Summary
During periods of stress, trophozoites of Entamoeba invadens (strain IP-1) undergo a process of differentiation (encystment) that results in a dormant cyst with a chitin containing cyst wall. Encystment can be induced by resuspension of trophozoites from growth medium into a diluted glucose free medium (47% LG) containing 5% adult bovine serum (ABS). ABS is thought to be a source of gal-terminated ligands that are required for high levels of encystment. After resuspension of trophozoites in 47% LG, encystment cultures were examined every 2h for responses to the (i) addition of 10 mM free-galactose, (ii) resuspension of cells to serum free medium, (iii) and dilution of encysting cultures to cell densities below that known to support full encystment (from 5 × 105 to 1 × 104 cells/ml). The role of serum components (and the gal-terminated ligand asialofetuin; ASF) adsorbed onto the surface upon which encystment proceeds, and their effect on the multi-cellular aggregation patterns formed during encystment, were also investigated. The addition of free galactose reduced the levels of encystment (compared with the control) even when added at 10 h after resuspension of trophozoites in 47% LG. The requirement for the presence of ABS during encystment was lost within 6 h, with levels of encystment of cells washed free of serum reaching 80% of the control. The ability of cells to encyst when diluted to a cell density below that normally thought to support encystment reached over 50% by 8 h. Efficient encystment could be obtained in 47% LG in the absence of ABS or ASF using pretreated glass culture tubes. Encystment (47% LG; 5% ABS) using ultra low attachment plates was poor, suggesting attachment of cells to a surface via gal-terminated ligands was important for efficient encystment.
The results suggest that ABS is probably not the only source of gal-terminated ligands necessary for high levels of encystment in 47% LG. While serum may provide a source of ligands which enhance the levels of encystment initially, other gal-terminated ligands possibly released by the encysting cells are still required for the completion of the encystment process and the formation of mature cysts. In addition, the gal-terminated ligands necessary for encystment efficiency may be adsorbed onto the glass surface of culture tubes and aid the initial aggregation process, as well as be involved in cell signaling during the encystment process.
Keywords: trophozoite, cyst, encystment, galactose lectin, gal-terminated ligands, adsorption
Introduction
Amebiasis is a potentially life threatening infection of the human gastrointestinal tract that accounts for approximately 70 thousand deaths each year worldwide and is associated with the protozoan parasite Entamoeba histolytica (Espinosa-Cantellano and Martinez-Palomo, 2000). Entamoeba invadens is a parasitic amoeba of reptiles, which has a life cycle very similar to E. histolytica and has served as an experimental model for the human parasite especially with regards to the differentiation process (Coppi and Eichinger, 1999; Coppi et al., 2002; Eichinger, 2001).
During periods of stress, trophozoites of E. invadens and E. histolytica undergo a process of differentiation (encystment) that results in a dormant cyst with a chitin containing cyst wall. The cyst is believed to be important in the transmission of the organism from one individual to another (Espinosa-Cantellano and Martinez-Palomo, 2000; Eichinger, 2001; Haque et al., 2003). The conditions within the infected colon that trigger the differentiation of the vegetative trophozoites are not known. While E. histolytica does not readily differentiate under axenic culture conditions in vitro, E. invadens (strain IP-1) can be induced to encyst by transfer of trophozoites from their growth medium (TYI-S-33) into a diluted glucose free medium (47% LG) containing 5% adult bovine serum (ABS). ABS is thought to provide a source of galactose (gal) -terminated ligands which have been shown to be required for efficient in vitro encystment of E. invadens (Coppi and Eichinger 1999; Eichinger, 2001). Mucin, galactosylated bovine serum albumin (galBSA), or asialofetuin (ASF) have been demonstrated to substitute for the requirement for ABS in 47% LG providing an alternative source of polyvalent gal-terminated ligands needed for efficient in vitro encystment. A narrow effective concentration range for efficient encystment was observed suggesting a specific ligand to lectin ratio was required within the encystment medium to convey the signal to proceed with encystment. [Coppi and Eichinger, 1999].
Upon transfer to the encystment medium trophozoites of E. invadens form multicellular aggregates within which differentiation proceeds. Gal-terminated ligands from the ABS are believed to be important for the initial aggregation phase of in vitro encystment and may act as a signal for the differentiation process acting via the galactose inhibitable cell surface lectins of E. invadens (Coppi and Eichinger, 1999; Eichinger, 2001). High concentrations (10 mM) of free-galactose or N-acetylglucosamine (glcNAc) inhibits in vitro encystment of E. invadens at temporally separated steps of the encystment pathway, with free-galactose inhibiting the initial aggregation phase of the encystment and glcNAc inhibiting the aggregated trophozoites from forming mature cysts (Cho and Eichinger 1998; Coppi and Eichinger, 1999). This is thought to be due to their ability to interfere with lectin function and the transmission of encystment-inducing signals to within the cell.
We were interested in the relationship between the need for gal-terminated ligands during encystment and their effect on the differentiation process itself. The effects of high concentrations of free-galactose and glcNAc during the encystment process were investigated, as was the effects of cell density within encystment cultures, which is also believed to be important for a high efficiency of encystment.
