Abstract
Background
Depressed patients show mood-congruent errors in the identification of emotion in facial expressions. Errors consist of impaired performance (recognition errors) and negative bias (seeing faces as sadder than they are). This abnormal processing may both reflect and contribute to the negative affective state. In this study, we administered an emotional recognition in facial expression task to women with premenstrual dysphoric disorder (PMDD) to determine whether processing errors similar to those in depression were present and whether they were confined to the luteal phase (i.e., state dependent).
Methods
The Facial Discrimination Task (FDT) was administered in the follicular and luteal phases to women with PMDD (n = 28) and asymptomatic controls (n = 27).
Results
ANOVA with repeated measures identified significantly increased negative judgments (both performance errors and bias) in women with PMDD during the luteal phase (more neutral to sad misjudgments and higher negative bias index) as well as impaired “specificity” of judgments [an inability to discriminate neutral from emotional stimuli] (diagnosis by phase interactions, p <.05), findings similar to those observed in depression. No menstrual cycle effects were seen in controls, and no differences between patients and controls were seen on a control task (age assessment of pictured subjects).
Limitations
The levels of significance obtained were modest and would not withstand correction for multiple comparisons.
Conclusion
Women with PMDD display a luteal phase-dependent impairment (negative bias) in the processing of non-verbal affective information. This negative bias may contribute to the generation of negative mood states during the luteal phase and could suggest the presence of dysfunction in those brain regions whose coordinated activity mediates the recognition of emotion in facial expression.
Introduction
Affective neuroscience requires the availability of cognitive or emotional probes that, in imaging studies, activate and help detect behaviorally meaningful neurocircuitry. While a variety of such probes have been validated, a challenge facing investigators is that of selecting probes that sample processes known to be affected in the disorder under study. One such probe that has been useful in studies of depression is the facial emotional discrimination task. Abnormalities in the identification of affects in facial expression have been repeatedly described in depressed patients and take two forms, either impaired performance (recognition errors) (George et al., 1998; Gur et al., 1992; Marcel et al., 1993; Mikhailova et al., 1996; Rubinow et al., 1992; Suslow et al., 2001) or negative bias (identification of more sadness in facial expressions) (Bouhuys et al., 1997; David et al., 1990; Gur et al., 1992; Hale, III 1998). It is the bias toward identifying faces as sad that is more commonly seen in major depression (versus bipolar depression) (Phillips et al., 2003), and this mood-congruent bias in the processing of emotions in faces has both contributed to hypotheses about the generation and perpetuation of depression and led to the use of affective recognition tasks to map out the areas of the facial detection circuitry that are dysfunctional in depression (Elliott et al., 2002; Lawrence et al., 2004; Phillips et al., 2003).
One affective disorder that is particularly well suited for study in this fashion – but to date conspicuously ignored in imaging studies - is premenstrual dysphoric disorder (PMDD). In this disorder, affective episodes occur in a regular and predictable fashion, thus permitting the performance of study procedures across affective states in relatively close temporal proximity and with the attendant opportunity to discriminate activation patterns related to state versus trait. Detection of disturbances in facial recognition in women with PMDD would be of value for several reasons: first, it would help explain the observed clinical phenomenon of affective “misperception” that informs behavioral responses in women with this disorder (Schmidt et al, l990). Second, it would help identify brain regions that could mediate this perceptual abnormality, given the extensive understanding of the neurocircuitry underlying facial perception. Third, it would permit the investigation of the relationship between affective symptoms and processing errors in an affective disorder distinct from major depression or bipolar disorder. To date, it is not known whether women with PMDD suffer impairments of facial recognition, either in a luteal-phase specific fashion or as a chronic trait characteristic.
