Abstract
In the elderly, systemic infection is associated with an increased frequency of behavioral and cognitive complications. We have reported that peripheral stimulation of the innate immune system with lipopolysaccharide (LPS) causes an exaggerated neuroinflammatory response and prolonged sickness/depressive-like behaviors in aged BALB/c mice. Therefore, the purpose of this study was to determine the degree to which LPS-induced neuroinflammation was associated with microglia-specific induction of neuroinflammatory mediators. Here, we show that peripheral LPS challenge caused a hyperactive microglial response in the aged brain associated with higher induction of inflammatory IL-1β and anti-inflammatory IL-10. LPS injection caused a marked induction of mRNA expression of both IL-1β and IL-10 in the cortex of aged mice compared to adults. In the next set of studies, microglia (CD11b+/CD45low) were isolated from the brain of adult and aged mice following experimental treatments. An age-dependent increase in major histocompatibility complex (MHC) class II mRNA and protein expression was detected in microglia. Moreover, peripheral LPS injection caused a more pronounced increase in IL-1β, IL-10, Toll-like Receptor (TLR)-2, and indoleamine 2, 3 dioxygenase (IDO) mRNA levels in microglia isolated from aged mice than adults. Intracellular cytokine protein detection confirmed that peripheral LPS caused the highest increase in IL-1β and IL-10 levels in microglia of aged mice. Finally, the most prominent induction of IL-1β was detected in MHC II+ microglia from aged mice. Taken together, these findings provide novel evidence that age-associated priming of microglia plays a central role in exaggerated neuroinflammation induced by activation of the peripheral innate immune system.
Keywords: Aging, Microglia, Neuroinflammation, Cytokines, Lipopolysaccharide, Mice
1. Introduction
Microglia are part of the innate immune system in the central nervous system (CNS) and play an important role in receiving and propagating inflammatory signals in response to activation of the peripheral immune system (Nguyen et al. 2002). Microglia are bone marrow derived myeloid lineage cells (Simard and Rivest 2004), are interspersed throughout the CNS, and represent approximately 10-15% of the total CNS cell population. In the absence of inflammatory stimuli, microglia are quiescent and are involved in immune surveillance (Nimmerjahn et al. 2005). Once activated, microglia have macrophage-like capabilities including phagocytosis, inflammatory cytokine production, and antigen presentation (Garden and Moller 2006). Neuroinflammatory processes are normally transient with microglia returning to a resting state as the immune stimulus is resolved. Aging, however, may maintain a brain environment where microglia are “primed” or “reactive” to a peripheral immune challenge (Godbout and Johnson 2006; Perry et al. 2003).
Markers of glial reactivity or priming, such as major histocompatibility complex (MHC) class II, are increased in the brain during normal aging (Frank et al. 2006a; Godbout et al. 2005; Morgan et al. 1999; Nicolle et al. 2001; Ogura et al. 1994; Perry et al. 1993; Sheffield and Berman 1998; Sloane et al. 1999). Moreover, increased expression of scavenger receptors (Godbout et al. 2005; Wong et al. 2005), integrins (Perry et al. 1993; Stichel and Luebbert 2007), Toll-like-Receptors (Letiembre et al. 2007), and astrocytic markers (Godbout et al. 2005; Lee et al. 2000; Morgan et al. 1999) are also detected in the brain of older rodents. In a BALB/c murine model of aging, we have reported that a consequence of a reactive glial profile is an exaggerated neuroinflammatory cytokine response to either central (Abraham et al. 2008; Huang et al. 2007) or peripheral innate immune challenges (Chen et al. 2008; Godbout et al. 2005; Henry et al. 2008; Huang et al. 2007). In several rodent models of aging, the excessive neuroinflammatory cytokine response is coupled with a myriad of complications including cognitive impairment (Barrientos et al. 2006; Chen et al. 2008), exaggerated sickness behavior (Abraham et al. 2008; Godbout et al. 2005; Huang et al. 2007), and protracted depressive-like behavior (Godbout et al. 2007). Although these data support the notion that microglial hyperactivity plays an integral role in exaggerated neuroinflammation and protracted behavioral changes, microglial specific responses in the BALB/c model of aging have not been determined.
Studies with minocycline, an anti-inflammatory agent and purported microglia inhibitor, (Nikodemova et al. 2007) also provide evidence that microglia contribute to age-associated neuroinflammation. For example, in aged rats minocycline reduces the aged-associated increase in brain MHC II and attenuates the age-related impairments in long-term potentiation (Griffin et al. 2006). Moreover, we recently reported that minocycline normalizes the LPS-induced exaggerated mRNA expression of neuroinflammatory markers including IL-1β, Toll like-receptor (TLR)-2, and indoleamine 2, 3 dioxygenase (IDO) in aged BALB/c mice (Henry et al. 2008). IDO activation is relevant because IDO-dependent tryptophan metabolism is associated with inflammatory-mediated depressive-like behavior (Dantzer et al. 2008; Godbout et al. 2007; O’Connor et al. 2008).
IL-1β, a major neuroinflammatory signal (Allan et al. 2005) that mediates several key physiological and behavioral changes associated with sickness (Kelley et al. 2003), is regulated by IL-10 (Heyen et al. 2000; Pousset et al. 1999). For instance, IL-10 suppresses cytokine mediated sickness behavior (Bluthe et al. 1999) and reverses impaired long term potentiation associated with increased IL-1β expression (Kelly et al. 2003; Lynch et al. 2004). Decreased anti-inflammatory IL-10 regulation of microglia activation during aging (Frank et al. 2006a; Ye and Johnson 2001), may lead to increased glial priming and increased IL-1β expression (Chen et al. 2008; Maher et al. 2004; Murray and Lynch 1998). In support of this notion, LPS treatment of cultured brain slices isolated from aged mice showed exaggerated IL-1β production and reduced IL-10 production compared to adult brain slices (Ye and Johnson 2001).
The present study investigated the degree to which LPS-induced amplified neuroinflammation in aged mice was associated with microglia-specific induction of neuroinflammatory mediators. Because exaggerated neuroinflammation in the aged brain may be related to imbalanced microglia-mediated cytokine production, we focused our attention on inflammatory IL-1β and anti-inflammatory IL-10. Our data show that microglia isolated from aged mice expressed markedly higher MHC II levels than microglia isolated from adults. Moreover, peripheral LPS injection caused exaggerated mRNA expression of both IL-1β and IL-10 in the cortex of aged mice compared to adults. This LPS-stimulated hyper-induction of IL-1β and IL-10 was also detected in microglia isolated from aged mice. Finally, LPS challenge elicited a robust induction of IL-1β in MHC II+ microglia from aged mice.