Materials and Methods
Materials
24-well ultra low attachment plates (Corning Costar), 24-well tissue culture treated plates (Corning Costar) and 9 ml borosilicate glass screw-cap culture tubes were obtained from Fisher Scientific (Pittsburgh, Pennsylvania, USA). Asialofetuin (ASF), D-galactose, N-acetylglucosamine (glcNAc), as well as all other reagents and media were obtained from Sigma-Aldrich (St. Louis, Missouri, USA).
Organism and culture
Entamoeba invadens (strain IP-1) was purchased from the American Type Culture Collection and maintained at 25°C in 50 ml culture flaks containing 50 ml of TYI-S-33 medium (Diamond, 1987) supplemented with 10% adult bovine serum (ABS).
Encystment method
Growth phase trophozoites (cultured as above) were harvested (800 g for 5 min) from 50 ml culture flasks, which had previously been chilled on ice for 10 min. The harvested trophozoites were washed with 50 ml ice-cold LG (TYI-S-33 without glucose) and resuspended in 9 ml of encystment media at a final concentration of 5 × 105 cells/ml, before transfer to 9 ml glass screw-cap culture tubes. The encystment media consisted of 47% v/v LG diluted with deionized water (47% LG only or 47% LG supplemented with either 5% v/v ABS or 1 μg/ml ASF). The tubes were incubated horizontally at 25°C for 48 h. After 48 h, the culture tubes were chilled on ice and a total cell count (trophozoites and cysts) was performed on 1ml samples from each tube.
Encystment efficiency
Samples (1 ml) from 48 h old encystment cultures were centrifuged at 800 g for 5 min, before the pellet was resuspended in 1 ml of ice-cold 0.1% g/v Sarkosyl and incubated on ice for 10 min. Cell counts were then performed using a haemacytometer and the total number of detergent-resistant cysts was determined. The percentage encystment was calculated as the percentage of an appropriate control culture or as a percentage of the total cell count (trophozoites and cysts) of a culture sample untreated with detergent. All encystment experiments were performed with duplicate or triplicate cultures for each induction condition, and data are presented as mean ± SD.
Time point analyses of the sensitivities to the addition of free galactose or N-acetylglucosamine (glcNAc), and the need for a specific cell density or serum
Encystment cultures using log phase trophozoites were prepared as described above. The cells were resuspended in 9 ml of encystment medium (47% LG) containing 5% ABS (time=0) to give a final cell density of 5 × 105 cells/ml. Culture tubes containing the suspensions were then incubated horizontally at 25 °C until required for sampling.
For convenience, two separate assessment periods were carried out, viz. 0–10 h and 12–24 h. Every 2 h, sample culture tubes were chilled on ice for 10 min and each was processed in one of the following ways: (i) after centrifugation (800 g for 5 min), 0.5 ml of medium was removed and a 0.5 ml solution of either free-galactose or glcNAc was added to give a final concentration of 10 mM after mixing; (ii) the cell cultures were washed twice in ice-cold LG (tubes were centrifuged; 800 g for 5 min) and resuspended in 9 ml of 47% LG containing no serum; (iii) after thorough mixing to disperse clumped cells, 1ml of the cell suspension was transferred to a 50 ml culture flask containing 49 ml of 47% LG (supplemented with 5% ABS) to give a final cell density of approximately 1 × 104 cells/ml. The processed culture tubes or flasks were then reincubated as described above. Control sample tubes for the above experiments were treated similar to each of the test samples, except and respectively for each of the above mentioned procedures: deionized water (0.5 ml) was added to control tubes as an alternative to the test solution; samples were resuspended in 47% LG supplemented with 5% ABS instead of 47% LG without ABS; and finally, no cells were removed from the control tubes before reincubation along side the culture flasks. Encystment cultures using 47% LG (supplemented either with or without ABS) were also setup at the start of each experiment in order to determine the overall effect of the individual procedures on encystment efficiency. After 48 h cell counts were performed and levels of detergent resistance (to 0.1% g/v Sarkosyl for 10 min on ice) were established as an indicator of cyst maturity (see above).
Pretreatment of culture tubes with adult bovine serum (ABS) or asialofetuin (ASF) prior to use with encystment cultures
Glass culture tubes were treated for 10 min at room temperature with 9ml of 47% LG supplemented with 5% v/v ABS. The tubes were then washed five times with deionized water (9 ml for each wash) prior to their use with encystment experiments (at a cell density of 5 × 105 cells/ml) as described previously. Encystment cultures using untreated glass culture tubes and medium (47% LG) supplemented either with or without 5% v/v ABS were also prepared as controls. All the encystment culture tubes were incubated horizontally at 25 °C and after 48 h cell counts were performed and levels of detergent resistance were established (see above).