To investigate whether women with PMDD demonstrate abnormal facial recognition, we administered the facial discrimination task (FDT), which was specifically developed for testing emotional processing (Gur et al., 1992). The reliability and validity of the FDT for emotion research has been amply demonstrated (Rojahn et al., 2000). A common version of this task involves distinguishing between happy, sad, and neutral faces. One component of this task is the correct identification of the expressions, whereas the other component is the emotional bias, i.e., whether the “errors” in identifying the expressions are more in the “sad” or the “happy” direction. The latter component is relatively independent of motivation and attention, as it is the direction (sad vs. happy) of the response rather than the accuracy of the response that counts.
In this study we tested the hypothesis that women with PMDD would show a bias towards negative mood, as has been reported in patients with depression. We also predicted such negatively-biased judgments would occur only in the luteal phase, and thus would be “state-linked,” as opposed to occurring during both follicular and luteal phases (“trait-linked”). A pure performance sub-task, that of age discrimination, was concurrently administered as a control for differences in non-mood related task performance.
Methods
Subject selection
Medication-free women with regular menstrual cycles were selected from respondents to newspaper advertisements for volunteers with a history of PMDD or without any history of menstrual cycle-related mood changes. Before entry into the study, prospective participants were screened with a daily visual analogue scale of self- ratings of affective symptoms (i.e., a 100 mm line bracketed by severity extremes [none to worst ever] for sadness and irritability/anxiety) (Rubinow et al., 1984). In at least two of three menstrual cycles, women diagnosed with PMDD (n = 28, age = 37.9. yrs, SD = 5.0) showed a 30% higher mean level (adjusted for the range of the scale employed) of sadness and/or irritability/anxiety symptoms in the week before menses than in the week following the end of menses. (Adjustment consists of dividing 30% by the percent of the scale spanned by the extreme high and low ratings (Smith et al., 2003).) This criterion operationalizes the DSM-IV severity and cyclicity criteria for PMDD (Smith et al., 2003), and all patient volunteers met DSM-IV criteria. On the daily ratings comparison women without PMDD (n = 27, age = 33.5 yrs, SD = 7.0) showed no evidence of mood changes related to menstrual cycle phase. All subjects were given a Structured Clinical Interview for DSM-III-R (SCID) (Spitzer et al., 1990) and a modified Schedule for Affective Disorders and Schizophrenia-Lifetime (SADS-L) (Spitzer et al., 1979); women with PMDD were required to have no current or recent (< 2 years) axis I condition, and comparison women without PMDD were required to have no current or past history of an axis I condition. The two year requirement eliminated subjects with significant current or recent comorbidities, which could serve as confounds, but did not eliminate the majority of subjects with PMDD with more remote histories of Axis I disorders. All gave written informed consent, and the protocol was approved by the National Institute of Mental Health institutional review board. All subjects reported menstrual cycles of regular length, ranging between subjects from 21 to 33 days, and none had any significant medical illness either at intake or at time of testing or within the previous year.
All women were tested at two points in the menstrual cycle, mid-follicular (days 5–11 after the onset of the last menstrual period) and mid to late-luteal (days 19–28). The order of menstrual cycle phase during which the FDT was administered was counter-balanced. All subjects completed two self-rating scales at each time of testing: the Beck Depression Inventory (BDI) (Beck et al., 1961) and the self-rated version of the Premenstrual Tension Syndrome Scale (PMTS-S) (Steiner et al., 1980).
Task Administration
The Facial Discrimination Task (Gur et al., 1992) consisted of the presentation of facial photographs expressing degrees of happy and sad emotion as well as neutral expressions. Twenty one faces were happy or sad and 19 were neutral. Two forms of the task were employed – one in each menstrual cycle phase – and were counter-balanced across subjects. Subjects were instructed to indicate on a 1 to 7-point scale whether the face presented was very happy, happy, mildly happy, neutral, mildly sad, sad, or very sad. Subjects were essentially asked to discriminate between happy and neutral, as well as between sad and neutral, as the discrimination between happy and sad was obvious. The polarity of the scale for the emotion-discrimination tasks (i.e., whether 1 or 7 represented the very happy rating) was counter-balanced across tasks and subjects.