2. Materials and methods
2.1 Animals
Male BALB/c mice (3-4 and 18-20 month-old) were purchased from the National Institute on Aging specific pathogen free colony (maintained at Charles River Laboratories, Inc., MA). The median lifespan for BALB/c mice is approximately 26 months (Morley and Trainor 2001), so to investigate changes that occur from adulthood to what is considered aged, 3-4-month-old (young adult) and 18-20-month-old (aged) male mice were used. Upon arrival, mice were individually housed in polypropylene cages and maintained at 21° C under a 12 h light: 12 h dark cycle with ad libitum access to water and rodent chow. At the end of each study, mice were examined postmortem for gross signs of disease (e.g., splenomeglia or tumors). Data from mice determined to be unhealthy were excluded from analysis (< 5%). All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Experimental protocols
Adult (3-4 month old) or aged (18-20 month old) male BALB/c mice were injected i.p. with saline or Escherichia coli LPS (0.33 mg/kg; serotype 0127:B8, Sigma, St. Louis, MO) and were euthanized by CO2 asphyxiation 4 (n=8) or 8 h (n=5) later. The LPS dosage was selected because it elicits a pro-inflammatory cytokine response in the brain resulting in mild transient sickness behavior in adult mice (Berg et al. 2004; Godbout et al. 2005; Henry et al. 2008). Brains were dissected and cortex samples were stored at -20°C in RNAlater (Qiagen, CA). Total RNA was isolated from the cortex and assayed using quantitative PCR. Plasma was also collected and stored (-80°C) until assaying.
In the second study, adult (3-4 month old) or aged (18-20 month old) male BALB/c mice were injected i.p. with saline or LPS and were euthanized by CO2 asphyxiation 4 h later. Brains were homogenized and microglia were isolated by discontinuous Percoll density gradient. Cells were used for either flow cytometric analyses (CD45, CD11b, and MHC II staining) or total RNA isolation/quantitative PCR (n=6-8).
In the final study, adult (3-4 month old) or aged (18-20 month old) male BALB/c mice were injected i.p. with saline or LPS and were euthanized by CO2 asphyxiation 4 h later. Brains were homogenized and microglia were isolated by discontinuous Percoll density gradient. Cells were used for flow cytometric analyses (CD11b, MHC II, IL-1β and IL-10 staining, n=3-6).
2.3 Isolation of microglia
Microglia were isolated from whole brain homogenates as described previously (Cardona et al. 2006; Frank et al. 2006a), but with several modifications. Mice were euthanized by CO2 asphyxiation and whole brains were collected. Brains were homogenized in Hank’s Balanced Salt Solution (HBSS), pH 7.4. Resulting homogenates were passed through a 70 μm nylon cell strainer and centrifuged at 500 g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-healthcare, Uppsala, Sweden) at room temperature. A discontinuous Percoll density gradient was set up as follows: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 minutes at 2000 g and microglia were collected from the interphase between the 70% and 50% Percoll layers (Frank et al. 2006a). Cells were washed and then re-suspended in sterile HBSS. The number of viable cells was determined using a hemacytometer and 0.1% trypan blue staining. For each extraction, approximately 6 × 105 viable cells were isolated.
2.4 RNA isolation and qPCR
RNA was isolated from either the cortex or from a microglial population collected by Percoll density gradient. In cortex samples, total RNA was isolated using the Tri-Reagent protocol (Sigma, MO) and subjected to a DNA-free/RNA clean up procedure (Ambion, TX). For Percoll separated cells, RNA was isolated using the RNeasy plus mini kit protocol (Qiagen, CA). In both RNA isolation procedures, RNA concentration was determined by spectrophotometry (Eppendorf, NY) and RNA was reverse transcribed to cDNA using a RT RETROscript kit (Ambion, TX).
Quantitative PCR was performed using the Applied Biosystems (Foster, CA) Assay-on-Demand Gene Expression protocol as previously described (Godbout et al. 2005). In brief, cDNA was amplified by real time PCR where a target cDNA (IL-1β, IL-10, MHC II, TLR2, or IDO) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5’ fluorescent reporter dye (6-FAM) and a 3’ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference.
2.5 Plasma cytokine measurement
Plasma levels of IL-1β and IL-10 were determined by ELISA (R&D Systems, MN). In brief, mice were euthanized by CO2 asphyxiation and blood was collected by cardiac puncture into EDTA coated syringes. Samples were centrifuged (6000 g for 15 min at 4 °C) and plasma was collected and stored frozen (-80°C) until assaying. Plasma samples were assayed for IL-1β and IL-10 according to the manufacturer’s instructions. Absorbance (450 nm) was determined using a Bio-Tek synergy HT microplate reader (Bio-Tek Instruments, VT). Assays were sensitive to 1.5 pg/ml of IL-1β and 10 pg/ml of IL-10, and inter- and intra-assay coefficients of variation were less than 10%.
2.6 Microglial staining and flow cytometry
Cells were assayed for microglial cell surface antigens by flow cytometry as previously described but with a few modifications (Henry et al. 2008; Nair and Bonneau 2006). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, CA). The cells were then incubated with anti-CD11b-APC, anti-CD45-FITC, and anti-MHC-II-PE antibodies (eBioscience, CA). Expression of these surface receptors was determined using a Becton-Dickinson FACSCaliber four color Cytometer. Ten thousand events were recorded and microglia were identified by CD11b+ and CD45low expression (Ford et al. 1995; Nair and Bonneau 2006). For each antibody, gating was determined based on appropriate negative isotype stained controls. Flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
2.7 Intracellular IL-1β and IL-10 detection
Microglial production of IL-1β and IL-10 was assayed by intracellular flow cytometric analysis based on BD Cytofix/Cytoperm Plus fixation/permeabilization protocol (BD biosciences, CA). To block cell secretion of cytokines, cells isolated by Percoll density gradient were incubated in 10% FBS DMEM media containing brefeldin A (BD Biosciences, CA) for 4 h at 37 °C. Next, cells were washed in FACS buffer (2% FBS in HBSS) and then Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, CA). After blocking, cells were stained with anti-CD11b-APC and anti-MHC-II-PE antibodies. Cells were then fixed and permeabilized with BD Fixation/Permeablization buffer for 20 min. Cells were washed with BD Perm/Wash™ buffer, re-suspended in BD Perm/Wash™ buffer, and incubated with either anti-IL-1β-FITC or anti-IL-10-FITC (eBioscience, CA) for 30 min. Cells were washed twice in BD Perm/Wash buffer and resuspended in FACS buffer. Surface and intracellular antigen expression were determined using a Becton-Dickinson FACSCaliber four color Cytometer. Ten thousand events were recorded. For each antibody, gating was determined based on appropriate negative isotype stained controls. Flow data were analyzed using FlowJo software (Tree Star, CA).