The procedure was similar for experiments involving the serial transfer of cell-free encystment medium from one 9 ml glass culture tube to another except the 9 ml of medium used for the conditioning of the culture tubes (47% LG containing either 5% v/v ABS or 1 μg/ml ASF) was not discarded (after incubation with the tube for 5 min at room temperature), but instead was transferred to a new 9 ml culture tube. The conditioned culture tubes were rinsed five times with 9 ml of deionized water after each transfer. The transfer of the same cell-free encystment medium between glass culture tubes was repeated through a series of four glass culture tubes prior to their use with encystment cultures as described above. Encystment cultures using untreated glass tubes and encystment medium (47% LG) supplemented either with or without 5% v/v ABS or 1 μg/ml ASF, were also prepared for comparisons of encystment efficiency. Cell counts were performed on the encystment cultures after 48 h of incubation (horizontally at 25 °C) and levels of detergent resistance were established as described above.
To investigate the effect on encystment of the transfer of cells to serum free medium at various times during encystment chilled (10 min on ice) log phase trophozoites were harvested from their growth medium (as described previously) before being resuspended (to give a final cell density of 5 × 105 cells/ml) in 9 ml of encystment medium (47% LG) supplemented with 5% ABS (time=0). The suspensions were then transferred to 9 ml glass culture tubes and incubated (tubes placed horizontally) at 25 °C until required for sampling. Encystment cultures both with and without 5% v/v ABS were also prepared as controls and for the establishment of encystment efficiency. Every 2 h (up to 10 h) sample culture tubes were chilled on ice for 10 min prior to the harvested cells being washed twice with, and finally being resuspended in, 9 ml of ice cold serum free 47% LG. The resuspended cells were then transferred either back to their original glass culture tube (rinsed twice with deionized water) or a new clean 9 ml glass culture tube before reincubated as described above. After 48 h cell counts were performed and levels of detergent resistance were established (see above). Data from this experimental procedure were analyzed for the statistical significance of any differences between the encystment efficiencies of the two treatments. The Student’s t-test was used with a probability value of p < 0.05 being considered as statistically significant.
Comparison of encystment efficiency using either tissue culture treated plates or ultra low attachment plates
Growth phase trophozoites were harvested for use in encystment cultures as described previously except cells were resuspended in encystment medium (47% LG; to a cell density of 5 × 105 cells/ml) containing various concentration of either ABS or ASF. Each well of a 24-well ultra low attachment plate (Corning Costar) or a 24-well tissue culture treated plate (Corning Costar) was filled with 3 ml of the above cell suspension. Control wells were filled with 3 ml of cells suspended in 47% LG without either ABS or ASF. After 48 h of incubation at 25°C cell counts were performed and detergent resistance levels of the mature cysts were established (see above).
Results
For the purpose of this study, percentage encystment refers to the percentage of cysts, compared with an appropriate control, able to withstand detergent treatment with 0.1% Sarkosyl for 10 min on ice. This is an arbitrary measurement of cyst maturity/resistance often employed in similar studies of Entamoeba differentiation.
Time point analyses of the sensitivities to the addition of free galactose or N-acetylglucosamine (glcNAc), and the need for a specific cell density or serum
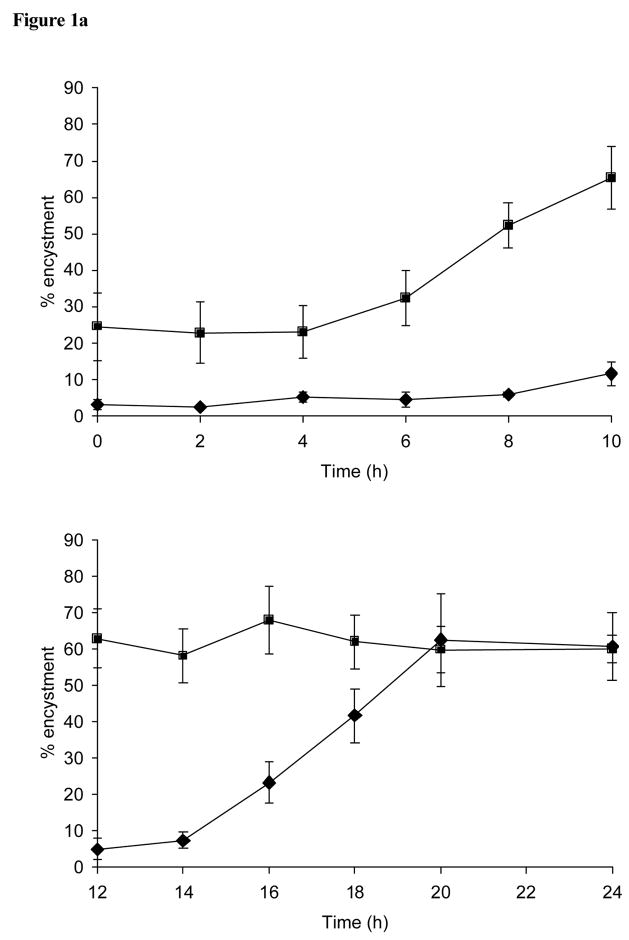
The addition of free galactose (10 mM) to encystment cultures of E. invadens at various times during encystment substantially reduced the levels of detergent resistant cysts counted 48 h later (compared with the control), even when added at 10 h after resuspension of trophozoites in to 47% LG containing 5% ABS (Figure 1a). The inhibitory effect of free galactose on the encystment process was eventually lost by 20 h. The inhibitory effect of the addition glcNAc (10 mM) at various times during encystment was lost within the first 10 h of encystment (Figure 1a).