As a control task, subjects were also given the age discrimination task, in which, using the same photographs, subjects were instructed to indicate the age of the poser by decade (1 = teens, 2 = twenties … 7 = seventies; the polarity of this scale was not reversed). Stimuli (photographs with a cardboard backing) were placed in front of the subjects, who indicated their answers verbally. Each task was self-paced with no time limit, and responses were obtained for each stimulus before the next trial was initiated. Subjects were presented stimulus sets of both sexes (i.e., male and female faces).
Data Analysis
In the happy vs. sad discrimination task, true positives (i.e., response in the happy range for a happy face and in the sad range for a sad face), false positives (neutral to sad [i.e., scores in the sad range for neutral stimuli] or neutral to happy [i.e., scores in the happy range for neutral stimuli]), true negatives (neutral responses to neutral faces), and false negatives (neutral responses to either happy or sad faces) were counted for each task as previously described (Gur et al., 1992). In addition, a negative bias was computed (happy to neutral false negatives + neutral to sad false positives divided by the total attempted neutral + total attempted happy) (Gur et al., 1992). Finally, again as per Erwin et al., (Erwin et al., 1992) sensitivity (true positives/true positives and false negatives) and specificity (true negatives/true negatives and false positives) scores were calculated. The first of these is a measure of “sensitivity” to the correct emotion, while specificity describes the ability of subjects to discriminate neutral from emotional stimuli. For the age discrimination task, the number of errors (more than one decade off) and the direction (older vs. younger) of the error were analyzed.
Statistical analysis was performed using the SYSTAT 9 (SPSS Inc, Chicago, IL) program and consisted of repeated measures analyses of variance (ANOVAs) on the primary dependent variables (false positives neutral to sad, sensitivity, specificity, negative bias, age discrimination), with diagnosis (women with PMDD vs. controls) as a between-subjects factor and menstrual cycle phase (follicular vs. luteal) as the within-subjects factor. When permitted by significant results on the ANOVA-R, post-hoc comparisons were performed using individually calculated Bonferroni t-tests employing appropriate mean square error terms (measures of random variation) from the ANOVA to maintain an overall Type I error of 0.05. The self-rating scales of depression (BDI) and PMDD symptoms (PMTS-S) were similarly analyzed (with ANOVA-R) to confirm that the women with PMDD were symptomatic at the time that they completed the FDT.
Secondary outcome measures were also analyzed with ANOVA-R and included positive bias, true positives (sad, happy), false negatives (sad, happy) and the remaining false positive (i.e., towards happy): these measures were not expected to show menstrual cycle or diagnostic effects.
To control for family-wise Type I error (the risk of finding significant differences where none exist), we performed multivariate analysis of variance (MANOVA) for each set of comparisons; i.e., four primary dependent variables (age discrimination, a control task, was not included) six secondary dependent variables, and two mood measures. Effect sizes were also calculated for the influence of menstrual cycle phase on test performance in patients and controls. ANOVAs were re-performed with sex of the face displayed as a repeated measure to determine whether diagnostic group effects differed for male versus female faces. Finally, for the women with PMDD, correlations between the mood ratings and FDT were calculated with Spearman rank order correlation coefficients. Values in the text are expressed as mean +/− standard deviation (SD).
Results
There was no significant difference between average day of testing in the follicular phase between controls (day = 8.2, SD = 2.0) and women with PMDD (day = 7.7, SD = 1.8), or in the luteal phase [controls (day = 22.6, SD = 2.2), women with PMDD (day = 24.5, SD = 2.4)].