2.8 Statistical analysis
To ensure a normal distribution, data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). Observations greater than 3 interquartile ranges from the first and third quartile were considered outliers and were excluded in the subsequent analysis. To determine significant main effects and interactions between main factors, data were analyzed using one- (Age, Treatment) or two- (Age × Treatment) way ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± standard error of the mean (SEM).
3. Results
3.1 Peripheral LPS-induced mRNA expression of IL-1β and IL-10 in the cortex
Peripheral LPS injection induced an exaggerated pro-inflammatory IL-1β response in the aged brain (Chen et al. 2008; Godbout et al. 2005; Henry et al. 2008). Thus, we sought to determine the degree to which brain IL-1β induction was associated with a corresponding reduction in IL-10 expression. To begin to address this question the cortex was used as a representation of the global neuroinflammatory response (Lee et al. 2000) and IL-1β and IL-10 mRNA expression were determined at two time points (4 & 8 h) post peripheral LPS challenge. Figs.1A&B show that i.p. injection of LPS increased IL-1β mRNA expression in the cortex 4 and 8 h post injection (P<0.001, for each). Moreover, LPS-induced IL-1β mRNA expression was significantly higher in aged mice compared to adults at 4 and 8 h. (Figs.1A&B). ANOVA of mRNA expression revealed a significant Age × LPS interaction for IL-1β at 4 h (F(1, 30)= 19.72, P<0.002) and at 8 h (F(1, 19)= 6.8, P<0.02). These data are consistent with our previous studies that peripheral LPS injection caused exaggerated expression of IL-1β in the aged brain (Chen et al. 2008; Godbout et al. 2005; Henry et al. 2008).
Fig 1. Peripheral LPS injection causes exaggerated mRNA expression of IL-1β and IL-10 in the cortex of aged mice compared to adults.
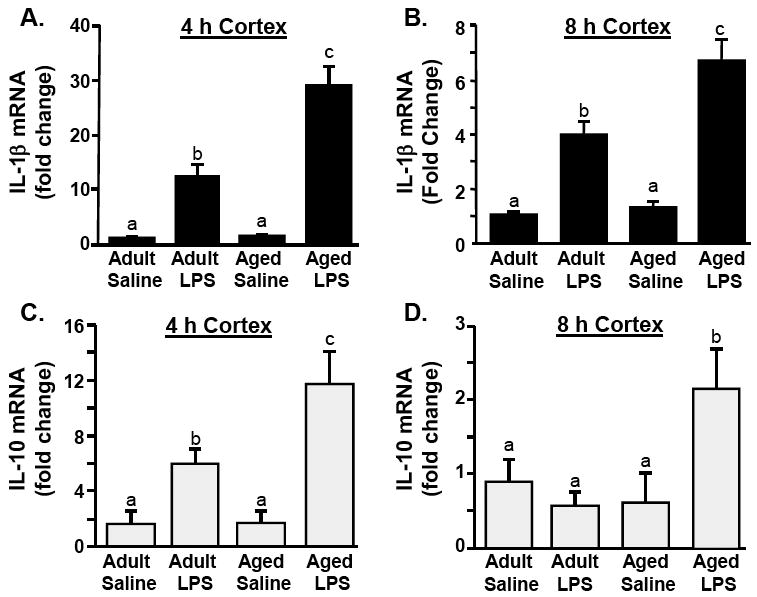
Adult (3-6 m) and aged (18-20 m) mice were challenged with saline or LPS i.p. and IL-1β mRNA levels were determined from the cortex collected A) 4 or B) 8 h later. From the same cortex samples, IL-10 mRNA levels were determined C) 4 or D) 8 h post experimental treatments. Bars represent the mean ± SEM (n=8 for 4 h, n=5 for 8 h). Means with different letters (a, b, or c) are significantly different (P<0.05) from each other.
Figs.1C&D show that i.p. injection of LPS increased IL-10 mRNA expression in the cortex at both time points (main effect of LPS, P<0.001, for each). Moreover, cortex mRNA levels of IL-10 were significantly higher after LPS injection in aged mice compared to adults (Fig.1C&D). ANOVA of mRNA expression revealed a significant Age × LPS interaction for IL-10 at 4 h (F(1, 28) 4.24, P<0.05) and at 8 h (F(1, 19)= 5.94, P<0.02). Taken together, these results indicate that i.p. LPS injection caused elevated and prolonged expression of both IL-1β and IL-10 in the cortex of aged mice.
3.2 LPS-induced circulating levels of IL-1β and IL-10
Because peripheral inflammatory cytokines can influence cytokine production in the CNS (Quan and Banks 2007), we next determined peripheral circulating levels of IL-1β and IL-10. Figs.2A&B show that LPS injection increased plasma levels of IL-1β at 4 (main effect of LPS, F(1,31)= 49.7 P<0.001) and 8 h (F(1,20)= 60.2, P<0.001). These data are consistent with our previous studies showing that LPS-induced plasma IL-1β levels were age-independent (Godbout et al. 2005; Henry et al. 2008). Figs.2C&D show that LPS injection was also associated with an increase in IL-10 at 4h (F(1,31)= 92.4, P<0.001) and at 8 h (F(1,20)= 56.8, P<0.001). Similar to the results for plasma IL-1β, LPS injection increased plasma IL-10 levels at 4 h in an age-independent manner. At 8 h, however, IL-10 levels were higher in LPS treated aged mice than LPS treated adults (Age × LPS interaction, F(1,20)= 6.15, P<0.03). These data indicate that the LPS-associated exaggerated inductions of IL-10 and IL-1β levels in the cortex of aged mice at 4 h (Fig.1) were not paralleled in the plasma.
Fig.2. LPS injection increases IL-1β and IL-10 and in the plasma independent of age.