Figure 1.
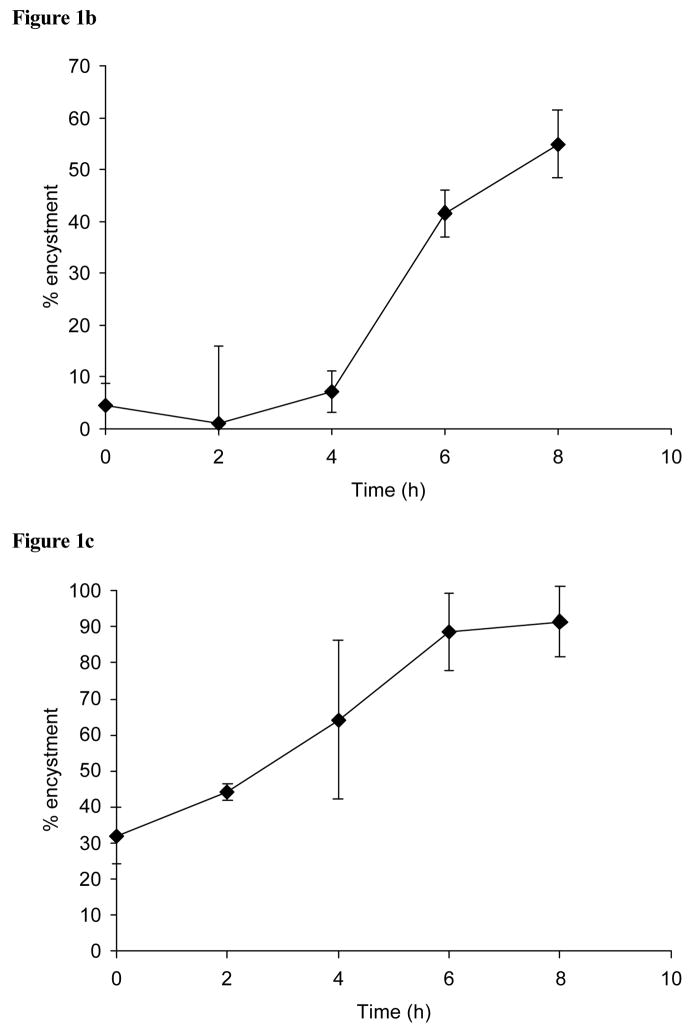
(a) Effect of the addition of free galactose and glcNAc during encystment. Shown is the percentage of encystment obtained at 48 h after free galactose (10 mM; diamond symbol) or glcNAc (10 mM; square symbol) were added at various times during encystment. (b) The loss of cell concentration dependant inhibition during encystment. Samples were removed from cultures (at an optimal density of 5 × 105 cells/ml) at various times during encystment, resuspended at a concentration that would normally be unable to support differentiation (1 × 104 cells/ml), and assessed for encystment after a total of 48 h. (c) Effect of the transfer of cells to serum-free medium (47% LG) during encystment. Cell samples taken at various times during encystment were washed free from ABS, resuspended in serum-free 47% LG, and assessed for encystment after a total of 48 h.
The ability of cells taken at various times during the assessment period to become detergent resistant after their adjustment to a cell density that would normally be unable to support encystment was greater than 50% by 8 h (Figure 1b). The requirement during encystment for the inclusion of ABS in the encystment medium (47% LG) was largely lost within 6 h, with the levels of encystment of cells washed free of serum at various times during encystment reaching 80% at this and a subsequent 8 h time point (Figure 1c).