ANOVAs
Symptoms
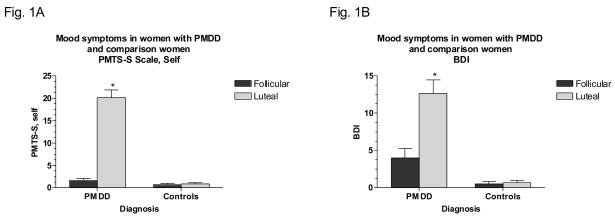
Mood ratings in women with PMDD and controls are shown in Figures 1A and B. As expected, women with PMDD showed a luteal phase increase in both symptom rating scales and low or absent symptoms in the follicular phase (group by phase effect: F1,56 = 97.9, p <.001 and F1,56 = 18.7, p <.001 for PMTS-S and BDI scales, respectively; luteal versus follicular phase in women with PMDD – Bonferroni t22 = 9.96, p <.001 and t22 = 4.31, p <.001 for PMTS-S and BDI scales, respectively). On both scales, controls showed low or absent symptoms in both phases.
Fig 1.
Significant luteal phase increase in symptom rating scale scores in women with PMDD but not in comparison women. 1A. PMTS-S: Steiner-Carroll Premenstrual Tension Syndrome Scale, self-rated version [*Bonferroni t in women with PMDD, p <.001, luteal phase vs. follicular phase]. 1B. BDI: Beck Depression Inventory [*Bonferroni t in women with PMDD, p <.001, luteal phase vs. follicular phase].
PMDD: Premenstrual Dysphoric Disorder
Task Performance
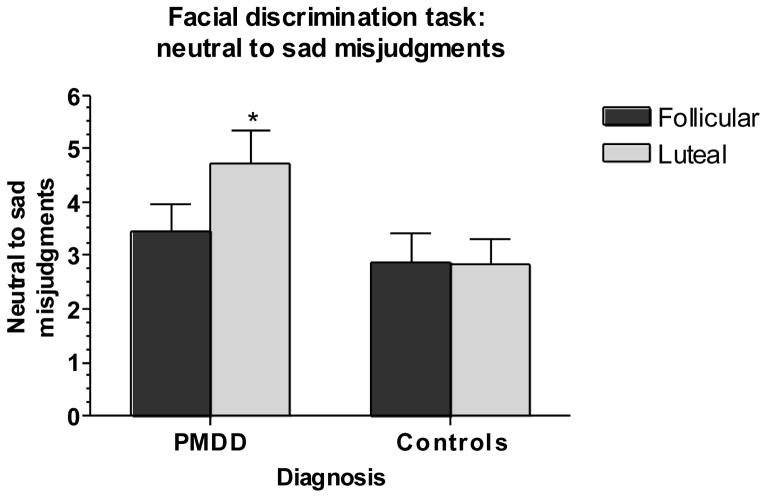
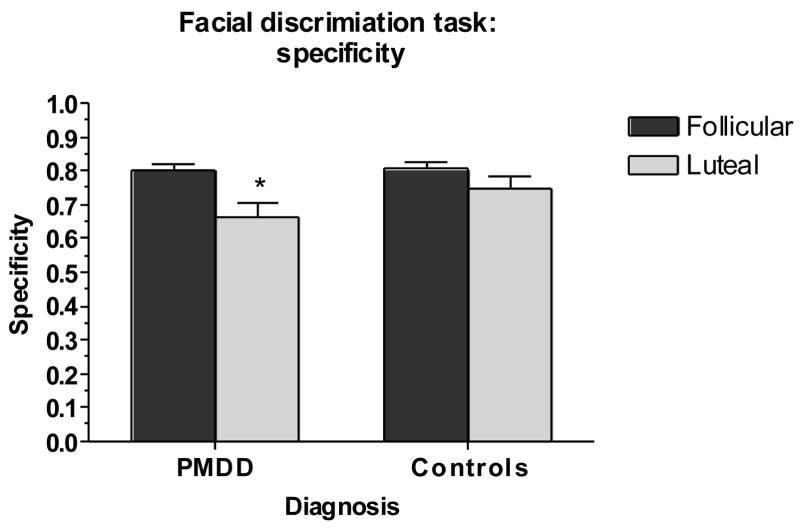
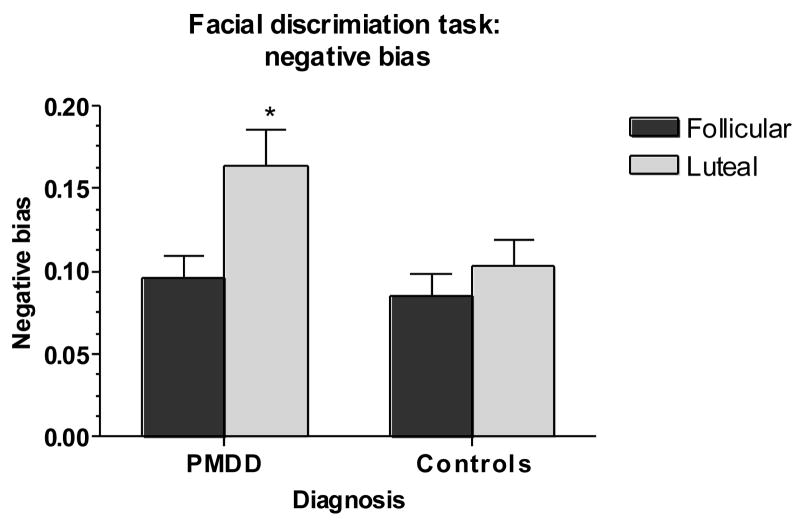
Main outcome variables were the precision of both groups in determining posers’ ages (AGE – a control task), the tendency to characterize neutral faces as sad (N → S), the sensitivity and specificity of responses, and the negative bias index (BIAS). Results of task