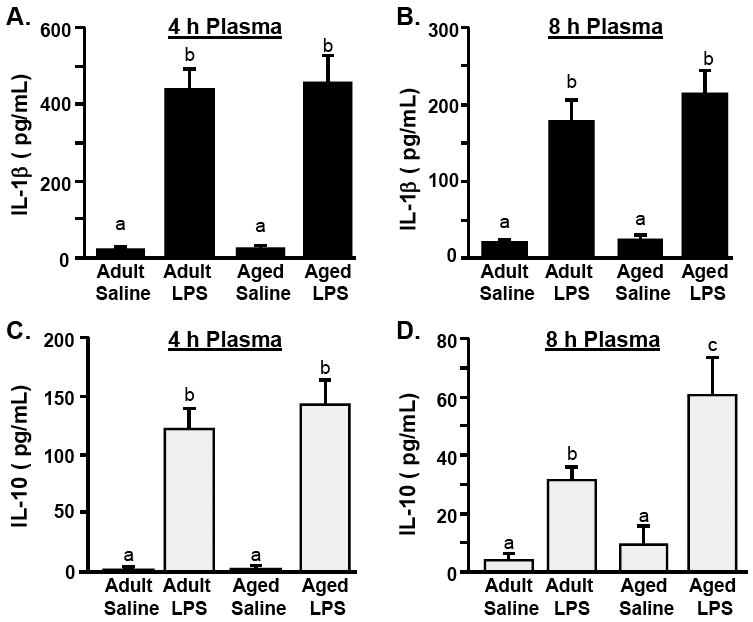
Adult (3-6 m) and aged (18-20 m) mice were challenged with saline or LPS i.p. and IL-1β protein levels were determined from the plasma collected A) 4 h or B) 8 h later. From the same plasma samples, IL-10 protein levels were determined C) 4 or D) 8 h post experimental treatments. Bars represent the mean ± SEM (n=8 for 4 h, n=5 for 8 h). Means with different letters (a, b, or c) are significantly different (P<0.05) from each other.
3.3 MHC II surface expression on microglia
Increased mRNA levels of inflammatory glial markers are detected in the aged brain (Godbout and Johnson 2006). Therefore, we determined MHC II surface expression on microglia isolated from adult and aged mice. To isolate a highly enriched microglial population from the brain we used a Percoll density gradient protocol (Fig.3A). Several groups have reported that this microglia isolation procedure yields a highly enriched population of microglia, that is devoid of astrocytes and macrophages (Cardona et al. 2006; Frank et al. 2006b).
Fig.3. Increased MHC II surface expression on microglia isolated from the brain of aged mice.
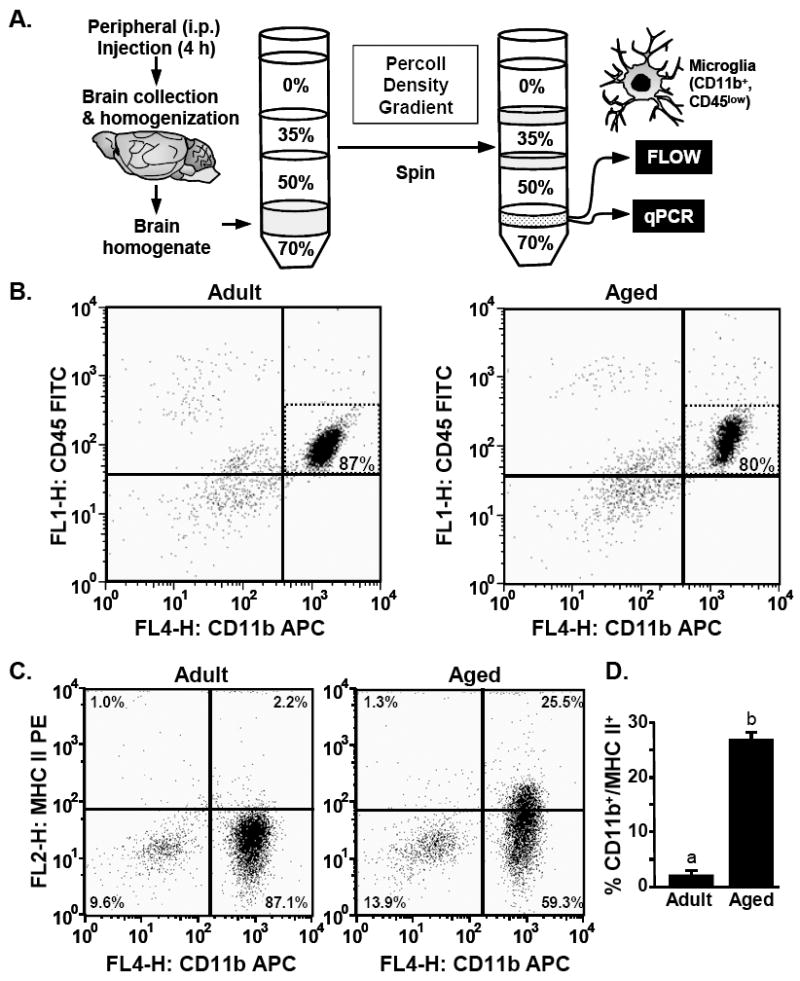
A) Illustration of the experimental protocol used to isolated microglia from adult (3-6 m) and aged (18-20 m) mice by Percoll density gradient. B) Representative bivariate dot plots of Percoll isolated cells stained with anti-CD11b-APC and anti-CD45-FITC. Microglia were identified by CD11b+/CD45low staining. C) Representative bivariate dot plots of Percoll isolated cells stained with anti-CD11b-APC and anti-MHC-II-PE. D) Average percentage of cells that were CD11b+/MHC II+. Bars represent the mean ± SEM (n=8). Means with different letters (a or b) are significantly different (P<0.05) from each other.
Microglia were isolated from adult and aged brains, stained with antibodies for CD11b, CD45 and MHC II, and then analyzed by flow cytometry. The representative bivariate dot plots show that the majority of the viable cells were CD11b+/CD45low in adult and aged mice (Fig.3B). The percentage of CD11b+/CD45low cells isolated from the brain was approximately 87-93% in adult mice and 80-86% in aged mice. In both age groups the isolated cell population contained less than 1% macrophages (CD11b+/CD45high) (Ford et al. 1995; Nair and Bonneau 2006). Based on these staining results, subsequent experiments identified microglia by CD11b+ staining. Collectively, these data indicate that Percoll gradient isolation yielded a highly enriched microglial population.