Pretreatment of culture tubes with adult bovine serum (ABS) or asialofetuin (ASF) prior to use with encystment cultures
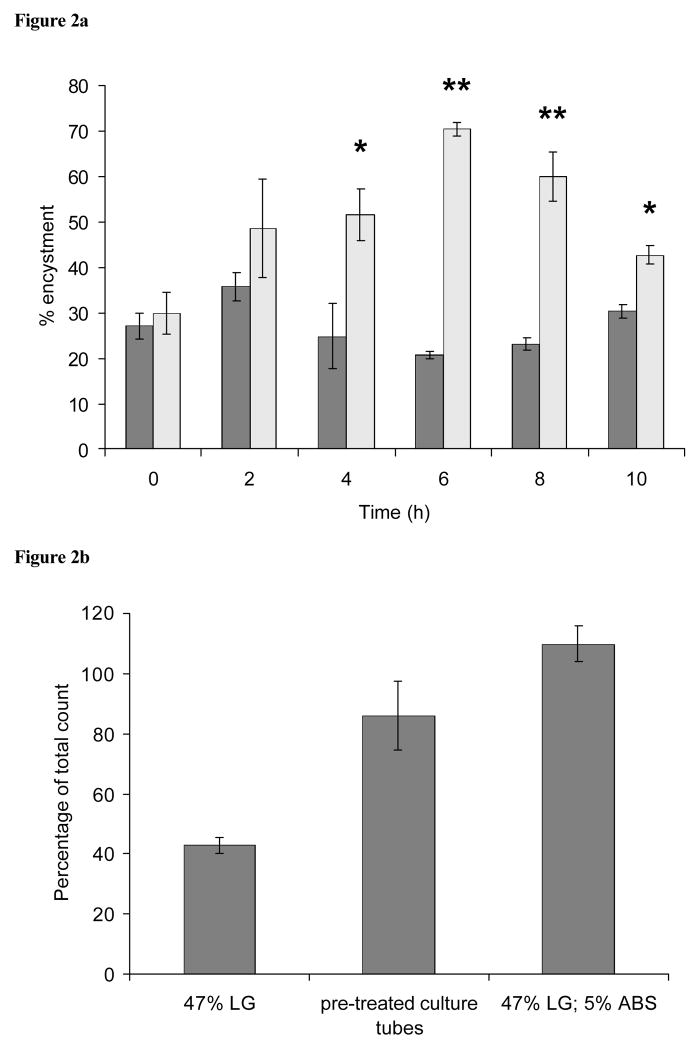
Another experiment examining the effect of the transfer of cells to serum free medium (47% LG) during encystment is shown in Figure 2a. In this case, cell samples taken at various times during encystment (with encystment medium containing 47% LG; 5% ABS) were washed free from serum, resuspended in 47% LG (no serum), and returned either to the original glass culture tube (rinsed twice with deionized water) or to a clean culture tube. Significant differences (p < 0.05) in encystment efficiency of the two treatments were observed after 2 h, with highly significant levels of encystment (p < 0.01) being observed by 6 h when differentiating cells were transferred back to their original culture tube. In contrast, cells transferred to new culture tubes produced low levels encystment, even after 10 h prior exposure to encystment media containing 5% ABS.
Figure 2.
(a) Effect of the transfer of cells to serum free medium (47% LG) and different tube types during encystment. Cell samples taken at various times during encystment were washed free from ABS and resuspended in serum-free 47% LG. They were then transferred either to the original glass culture tube (rinsed twice with LG; striped columns) or a new culture tube (grey columns). *p < 0.05, and **p < 0.01, compared to the encystment efficiency of samples transferred to new culture tubes (n = 3). (b) Encystment in glass culture tubes treated with ABS. Culture tubes were treated with 5% ABS for 10 minutes and washed 5 times with deionized water prior to the addition of cells. The first two columns show encystment of cells that were suspended in 47% LG (no serum). In the last column cells were suspended in 47% LG containing 5% ABS.
The previous results suggested that medium components provided a conditioning effect on the culture tubes that stimulated encystment. To directly test this, glass culture tubes were pre-treated with 47% LG, 5% ABS for 10 minutes, and washed 5 times with deionized water. Cells adjusted to 5 × 105 cells/ml in 47% LG (no serum) and placed in these tubes were capable of encysting with high efficiency, reaching ca. 80% of the control (cells suspended in 47% LG containing 5% ABS; Figure 2b).
To further test the conditioning effect, cell-free encystment medium (containing either 5% ABS or 0.1 μg/ml ASF), was serially transferred from one glass culture tube to another (with an exposure time of 5 min) prior to repeated washings with deionized water. There was a progressive loss of encystment efficiency when the tubes were then used to hold encystment cultures (Table 1). After 2 and 1 transfer step(s), for medium containing ABS and ASF, respectively, the levels of encystment obtained with the treated tubes diminished to values similar to that of the untreated control (47% LG; no serum).
Table 1.
Treatment of glass culture tubes by serial transfer of cell-free encystment medium from one tube to another. Tubes were pre-treated for 5 min with 47% LG containing either adult bovine serum (ABS) or asialofetuin (ASF) prior to washing and use with encystment cultures. First two rows of results show encystment efficiency using the conventional encystment method.
| Pre-treatment of glass culture tubes (percentage of control) | ||||
|---|---|---|---|---|
| Culture conditions | ABS 5% (v/v) | ASF 0.1 (μg/ml) | ||
| Controls (with cells) | No ABS or ASF | 27.0 ± 7.1 | 32.4 ± 3.9 | |
| With ABS or ASF | 82.8 ± 17.0 | 57.4 ± 4.6 | ||
| Transfer of cell-free medium along a series of glass culture tubes | Culture tube 1 |
|
65.1 ± 6.5 | 40.3 ± 6.2 |
| Culture tube 2 | 58.3 ± 3.2 | 26.5 ± 7.8 | ||
| Culture tube 3 | 40.9 ± 2.3 | 21.2 ± 8.0 | ||
| Culture tube 4 | 40.6 ± 1.6 | 24.0 ± 10.8 | ||
The cellular aggregation patterns observed in encystment cultures using tubes pre-treated with either ABS or ASF differed slightly from those observed during encystment with serum or ASF present. The multiple, discrete, multi-cellular aggregates normally observed within encystment cultures (when using either 5% ABS or 0.1 μg/ml ASF) were not present in the pretreated tubes. Instead, a more continuous layer of cells was initially observed which developed into a pavement-like pattern as encystment proceeded (within the first few hours of encystment).
