor
- TCR
T-cell receptor
- Th
T helper
- TLR
Toll-like receptor
- TRIF
TIR-domain-containing adapter protein-inducing interferon-beta
REFERENCES
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin 23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin 17. J Biol Chem. 2002;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling at IL17-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- Alayan J, Gemmell E, Ford P, Hamlet S, Bird PS, Ivanovski S, et al. The role of cytokines in a Porphyromonas gingivalis-induced murine abscess model. Oral Microbiol Immunol. 2007a;22:304–312. doi: 10.1111/j.1399-302X.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Alayan J, Ivanovski S, Farah CS. Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodontal Res. 2007b;42:97–103. doi: 10.1111/j.1600-0765.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T, Sugawara-Aoyagi M, Yamazaki K, Hara K. Interleukin 4 (IL-4) and IL-6-producing memory T-cells in peripheral blood and gingival tissue in periodontitis patients with high serum antibody titers to Porphyromonas gingivalis. Oral Microbiol Immunol. 1995;10:304–310. doi: 10.1111/j.1399-302x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Baker P. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova J, Kratka-Opatrna Z, Prochazkova J, Krejsa O, Duskova J, Mrklas L, et al. Th1 and Th2 cytokine profile in patients with early onset periodontitis and their healthy siblings. Mediators Inflamm. 2000;9:115–120. doi: 10.1080/096293500411587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beertsen W, Willenborg M, Everts V, Zirogianni A, Podschun R, Schroder B, et al. Impaired phagosomal maturation in neutrophils leads to periodontitis in lysosomal-associated membrane protein-2 knockout mice. J Immunol. 2008;180:475–482. doi: 10.4049/jimmunol.180.1.475. [DOI] [PubMed] [Google Scholar]
- Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Liljenberg B, Lindhe J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol. 2002;29:705–709. doi: 10.1034/j.1600-051x.2002.290807.x. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Liou HC, Chen YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J Immunol. 2007;178:186–191. doi: 10.4049/jimmunol.178.1.186. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Chen Z, O’Shea JJ. Regulation of IL-17 production in human lymphocytes. Cytokine. 2008;41:71–78. doi: 10.1016/j.cyto.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- Colic M, Vasilijic S, Gazivoda D, Vucevic D, Marjanovic M, Lukic A. Interleukin-17 plays a role in exacerbation of inflammation within chronic periapical lesions. Eur J Oral Sci. 2007;115:315–320. doi: 10.1111/j.1600-0722.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Arbabi S, Garcia I, Bainbridge B, Maier RV. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect Immun. 2002;70:1867–1873. doi: 10.1128/IAI.70.4.1867-1873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998;27:101–105. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Evans RI, Mikulecky M, Seymour GJ. Effect of initial treatment of chronic inflammatory periodontal disease in adults on spontaneous peripheral blood lymphocyte proliferation. J Clin Periodontol. 1989;16:271–277. doi: 10.1111/j.1600-051x.1989.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Farah C, Hu Y, Riminton S, Ashman R. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene targeting. Oral Microbiol Immunol. 2006;21:252–255. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- Fokkema SJ, Loos BG, de Slegte C, Burger W, Piscaer M, Ijzerman Y, et al. Increased release of IL-12p70 by monocytes after periodontal therapy. J Clin Periodontol. 2003;30:1091–1096. doi: 10.1046/j.0303-6979.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. Selected Th1 and Th2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin Exp Immunol. 1996;103:422–428. doi: 10.1111/j.1365-2249.1996.tb08297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. In: Litwack G, editor. Vitamins and hormones. London: Academic Press; 2006. pp. 255–282. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13:17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- Gemmell E, Drysdale KE, Seymour GJ. Gene expression in splenic CD4 and CD8 cells from BALB/c mice immunized with Porphyromonas gingivalis. J Periodontol. 2006;77:622–633. doi: 10.1902/jop.2006.050211. [DOI] [PubMed] [Google Scholar]