Fig.3C shows representative bivariate dot plots of CD11b and MHC II staining. The microglia isolated from aged mice had significantly higher MHC II surface expression (CD11b+/MHC II+) compared to the microglia isolated from adults (main effect of Age, F(1,13)= 287, P<0.001). While less than 3% of the microglia isolated from adult mice were MHC II+, approximately 25% of microglia isolated from aged mice were MHC II+ (Fig.3D). Based on these findings, MHC II was used as a marker of primed or reactive microglia in subsequent analysis.
3.4 Peripheral LPS-induced mRNA expression of inflammatory markers in microglia
Based on previous studies we hypothesized that the exaggerated pro-inflammatory response in the aged brain was associated with microglia hyperactivity (Chen et al. 2008; Godbout et al. 2005; Henry et al. 2008; Huang et al. 2007). Therefore, we determined the expression of IL-1β, IL-10, and several microglial inflammatory markers including MHC II (Perry et al. 2003), TLR2 (Nguyen et al. 2004; Nguyen et al. 2002), and IDO (Dantzer et al. 2008; Guillemin et al. 2005), in the enriched microglial population isolated 4 h following peripheral LPS challenge. In the cell layer containing microglia, MHC II mRNA was detected in all samples and was approximately 2.5 fold higher in aged mice compared to adults (main effect of Age, P<0.001) (Fig.4A). These data are consistent with the age-associated increase in MHC II surface expression (Fig.3C). Similar to previous studies (Godbout et al. 2005; Henry et al. 2008; Huang et al. 2007), LPS injection did not increase MHC II mRNA or surface expression levels in the brain of either age-group (data not shown). Figs.4B&C show that peripheral LPS injection increased mRNA levels of TLR2 and IDO in the enriched microglia populations (P<0.001, for each). Because IDO mRNA was undetected in saline treated mice, IDO fold change was expressed relative to the IDO mRNA levels in LPS treated adults. ANOVA of mRNA expression revealed a significant Age × LPS interaction for IDO (F(1, 26)= 20.7, P<0.001) and a tendency for Age × LPS interaction for TLR2 (F(1,25)= 2.8 P =0.11).
Fig.4. Peripheral LPS injection causes exaggerated mRNA levels of inflammatory mediators in microglia of aged mice compared to adults.
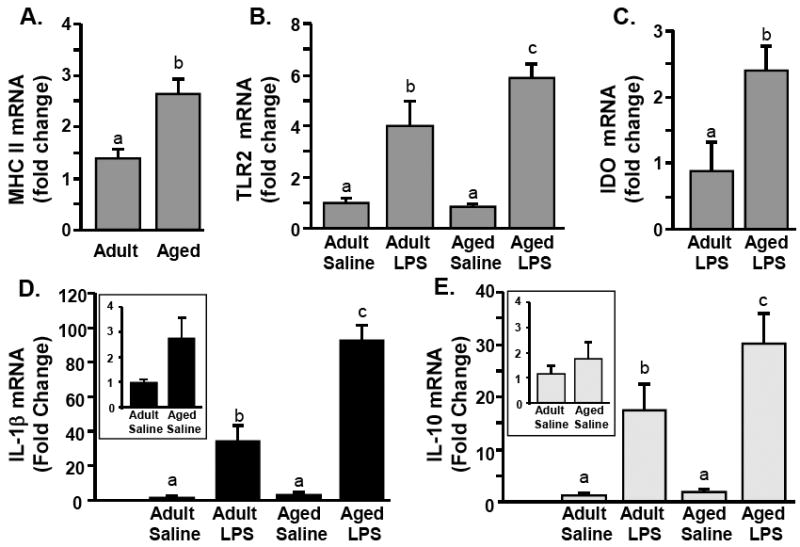
Adult (3-6 m) and aged (18-20 m) mice were challenged with saline or LPS i.p. and A) MHC II, B) TLR2, C) IDO, D) IL-1β and E) IL-10 mRNA levels were determined from enriched microglia isolated 4 h later. Bars represent the mean ± SEM (n=8). Means with different letters (a, b, or c) are significantly different (P<0.05) from each other.
Figs.4D&E show the effects of peripheral LPS injection on IL-1β and IL-10 mRNA expression in the enriched microglial population. There was a significant main effect of age for IL-1β mRNA (F(1, 26)= 23.3, P<0.001) and a tendency for IL-10 mRNA (F(1,24)= 2.9, P =0.09). To highlight the age-related differences in cytokine mRNA expression, the insets in Figs.4D&E show comparisons of microglial mRNA expression of IL-1β and IL-10 between saline treated adult and aged mice. There was a tendency for an age-related increase in IL-1β expression (F(1,13)= 4.45, P=0.06), but no significant main effect of age on IL-10 expression. These findings indicate that there is a modest age-related increase in IL-1β mRNA expression in microglia.
Parallel with the data presented in Fig.1, LPS injection caused a robust increase in Mrna levels of IL-1β and IL-10 in the enriched microglial population 4 h post injection (Fig.4 D&E). LPS elicited a more pronounced mRNA induction of both IL-1β and IL-10 in aged microglia than adult microglia. ANOVA of mRNA expression revealed a significant Age × LPS interaction for IL-1β (F(1, 26)= 20.7, P<0.001) and a tendency for Age × LPS interaction for IL-10 (F(1,24)= 2.5, P=0.10). Taken together, these data indicate that peripheral LPS injection caused greater induction of both IL-1β and IL-10 in aged microglia than adult microglia.
3.5 LPS-induced microglial production of IL-1β and IL-10
To confirm the cytokine mRNA findings in Fig.4, we determined intracellular protein expression of IL-1β and IL-10 in microglia isolated from adult and aged mice 4 h post i.p. LPS injection. Fig.5A shows representative bivariate dot plots of enriched microglia from adult and aged mice stained with CD11b-APC and intracellular IL-1β-FITC following i.p. LPS injection. It is important to note that the saline control group represents adult and aged saline treated mice. As expected, peripheral LPS injection increased the percentage of IL-1β+ microglia (CD11b+/IL-1β+) in both age groups (Fig.5A). In a comparison between LPS treated adult and aged mice, there was a greater percentage of IL-1β+ microglia (CD11b+/IL-1β+) in aged mice (Fig.5C). Fig.5B shows representative bivariate dot plots of enriched microglia stained with CD11b-APC and intracellular IL-10-FITC following peripheral LPS injection. LPS injection increased the percentage of IL-10+ microglia (CD11b+/IL-10+), but only in aged mice (Figs.5B&5D). Taken together, these cytokine protein data indicate that peripheral LPS injection caused microglial hyperactivity in aged mice associated with increased production of both IL-1β and IL-10.