Comparison of encystment efficiency using either tissue culture treated plates or ultra low attachment plates
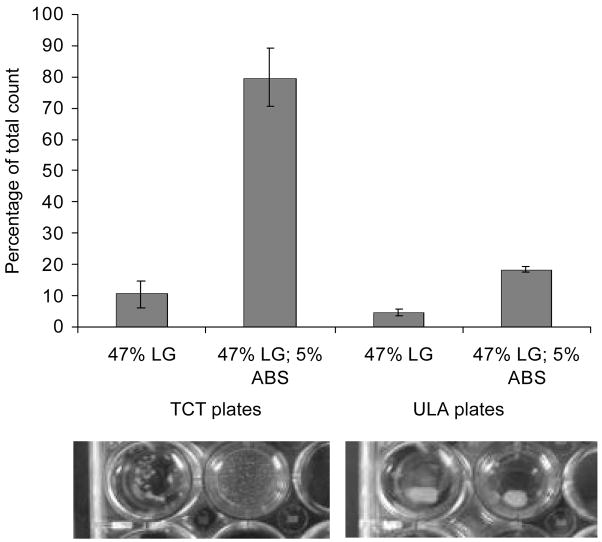
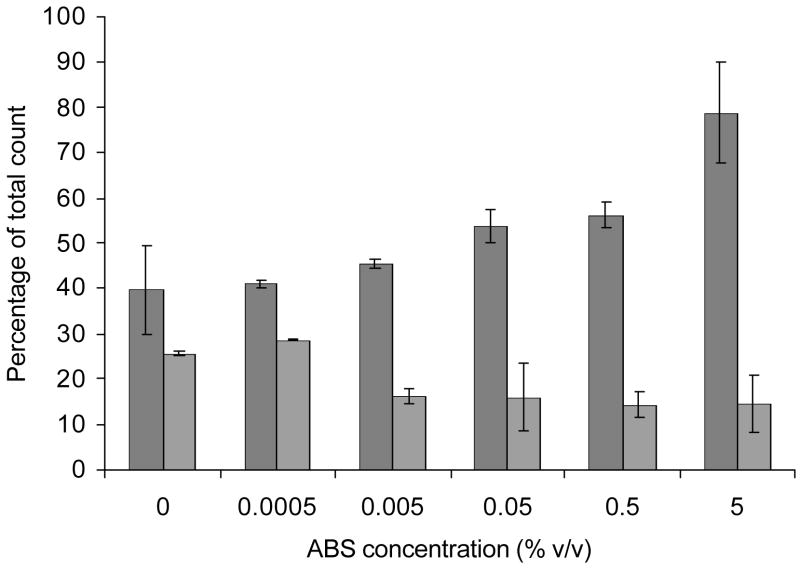
A comparison of encystment efficiency of E. invadens obtained when using either tissue culture treated (TCT) plates or commercially available ultra low attachment (ULA) plates which have a covalently bonded hydrogel surface (that is hydrophilic and neutrally charged) are displayed in Figure 3a and 3b. ULA plates were unable to support efficient encystment, even when 5% ABS or 0.1 μg/ml ASF (not shown) was present in the encystment medium (47% LG). The levels of encystment obtained were similar to TCT plates when no serum was included in the medium. The multi-cellular aggregation patterns formed in the ULA plates differed from those formed in the TCT plates. The encystment-induced trophozoites attached to the bottom of TCT plates and formed numerous relatively uniform-sized discrete multi-cellular aggregates. However, at no point during encystment did the cells attach to the covalently bonded hydrogel surface of the ULA plates. Instead, the majority of the cells formed a single, large clump (Figure 3a).
Figure 3.
(a) Comparison of encystment efficiency using either tissue culture treated (TCT) plates or ultra low attachment (ULA) plates. First two columns show encystment efficiency using TCT plates and the last two columns show encystment efficiency using ULA plates (cells suspended in 47% LG either supplemented with or without 5% ABS). (b) Comparison of encystment efficiency in 47% LG using either TCT plates (gray columns) or ULA plates (striped columns) with various ABS concentrations. Shown is the percentage of encystment obtained at 48 h as a percentage of the total cell count.
Discussion
The human parasite E. histolytica is believed to proliferate within the mucus layer of the colon (Eichinger, 2001). Trophozoites express a heterodimeric galactose/N-acetylgalactosamine (gal/galNAc)-specific lectin on their surface which has a high affinity for the macroclusters of ligand sugars present on the colonic forms of mucin, the major glycoprotein of the mucus layer (Chadee et al., 1987; Alder et el., 1995). Integrin-like sequences found in the cytoplasmic domain of the heavy subunit of the gal/galNAc lectin suggests a transmembrane signaling function for the lectin (Vines et al., 1998). An equivalent lectin (gal lectin) is also expressed on the surface of trophozoites of the reptile parasite E. invadens, which, however, has a specificity limited to galactose (Coppi and Eichinger, 1999). It has been proposed that as the cell density increases, the colonic mucus layer is depleted by enzymes expressed by the amoeba and a point is reached where by encystment is triggered by the sensing of the decreased mucin concentration via clustering of lectin molecules on the trophozoites surface (Eichinger, 2001).