performance in women with PMDD and controls are shown in Figures 2–5. For N → S errors, there was a significant group-by-phase interaction (F1,50 = 4.54, p <.05) (Figure 2), reflecting an increase in false positives (N → S) in the luteal phase in women with PMDD (t27 = 2.80, p <.05) but not controls. A similar diagnosis-by-phase-related impairment was seen for specificity but not for sensitivity (F1,53 = 4.61, p <.05) (Figure 3), accounted for largely by the mislabeling of neutral stimuli as sad by patients during the luteal phase (t27 = 4.71, p <.001). For BIAS, there were significant phase and group-by-phase interaction effects (F1,53 = 5.93, p <.05) (Figure 4), again reflecting an increase in negative bias in the luteal phase only in women with PMDD (t27 = 4.52, p <.001). For all three measures [(N → S) false positives, specificity, and negative bias], Bonferroni t-tests revealed significant menstrual cycle phase effects (p <.05, p <.001, and p <.001, respectively) in women with PMDD but not in controls. The tendency to characterize neutral faces as happy or very happy (positive bias) showed no significant group, phase or interactive effects.
Fig 2.
Significant luteal phase-related increase in neutral to sad misjudgments in women with PMDD but not in comparison women [*Bonferroni t in women with PMDD, p <.05, luteal phase vs. follicular phase].
PMDD: Premenstrual Dysphoric Disorder
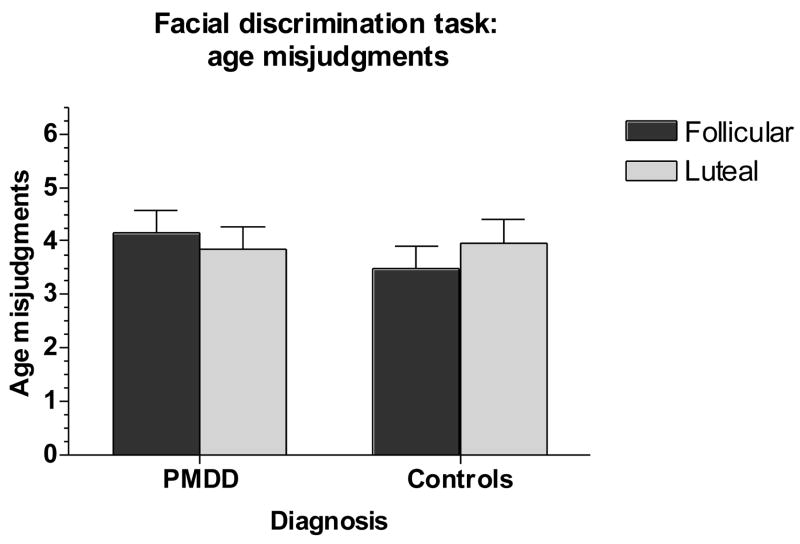
Fig 5.