Fig.5. Higher percentage of IL-1β+ and IL-10 positive microglia in aged mice compared to adults following peripheral LPS injection.
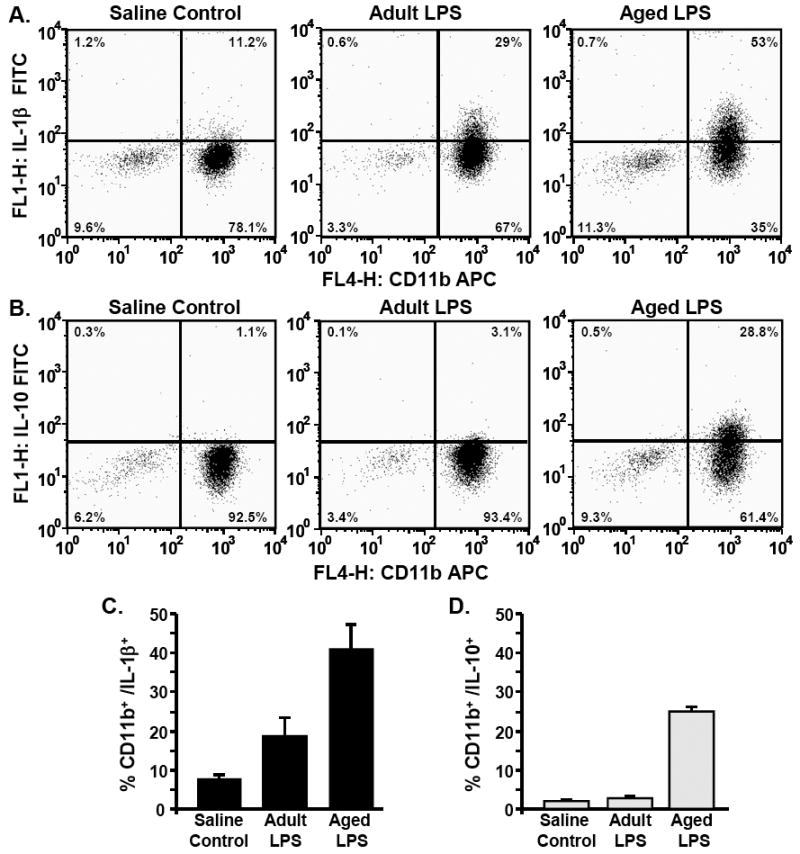
Adult (3-6 m) and aged (18-20 m) mice were challenged with saline or LPS i.p. and microglia were isolated 4 h later by Percoll density gradient. Cells were subjected to the BD Cytofix/Cytoperm Plus fixation/permeabilization protocol as described in the Methods. Representative bivariate dot plots of Percoll isolated cells stained with A) anti-CD11b-APC and anti-IL-1β-FITC or B) anti-CD11b-APC and anti-IL-10-FITC. Average percentage of cells that were C) CD11b+/IL-1β+ and D) CD11b+/IL-10+. Bars represent the mean ± SEM (n=3-6).
To determine the extent to which MHC II+ microglia in aged mice (Fig.3) were also IL-1β+ following LPS injection, CD11b+ cells (Fig.5A) were analyzed for MHC II and IL-1β. Fig.6A shows representative bivariate contour plots of enriched microglia from LPS treated adult and aged mice. While LPS treated adult and aged mice exhibited a similar percentage of MHC IIneg/IL-1β+ microglia (Fig.6B, white bars), LPS treated aged mice had a considerable percentage of MHC II+/IL-1β+ microglia (Fig.6B, black bars). It is important to note that these comparisons could not be made in the IL-10 samples (Fig.5B) because those samples were not stained with anti-MHC II-PE. To further analyze microglia isolated from LPS treated aged mice, CD11b+ cells were gated for MHC II and analyzed for IL-1β. Fig.6C shows that in aged mice 31% of MHC II neg microglia were IL-1β+ whereas 95% of MHC II+ microglia were IL-1β+. Collectively, these data indicate that primed (MHC II+) microglia in aged mice are activated to a greater extent by peripheral LPS injection than MHC II neg microglia.
Fig.6. MHC II positive microglia from aged mice have a robust IL-1β induction following peripheral LPS injection.
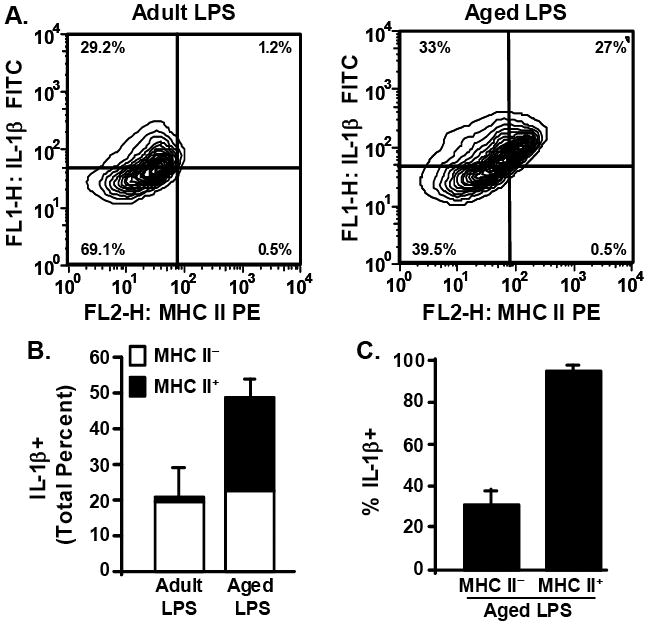
A subset of Percoll isolated microglia (Fig.5) were also stained with anti-MHC-II-PE. Cells were gated by CD11b+ staining. A) Representative bivariate contour plots of CD11b+ cells that were stained with anti-IL-1β-FITC and anti-MHC-II-PE. B) Average total percent of microglia that were IL-1β+ following peripheral LPS injection, differentiated based on whether they were MHC IIneg (white bars) or MHC II+ (black bars). Bars represent the mean ± SEM (n=3). C) Average percent of MHC IIneg and MHC II+ microglia that were also IL-1β+. Bars represent the mean ± SEM (n=3).