In vitro, encystment of E. invadens can be stimulated by a change in either osmotic conditions and/or removal of glucose from the growth medium (Vazquezdelara-Cisneros and Arroyo-Begovich, 1984; Avron et al., 1986; Sanchez et al., 1994). An optimum concentration of cells harvested from an exponential growth phase culture, and the inclusion of serum (5% bovine serum) in the encystment medium, are required for efficient differentiation of E. invadens (Coppi and Eichinger, 1999). Gal-terminated ligands such as mucin, galBSA, or asialofetuin (ASF) have been shown to functionally replace the requirement for serum when combined with the above stimuli. Coppi and Eichinger, (1999) demonstrated that during the induction phase of encystment high concentrations of free-galactose (10 Mm) prevented the formation of multi-cellular aggregates and the accumulation of three gene transcripts that were usually upregulated during encystment. High concentrations of GlcNAc prevented accumulation of one of the three encystment-marker transcripts, and multi-cellular aggregates formed but did not go on to yield detergent-resistant cysts (Coppi and Eichinger, 1999).
The inhibitory effect on encystment of the two sugars when added at various times during encystment (Figure 1a) imply that at high concentrations glcNAc affects a process that occurs after the formation of multi-cellular aggregates but does not effect cell wall synthesis once it has been initiated. Where as, loss of free galactose-mediated inhibition was observed during a period associated with increases in detergent resistance (by 20 h) - presumably after the chitin wall synthesis phase had already been initiated. Further to this, even though the differentiation process resulting in a mature dormant cyst may take several days to complete (Sanchez et al., 1994; Silberman et al., 1999), the induction phase probably lasts a relatively short period early on in the encystment process, after which differentiation may proceed in the absence of serum or direct cell to cell contact (Figure 1b and 1c).
The differing timing of the inhibitory effects on encystment of galactose and glcNAc could suggest that ABS is not the only source of ligands necessary for high levels of encystment in 47% LG. While the serum may provide a source of ligands which enhance the levels of encystment initially (and can be substituted by gal-terminated molecules), other carbohydrate-terminated ligands, possibly released by the encysting cells or contained in the medium, are still required for the completion of the encystment process and the formation of mature cysts. Alternatively free galactose may interfere with the synthesis or assembly of the chitin-containing cyst wall, rendering cells less resistant to detergent treatment. A cyst wall glycoprotein with an apparent molecular mass of ~100 kDa and a ladder-like series of Cys-rich, chitin-binding domains has been identified in the cyst wall of E. invadens (Frisardi et al., 2000; Van Dellen et al., 2002). Frisardi et al. (2000) suggested that free galactose interferes with wall synthesis by preventing the cell surface gal lectin from binding to the glycoprotein (and possibly other cyst wall glycoproteins). However, free galactose may also have an effect on glycoprotein and chitinase secretion during encystment (Frisardi et al., 2000).
The significant differential encystment response noted when cell samples taken during encystment were washed free from ABS and then transferred either back into their original glass culture tube or to a clean glass culture tube suggested that a component of the ABS was being adsorbed onto the glass surface (Figure 2a). Pre-treatment of the glass culture tubes prior to their use for encystment cultures was also able to support efficient encystment (Figure 2b). Further to this, the serial transfer of cell-free encystment medium (containing either 5% ABS or 01 μg/ml ASF) from one glass culture tube to another prior to washing and use with encystment cultures further suggested that the positively charged (hydrophilic) glass surface of the culture tubes was adsorbing gal-terminated ligands from the medium (Table 1). The pavement-like pattern of aggregation of induced trophozoites observed during encystment using treated tubes was presumably due to the cells binding to the ligand-covered surface preferentially, rather than to each other by either direct cell-to-cell interactions or crosslinking interactions via serum components or multivalent gal-terminated ligands.
A comparison of encystment efficiencies as well as the aggregation patterns observed using TCT and ULA plates suggests that attachment of the cells to a surface, via gal-terminated ligands, is important for efficient encystment in vitro (Figure 3a and 3b). Further, adsorption of serum components or gal-terminated ligands to the surface upon which encystment is proceeding may be more important than gal-terminated ligands in solution. This being the case, then only those cells in contact with the adsorbed ligands would be competent to encyst and the majority of cells within the multi-cellular aggregates (to which the adsorbed ligands are unavailable) would not be expected to encyst. The high encystment efficiency would suggest that the encystment-competent attached cells maybe able to influence the encystment efficiency of other cells within the multi-cellular aggregate. The nature of this possible signaling event was not determined during this study, but a signaling mechanism has been proposed involving a presumed catecholamine-type receptor that functions downstream of the gal lectin and upstream of adenylyl cyclase during encystment of E. invadens (Coppi et al., 2002).