No significant differences in age misjudgments.
PMDD: Premenstrual Dysphoric Disorder
Fig 3.
Significant luteal phase-related decrease in specificity in women with PMDD but not in comparison women [*Bonferroni t in women with PMDD, p <.001, luteal phase vs. follicular phase].
PMDD: Premenstrual Dysphoric Disorder
Fig 4.
Significant luteal phase-related increase in negative bias in women with PMDD but not in comparison women [*Bonferroni t in women with PMDD, p <.001, luteal phase vs. follicular phase].
PMDD: Premenstrual Dysphoric Disorder
While no other interactive effects were observed, significant diagnostic effects were seen for true positive happy items (correct identification of happy faces) (decreased in patients) (group effect, F1,53 = 4.98, p =.03) and false negative happy items (H → N misjudgments) (increased in patients) (group effect, F1,53 = 4.98, p =.03). There were no significant effects or trends for AGE (number or direction of errors) (group, phase, and group by phase interactions, F’s =.09–1.4, p = NS) (Figure 5) (data not shown for direction of errors). Results observed were not affected by the sex of the faces in the FDT.
As six subjects with PMDD had past histories of major depression, the two analyses showing a diagnostic effect (true positive happy and false negative happy) were repeated with these subjects removed. The F values obtained increased for both measures and remained significant (p =.014 for both).
In the MANOVAs performed to control for family-wise Type I error, a significant time-by-diagnosis effect (F1,53 = 4.45, p <.05) was observed for the primary variables (false positives neutral to sad, sensitivity, specificity, negative bias), confirming the significant phase-by-diagnosis effects seen with the univariate F tests. In contrast, no significant diagnostic or phase-by-diagnosis effects were seen with the MANOVA for the secondary variables. As expected, the phase-by-diagnosis effects (as well as the primary effects) were highly significant for the MANOVA for the mood variables (F1,56 = 25.6, p <.001).
Menstrual cycle effect sizes for the three significant primary measures in women with PMDD - false positive sad, specificity, and negative bias - were.42,.90, and.74, respectively. Corresponding effect sizes for controls were.03,.39, and.22, and effect sizes for secondary measures were all small (.3 or less) in both subject groups.
Correlations
There were no significant correlations between mood ratings and any task performance variable. Spearman correlation coefficients ranged from.0001 to.415, p = NS.
Discussion
Women with PMDD significantly differed from control women in displaying a luteal phase-related increase in negative judgments of mood in the facial expression of the photos, both N → S false positives and negative bias. The FDT task was untimed and was based on subjective judgments that do not require any concerted effort of concentration, so results are unlikely to have been influenced by impairments in attention or lack of motivation potentially consequent to the symptoms experienced by women with PMDD during the luteal phase. This inference is supported by the lack of any significant differences between patients and controls on the age discrimination task.