5. Discussion
In the elderly, systemic infection is associated with an increased frequency of behavioral and cognitive complications (Evans et al. 2005; Penninx et al. 2003). We have reported that stimulation of the peripheral innate immune system in aged BALB/c mice caused exaggerated neuroinflammation (Henry et al. 2008) that was paralleled by prolonged sickness behavior (Godbout et al. 2005), impaired working memory (Chen et al. 2008), and protracted depressive-like behavior (Godbout et al. 2007). Here, we extend our previous work with several novel findings. First, we show that MHC II expression was increased specifically in aged microglia (Fig.3C&4A). Second, peripheral LPS injection caused exaggerated microglial mRNA and protein induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 in aged mice compared to adults (Figs.4-5). Finally, the most prominent induction of IL-1β was detected in MHC II+ microglia from aged mice. (Fig.6). These data are relevant because they demonstrate microglial-hyperactivity in the aged brain and indicate that MHC II+ microglia are significant contributors to the exaggerated neuroinflammation in aged mice following peripheral immune challenge.
One important finding of this study was that there was an age-related increase in MHC II expression in microglia (Figs.3C&4A). We have previously reported an age-associated increase in MHC II mRNA levels in whole brain and hippocampal homogenates from older BALB/c mice (Godbout et al. 2005; Henry et al. 2008; Huang et al. 2007), but the current study provides novel evidence for increased MHC II mRNA and protein expression specifically on microglia (CD11b+) isolated from aged BALB/c mice (Figs.3C-D&4A). For example, approximately 25% of aged microglia were positive for MHC II expression compared to 2% for adult microglia (Fig.3D). These data indicate that microglia from older BALB/c mice maintain a primed or reactive phenotype.
Along with the age-associated increase in MHC II mRNA expression in microglia, we detected an age-related increase in IL-1β expression (Fig.4D, inset), but did not detect any age related change in IL-10 expression (Fig.4E, inset). An age-related increase in brain IL-1β is consistent with other studies (Chen et al. 2008; Maher et al. 2004; Murray and Lynch 1998; Sierra et al. 2007). Moreover, our results are in line with findings showing higher microglial-specific IL-6 induction in mixed glia cultures established from aged mice than adults (Ye and Johnson 1999). Our IL-10 findings, however, are inconsistent with previous reports of an age-related decrease in brain IL-10 (Frank et al. 2006a; Ye and Johnson 2001). This inconsistency with IL-10 may be associated with species variation (Frank et al. 2006a) or age (Ye and Johnson 2001). For instance, the BALB/c mice used in the present study were approximately 4 m younger than the mice used previously (Ye and Johnson 2001). Moreover, age-related decreases in IL-10 were detected in brain slice cultures and mixed glial cultures. In the present study, however, we determined IL-10 expression in the cortex and in enriched microglia isolated following in vivo activation by peripheral LPS challenge. One final point to highlight is that we detected an age-associated increase in IL-1β in the enriched microglial mRNA samples (Fig.4D), but not in cortex mRNA (Figs.1A&B) or cortex protein samples (data not shown). Despite only marginally higher levels of IL-1β and no change in IL-10 in the brain of older mice, peripheral LPS injection still caused an exaggerated neuroinflammatory response in aged mice (Figs.1,4, & 5). We interpret these data to indicate that glial markers of reactivity, including MHC II (Fig.3C), are more consistent predictors of an amplified neuroinflammatory response than small alterations in cytokine expression.
Because microglia have different activation states that depend on the specific inflammatory stimulus (Carson et al. 2004), we determined the microglia-related mRNA expression of several neuroinflammatory markers including MHC II, TLR2, and IDO. Peripheral LPS injection caused microglial-related over-expression of TLR2 and IDO in aged mice compared to adults (Fig.4). LPS injection, however, did not affect either mRNA or surface expression of MHC II at 4 h (data not shown). These data support our hypothesis that there is increased microglia activation in the aged brain compared to adults after peripheral LPS challenge.
A critical finding of this study was that peripheral LPS injection caused higher microglia-related induction of both IL-1β and IL-10 in aged mice compared to adults. Based on previous ex vivo findings in older BALB/c mice (Ye and Johnson 2001), we anticipated that LPS injection would be associated with over-production of IL-1β and under-production of IL-10. As expected in LPS treated mice, IL-1β was induced at a higher level in the brain and plasma than IL-10 (Figs.1, 2, 4, & 5). Contrary to our expectations, however, both IL-1β and IL-10 cortex mRNA levels were significantly higher 4 and 8 h post peripheral LPS injection in aged mice compared to adults (Fig.1). The simultaneous induction of IL-10 and IL-1β by LPS was also detected in the plasma (Fig.2). The LPS-induced plasma cytokine levels were age-independent (Figs.2A-C) with exception of IL-10 levels 8 h post LPS (Fig.2D). The Age × LPS interaction for plasma IL-10 may be related to previous reports showing that splenic macrophages from older mice produced less inflammatory cytokines (e.g., IL-1, IL-6, and TNFα) and more anti-inflammatory IL-10 following LPS activation than adults (Chelvarajan et al. 2005; Chelvarajan et al. 2006). In the brain, peripheral LPS injection caused an exaggerated induction of IL-1β and IL-10 in enriched microglia isolated from aged mice (Figs.4D&E). These results are similar to a recent study showing that microglia of aged transgenic p.7.2fms-EGFP (C57BL/6X CBA background) mice had the highest mRNA induction of both IL-1β and IL-10 after peripheral LPS injection (Sierra et al. 2007). Consistent with the mRNA data presented in Fig.4, there was a higher percentage of IL-1β+ and IL-10+ microglia (CD11b+) in aged mice following peripheral LPS injection than adults (Figs.5).
The coupled induction of IL-1β and IL-10 is possible because these cytokine genes have several promoter regions in common, including SP1, C/EBPβ, and NFκB binding sites, that are activated by Toll-like receptor and cytokine stimulation (Allan et al. 2005; Amaral et al. 2006; Basak et al. 2005; Brightbill et al. 2000; Liu et al. 2006; Murray 2006). It is plausible that this IL-10 induction provides negative feedback on IL-1β, so it is unclear why IL-1β mRNA expression remained elevated at 8 h in the cortex of aged mice (Fig.1D) despite the high levels of IL-10 (Fig.1C & Fig.5C). The inability of IL-10 to modulate IL-1β production in the aged brain may be associated with down regulation of the IL-10 receptor or decreased sensitivity to IL-10. Whatever the specific deficiency, we interpret our results to indicate that microglial hyperactivity in the aged brain after peripheral LPS injection is not associated with reduced microglial induction of IL-10.