- Gemmell E, Yamazaki K, Seymour G. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ouyang W. Targeting the development and effector functions of Th17 cells. Semin Immunol. 2007;19:383–393. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney A, de Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- Glimcher L. Trawling for treasure: tales of t-bet. Nat Immunol. 2007;8:448–450. doi: 10.1038/ni0507-448. [DOI] [PubMed] [Google Scholar]
- Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- Gowen M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986;136:2478–2482. [PubMed] [Google Scholar]
- Gowen M, Nedwin GE, Mundy GR. Preferential inhibition of cytokine-stimulated bone resorption by recombinant interferon gamma. J Bone Miner Res. 1986;1:469–474. doi: 10.1002/jbmr.5650010511. [DOI] [PubMed] [Google Scholar]
- Granet C, Miossec P. Combination of the pro-inflammatory cytokines IL-1, TNF-alpha and IL-17 leads to enhanced expression and additional recruitment of AP-1 family members, Egr-1 and NF-kappaB in osteoblast-like cells. Cytokine. 2004;26:169–177. doi: 10.1016/j.cyto.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models of investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Martin M, Schifferle RE, Genco RJ. Counteracting interactions between lipopolysaccharide molecules with differential activation of Toll-like receptors. Infect Immun. 2002;70:6658–6664. doi: 10.1128/IAI.70.12.6658-6664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S, Ratti P, Schifferle RE, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, et al. Cutting edge: Roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Han X, Yamashita K, Kawai T, Eastcott JW, Smith DJ, et al. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J Periodontal Res. 2006;41:101–107. doi: 10.1111/j.1600-0765.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Heasman PA, Benn DK, Kelly PJ, Seymour RA, Aitken D. The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease. J Clin Periodontol. 1993;20:457–464. doi: 10.1111/j.1600-051x.1993.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001;166:4915–4921. doi: 10.4049/jimmunol.166.8.4915. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–181. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammaT directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Ivanyi L, Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970;15:1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Trinchieri G. IL-10 or not IL10: That is the question. Nat Immunol. 2007;8:1281–1283. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Serio FG. Interleukin-18 concentrations and the pathogenesis of periodontal disease. J Periodontol. 2005;76:785–790. doi: 10.1902/jop.2005.76.5.785. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Reboul P, He Y, Jolicoeur FC, et al. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J Rheumatol. 2001;28:712–718. [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. published erratum in Science 275 :1053, 1997. [DOI] [PubMed] [Google Scholar]
- Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Stashenko P. Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch Oral Biol. 1999;44:55–66. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun. 2004;72:5089–5096. doi: 10.1128/IAI.72.9.5089-5096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Demuth DR, Gorr SU, Hajishengallis GN, Martin MH. Human variability in innate immunity. Periodontol 2000. 2007;45:14–34. doi: 10.1111/j.1600-0757.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Zhang Z. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2005;352:627–628. [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Gaffen S. Interleukin-17: a new paradigm in inflammation, autoimmunity and therapy. J Periodontol. 2007;78:1083–1093. doi: 10.1902/jop.2007.060392. [DOI] [PubMed] [Google Scholar]
- Kuestner R, Taft D, Haran A, Brandt C, Brender T, Lum K, et al. Identification of the IL-17 receptor related molecule, IL-17RC, as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin DF, MacLeod CP, Kerr A, Mitchell T, Kinane DF. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin Exp Immunol. 2001;123:294–300. doi: 10.1046/j.1365-2249.2001.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, O’Shea J. Th-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78:1545–1550. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci USA. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Manhart SS, Reinhardt RA, Payne JB, Seymour GJ, Gemmell E, Dyer JK, et al. Gingival cell IL-2 and IL-4 in early-onset periodontitis. J Periodontol. 1994;65:807–813. doi: 10.1902/jop.1994.65.9.807. [DOI] [PubMed] [Google Scholar]
- Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Yamaguchi H, Klein TW, Friedman H, Yamamoto Y. Legionella pneumophila suppresses macrophage interleukin-12 production by activating the p42/44 mitogen-activated protein kinase cascade. Infect Immun. 2003;71:6672–6675. doi: 10.1128/IAI.71.11.6672-6675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Shitashige M, Yanai M, Morita I, Nishihara T, Murota S, et al. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18:30–36. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21:256–260. doi: 10.1111/j.1399-302X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Prabhu A, Michalowicz BS, Mathur A. Detection of local and systemic cytokines in adult periodontitis. J Periodontol. 1996;67:515–522. doi: 10.1902/jop.1996.67.5.515. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect Immun. 2004;72:1166–1168. doi: 10.1128/IAI.72.2.1166-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Shen F, Smith J, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004a;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer binding protein family members. J Biol Chem. 2004b;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32 Suppl 6:108–129. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–3630. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Balto K, Kawashima N, Eastcott J, Hoshino K, Akira S, et al. Gamma interferon (IFN-gamma) and IFN-gamma-inducing cytokines interleukin-12 (IL-12) and IL-18 do not augment infection-stimulated bone resorption in vivo. Clin Diagn Lab Immunol. 2004a;11:106–110. doi: 10.1128/CDLI.11.1.106-110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004b;39:432–441. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Suzuki N, Kent R, Jr, Kawashima N, Takeda J, Stashenko P. T cell response mediated by myeloid cell-derived IL-12 is responsible for Porphyromonas gingivalis-induced periodontitis in IL-10-deficient mice. J Immunol. 2008;180:6193–6198. doi: 10.4049/jimmunol.180.9.6193. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861–868. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- Sigusch B, Klinger G, Glockmann E, Simon HU. Early-onset and adult periodontitis associated with abnormal cytokine production by activated T lymphocytes. J Periodontol. 1998;69:1098–1104. doi: 10.1902/jop.1998.69.10.1098. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Stashenko P, Goncalves RB, Lipkin B, Ficarelli A, Sasaki H, Campos-Neto A. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am J Pathol. 2007;170:203–213. doi: 10.2353/ajpath.2007.060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. erratum in Nat Med 13:385, 2007. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76 Suppl 11:2033S–2041S. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YT. Mixed periodontal Th1-Th2 cytokine profile in Actinobacillus actinomycetemcomitans-specific osteoprotegerin ligand (or RANK-L)-mediated alveolar bone destruction in vivo. Infect Immun. 2002;70:5269–5273. doi: 10.1128/IAI.70.9.5269-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol. 1997;107:166–174. doi: 10.1046/j.1365-2249.1997.d01-880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001;46:901–908. doi: 10.1016/s0003-9969(01)00057-7. [DOI] [PubMed] [Google Scholar]
- van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Shakhatreh MA, James D, Liang S, Nishiyama S, Yoshimura F, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Murphy KM. T-cell subsets: the more the merrier. Curr Biol. 2007;17:61–63. doi: 10.1016/j.cub.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Onodera A, Nakayama T. Immune mechanisms of allergic airway disease: regulation by transcription factors. Crit Rev Immunol. 2007;27:539–546. doi: 10.1615/critrevimmunol.v27.i6.40. [DOI] [PubMed] [Google Scholar]
- Yang CS, Song CH, Lee JS, Jung SB, Oh JH, Park J, et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–1171. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human Toll-like receptor 4. Infect Immun. 2002;70:218–225. doi: 10.1128/IAI.70.1.218-225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Ruddy M, Wong G, Sfintescu C, Baker P, Smith J, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun PL, Decarlo AA, Collyer C, Hunter N. Hydrolysis of interleukin-12 by Porphyromonas gingivalis major cysteine proteinases may affect local gamma interferon accumulation and the Th1 or Th2 T-cell phenotype in periodontitis. Infect Immun. 2001;69:5650–5660. doi: 10.1128/IAI.69.9.5650-5660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hinrichs D, Lu H, Chen H, Zhong W, Kolls J. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol. 2007;7:409–416. doi: 10.1016/j.intimp.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]