The potential import of these findings is as follows: first, the negative bias on the FDT represents one of several measures that show menstrual cycle-related variation in patients with PMDD but not controls. Other examples include luteal phase-related increases in measures of personality dysfunction (Berlin et al., 2001) and the reporting of negative life events (Schmidt et al., 1990). Further, unlike some cognitive measures that have shown abnormalities in patients with PMDD independent of menstrual cycle phase (Keenan et al., 1995), the bias in affective assessment in PMDD appears to be a state-linked rather than a trait-linked characteristic. Consequently, we can postulate that in women with PMDD, events related to the luteal phase trigger a functional dysregulation in specific brain regions known to play a role in the recognition of emotional facial expression (e.g., amygdala, fusiform gyrus, orbital frontal cortex (OFC), superior temporal sulcus) (Izquierdo et al., 2004; Killgore et al., 2004; LaBar et al., 2003). Disturbances in these same brain regions have been implicated in depression (Elliott et al., 2002; Lawrence et al., 2004; Phillips et al., 2003), and the FDT abnormalities in PMDD during the luteal phase are similar to those in major depression (i.e., decreased specificity and increased negative bias) (Surguladze et al., 2004). The relevance of luteal phase endocrine changes for the modulation of affective processing is supported by recent fMRI findings that anterior-medial OFC activity for negative vs. neutral stimuli increased premenstrually and decreased postmenstrually (Protopopescu et al., 2005). Interestingly, performance in our study was not related to severity of mood measures in either patients or controls. While correlations between performance on the FDT and measures of either depression (Gur et al., 1992) or anxiety (Bouhuys et al., 1997) have been reported previously, so has the absence of relationship to either mood measure (Elliott et al., 2002; Lawrence et al., 2004).
Second, the bias in affect assessment that we observed may reflect a critical element of the phenomenology of these patients, who report that their perceptions and elicited behaviors differ as a function of menstrual cycle phase. This tendency for women with PMDD to view emotions expressed in faces more negatively during the luteal phase has three possible attendant implications. First, it may significantly contribute to both the generation and perpetuation of the negative affective state during the luteal phase. As has been postulated in MDD, a negative bias in the processing of emotion in facial expression reflects the experience of stimuli as both more emotional and more negative, with consequent elicited behaviors more likely to reinforce rather than reverse the negative affective state (Fu et al., 2004; Phillips et al., 2003). Second, it suggests additional avenues of investigation that may more precisely define both the nature of the affective disturbance in PMDD as well as its underlying neurophysiologic basis. For example, Hooker et al. (Hooker et al., 2004) show that an inability to perform reversal learning with emotionally charged faces is associated with an oversensitivity to negative social cues, cognitive inflexibility, and a differential pattern of brain regional activation on fMRI (Hooker et al., 2004). Studies by Schultz and colleagues (Schultz 2004) demonstrate that a reward that is smaller than that expected will actually inhibit the firing of dopamine neurons associated with the expectation of reward. Hence, an affective misperception may contribute to as well as reflect the experience of anhedonia or dysphoria. Third, the state-dependent nature of the negative bias should, in a disorder with rapid (monthly), predictable state changes, permit a more precise delineation of the underlying neurocircuitry than a disorder with much less frequent or predictable state changes.
While the major outcome measures showed diagnostic by phase effects, two secondary measures revealed diagnostic effects independent of menstrual cycle phase. Comparison women showed better performance identifying true positive happy items and in avoiding false negative happy judgments. These findings are consistent with the demonstration that just as depressed patients are biased towards sad information, controls may be biased toward happy information (Elliott et al., 2002) (although differences specifically in positive bias did not reach significance in our study). Indeed it has been suggested that the medial frontal region may respond differentially to sad targets in depressed patients and to happy targets in controls (Elliott et al., 2002). It is possible that the recurrence of negative affective states in women with PMDD may be accompanied by perceptual residua that eliminate the normal tendency to “over-identify” happy items even during the asymptomatic phase of the menstrual cycle. The differences in true positive happy and false negative happy items observed between women with PMDD and comparison women did not appear confounded by a past history of depression, as the diagnostic effects remained significant when the analyses were repeated without the six subjects with a past history of depression.
This study has several limitations First, the levels of significance obtained were modest and, while consistent, would not have withstood the most conservative correction for multiple comparisons. Second, we did not have adequate numbers of PMDD women with a past history of MDD to determine whether such a history would predict a different pattern of results. Third, despite reasonable sample sizes, our findings must be viewed as preliminary until replicated in an independent sample. If replicated, the observation of menstrual cycle phase-related, mood-congruent, negative processing biases in women with PMDD would suggest that (luteal phase-specific) affective symptoms in PMDD potentially share a neurobiological commonality with depression. As such, future studies of disturbed perception of emotion in facial expressions in women with PMDD may not only help define the neurocircuitry (with functional imaging) of PMDD but may as well exploit the presence of a specific physiologic variable - the change in reproductive steroids - that is directly relevant to changes in affective state and hence may promote a more detailed general understanding of the physiological disturbances and substrates of affective dysregulation.