To determine intracellular IL-1β and IL-10 expression (Fig.5) it was necessary to inhibit cell protein secretion with a 4 h brefeldin A incubation. During this incubation step, no reagents were added to stimulate cytokine production (i.e., secondary exposure to LPS). Therefore, the enriched microglia isolated from adult and aged mice correspond with a time point between 4-8 h post peripheral LPS challenge. This incubation step, however, may explain why IL-10 mRNA levels were elevated in adult microglia 4 h post LPS (Fig.4E), but IL-10 protein was undetected by intracellular staining (Fig.5B). This notion is supported by the results showing that cortex IL-10 mRNA levels returned to baseline in adult mice by 8 h post LPS injection (Fig.1D). It is important to highlight that only CD11b+ cells had detectable protein levels of cytokines (Figs.5A&B) and MHC II (Fig.3C). Therefore, the modest increase in CD11bneg cells isolated from the aged brain (Fig.3B) is unlikely to confound the mRNA data (Fig.4). It is also important to mention that we had a limited number of mice available for intracellular staining so our experiments were imbalanced to focus on the effect of peripheral LPS injection on microglial induction of cytokines (Aged LPS vs Adult LPS). We acknowledge that age-dependent increases in cytokine protein in microglia are possible, but these were not evident in our small sample pool of saline treated mice. Nonetheless, our data indicate that LPS caused the most prominent induction of IL-1β and IL-10 (mRNA and intracellular protein) in aged mice compared to all other treatment groups (Fig.1, 4, & 5).
A final original finding was that primed (MHC II+) microglia in aged mice were activated to a greater extent by peripheral LPS injection than MHC II neg microglia (Fig.6). In LPS treated adult mice approximately 20% percent of the microglia were IL-1β+ whereas in LPS treated aged mice approximately 40% percent of the microglia were IL-1β+ (Fig.5C). In LPS treated aged mice, 95% of the MHC II+ microglial population were also IL-1β+ (Fig.6C). These data support the hypothesis that primed microglia (in aging or neurological disease) are highly responsive to peripheral innate immune stimulation (Perry et al. 2003). For instance, our data are consistent with findings in a model of pre-clinical prion disease (Betmouni et al. 1996) where robust IL-1β induction was detected in primed microglia after peripheral LPS injection (Cunningham et al. 2005). Taken together, our findings indicate that peripheral LPS challenge caused microglial hyperactivity in the brain of aged mice associated with marked induction of IL-1β by MHC II+ microglia.
In conclusion, the present study demonstrates that there was a pronounced age-related increase in MHC II mRNA and protein expression in microglia. Moreover, peripheral LPS injection promoted an inflammatory response in the aged brain that was associated with exaggerated microglial induction of inflammatory markers (TLR2 and IDO), inflammatory IL-1β, and anti-inflammatory IL-10. These data are significant because they show microglia-specific hyperactivity in the aged brain and indicate that MHC II+ microglia are significant contributors to the exaggerated neuroinflammation in aged mice following peripheral LPS challenge.
Acknowledgments
This research was supported by an NIH grant R21-MH077817 to J.P.G. C.J.H. was supported by an NIH T32-AI-05-5411 Training Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29(4):614–21. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Amaral ME, Barbuio R, Milanski M, Romanatto T, Barbosa HC, Nadruz W, Bertolo MB, Boschero AC, Saad MJ, Franchini KG, Velloso LA. Tumor necrosis factor-alpha activates signal transduction in hypothalamus and modulates the expression of pro-inflammatory proteins and orexigenic/anorexigenic neurotransmitters. J Neurochem. 2006;98(1):203–12. doi: 10.1111/j.1471-4159.2006.03857.x. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Basak C, Pathak SK, Bhattacharyya A, Mandal D, Pathak S, Kundu M. NF-kappaB- and C/EBPbeta-driven interleukin-1beta gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1beta release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280(6):4279–88. doi: 10.1074/jbc.M412820200. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain Behav Immun. 2004;18(2):149–57. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74(1):1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24(3):301–11. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164(4):1940–51. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Huang D, Sasse ME, Ransohoff RM. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat Protoc. 2006;1(4):1947–51. doi: 10.1038/nprot.2006.327. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Lo D. Analysis of microglial gene expression: identifying targets for CNS neurodegenerative and autoimmune disease. Am J Pharmacogenomics. 2004;4(5):321–30. doi: 10.2165/00129785-200404050-00005. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77(4):503–12. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79(6):1314–27. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301–11. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154(9):4309–21. [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006a;27(5):717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006b;151(2):121–30. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1(2):127–37. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24(3):521–38. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, Connor JO, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging Exacerbates Depressive-like Behavior in Mice in Response to Activation of the Peripheral Innate Immune System. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301649. Epub: Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99(4):1263–72. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res. 2000;77(1):138–47. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.012. Epub: May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(1 Suppl):112–8. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278(21):19453–62. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146(1):248–54. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chen CC, Tseng HP, Chang WC. Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-kappaB-induced CCAAT/enhancer-binding protein delta in mouse macrophages. Cell Signal. 2006;18(9):1492–500. doi: 10.1016/j.cellsig.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10--a role for IL-1 beta? J Neurochem. 2004;88(3):635–46. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Maher FO, Martin DS, Lynch MA. Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging. 2004;25(6):795–806. doi: 10.1016/j.neurobiolaging.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89(3):687–99. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2(2):109–12. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998;273(20):12161–8. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6(4):379–86. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171(12):72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24(6):1340–9. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning- impaired rodents. Neuroscience. 2001;107(3):415–31. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282(20):15208–16. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5(10):1224–6. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7(1):60–7. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pousset F, Cremona S, Dantzer R, Kelley K, Parnet P. Interleukin-4 and interleukin-10 regulate IL1-beta induced mouse primary astrocyte activation: a comparative study. Glia. 1999;26(1):12–21. doi: 10.1002/(sici)1098-1136(199903)26:1<12::aid-glia2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–35. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19(1):47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb J. 2004;18(9):998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20(4):395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28(10):1507–21. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJ, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci Lett. 2005;390(2):76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93(12):139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9(4):183–92. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]