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIMH Grant #1 ZO1 MH002765-09 Reproductive Endocrine Related Mood Disorders--Differential Sensitivity; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The research was supported by the Intramural Research Programs of the NIMH and NINDS.
Footnotes
Contributors: Authors Rubinow and Schmidt designed the study and wrote the protocol. Authors Dancer, Schenkel, and Smith performed the testing and undertook the statistical analysis, author Smith wrote the first draft of the manuscript, and author Rubinow wrote all subsequent drafts. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berlin RE, Raju JD, Schmidt PJ, Adams LF, Rubinow DR. Effects of the menstrual cycle on measures of personality in women with premenstrual syndrome: a preliminary study. J Clin Psychiatry. 2001;62:337–342. doi: 10.4088/jcp.v62n0505. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Mersch PP. Relationship between perception of facial emotions and anxiety in clinical depression: does anxiety-related perception predict persistence of depression? J Affective Disord. 1997;43:213–223. doi: 10.1016/s0165-0327(97)01432-8. [DOI] [PubMed] [Google Scholar]
- David AS, Cutting JC. Affect, affective disorder and schizophrenia: A neuorpsychological investigation of right hemisphere function. Br J Psychiatry. 1990;156:491–495. doi: 10.1192/bjp.156.4.491. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- George MS, Huggins T, McDermut W, Parekh PI, Rubinow D, Post RM. Abnormal facial emotion recognition in depression: serial testing in an ultra-rapid-cycling patient. Behav Modif. 1998;22:192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hale WW., III Judgment of facial expressions and depression persistence. Psychiatry Res. 1998;21:265–274. doi: 10.1016/s0165-1781(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Germine LT, Owen E, Knight RT, D’Esposito M. Neural mechanisms involved in learning from happy and fearful facial expressions; Abstr. 34th Annu. Meeting Soc. Neurosci; 2004. p. 203.6. [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Lindamer LA, Jong SK. Menstrual phase-independent retrieval deficit in women with PMS. Biol Psychiatry. 1995;38:369–377. doi: 10.1016/0006-3223(94)00303-K. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Marcel BB, Samson J, Cole JO, Schatzberg AF. Discrimination of facial emotion in depressed patients with visual-perceptual disturbances. J Nerv Ment Dis. 1993;181:583–584. doi: 10.1097/00005053-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiatry. 1996;40:697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojahn J, Gerhards F, Matlock ST, Kroeger TL. Reliability and validity studies of the Facial Discrimination Task for emotion research. Psychiatry Res. 2000;95:169–181. doi: 10.1016/s0165-1781(00)00169-4. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM. Impaired recognition of affect in facial expression in depressed patients. Biol Psychiatry. 1992;31:947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roy-Byrne PP, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141:684–686. doi: 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Grover GN, Hoban MC, Rubinow DR. State-dependent alterations in the perception of life events in menstrual-related mood disorders. Am J Psychiatry. 1990;147:230–234. doi: 10.1176/ajp.147.2.230. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Schmidt PJ, Rubinow DR. Operationalizing DSM-IV criteria for PMDD: selecting symptomatic and asymptomatic cycles for research. J Psychiatr Res. 2003;37:75–83. doi: 10.1016/s0022-3956(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1979. Schedule for affective disorders and schizophrenia - lifetime version. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1990. Structured clinical interview for DSM-III-R, patient edition. [Google Scholar]
- Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand. 1980;62:177–190. doi: 10.1111/j.1600-0447.1980.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills. 2001;92:857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]