An individual infected with Entamoeba may produce hundreds of thousands of cysts per day. However, the conditions that trigger encystment in the infected colon are not known (Mathur and Kaur, 1973). It is also unknown whether the cellular aggregation phase observed prior to encystment in vitro would occur in vivo. Galactose-terminated molecules have been shown previously to regulate in vitro encystment of E. invadens (Coppi and Eichinger, 1999). The effectiveness of each of the molecules was confined to a specific narrow concentration range and it was suggested that galactose-binding molecules on the amoeba’s surface monitor and transmit signals in response to changes in the concentration of gal-terminated ligands (e.g. mucin) in the infected reptile colon (Coppi and Eichinger, 1999; Eichinger, 2001). Alternatively, the ligand concentration required for efficient encystment may reflect a concentration needed for a sufficient amount of adsorption of the ligand to the surface upon which the encysting cells attach. Concentrations above and below this may interfere with attachment to the surface and the formation of aggregates.
The results of this study confirm that the aggregation phase of the encystment process is an important period in which differentiation-specific ligand-induced signaling takes place. In addition, even though the differentiation process may take several days to complete, the induction phase probably lasts a relatively short period of time (8–10 h), after which differentiation may proceed in the absence of serum or direct cell-to-cell contact. The gal-terminated ligands which are important for encystment efficiency may be adsorbed onto the glass surface of culture tubes and aid the initial aggregation process, as well as be involved in cell signaling during the encystment process. A possible explanation for the requirement of gal-terminated ligands during encystment of Entamoeba may be that for the cyst stage in the life cycle to be a useful means for survival in the open environment a certain level of maturity would need to be acquired before being expelled from their host. Since the encystment process may require up to two days for completion (in vitro) one could speculate that upon local unfavorable conditions within the colon the attachment and retention of a colony to the colonic mucin layer may allow the differentiating cells to attain a certain level of maturity before their expulsion into the environment. A prerequisite of attachment to the mucin layer during encystment may therefore prevent cysts being passed which are too immature to survive in the environment outside of the host.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler P, Wood SJ, Lee YC, Lee RT, Petri WAJ, Schnaar RL. High affinity binding of the Entamoeba histolytica lectin to polyvalent N-acetylgalactosaminides. Journal of biological chemistry. 1995;2780:5164–5171. doi: 10.1074/jbc.270.10.5164. [DOI] [PubMed] [Google Scholar]
- Avron B, Stolarsky T, Chayen A, Mirelman D. Encystation of Entamoeba invadens IP-1 is induced by lowering the osmotic pressure and depletion of nutrients from the medium. Journal of protozoology. 1986;33:522–525. doi: 10.1111/j.1550-7408.1986.tb05655.x. [DOI] [PubMed] [Google Scholar]
- Chadee K, Petri WAJ, Innes DJ, Ravdin JI. Rat and human colonic mucins bind to and inhibit the adherence lectin of Entamoeba histolytica. Journal of clinical investigation. 1987;80:1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Eichinger D. Crithidia fasciculata induces encystation of Entamoeba invadens in a galactose-dependent manner. Journal of parasitology. 1998;84:705–710. [PubMed] [Google Scholar]
- Coppi A, Eichinger D. Regulation of Entamoeba invadens encystation and gene expression with galactose and N-acetylglucosamine. Molecular and biochemical parasitology. 1999;102:67–77. doi: 10.1016/s0166-6851(99)00085-7. [DOI] [PubMed] [Google Scholar]
- Coppi A, Merali S, Eichinger D. The enteric parasite Entamoeba uses an autocrine catecholamine system during differentiation into the infectious cyst stage. Journal of biological chemistry. 2002;277:8083–8090. doi: 10.1074/jbc.M111895200. [DOI] [PubMed] [Google Scholar]
- Diamond LS. Entamoeba. In: Taylor AE, Baker JR, editors. In Vitro Methods for Parasite Cultivation. Academic Press; London: 1987. pp. 1–17. [Google Scholar]
- Eichinger D. A role for a galactose lectin and its ligands during encystment of Entamoeba. Journal of eukaryotic microbiology. 2001;48:17–21. doi: 10.1111/j.1550-7408.2001.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Espinosa-Cantellano M, Martinez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clinical microbiology reviews. 2000;13:318–331. doi: 10.1128/cmr.13.2.318-331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisardi M, Ghosh SK, Field J, Van Dellen K, Rogers R, Robbins P, Samuelson J. The most abundant glycoprotein of amebic cyst walls (Jacob) is a lectin with five Cys-rich, chitin-binding domains. Infection and immunity. 2000;68:4217–4224. doi: 10.1128/iai.68.7.4217-4224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. New England journal of medicine. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- Mathur TN, Kaur J. The frequency of excretion of cysts of Entamoeba histolytica in known cases of non-dysenteric amoebic colitis based on 21 stool examinations. Indian journal of medical research. 1973;61:330–334. [PubMed] [Google Scholar]
- Sanchez L, Enea V, Eichinger D. Identification of a developmentally regulated transcript expressed during encystation of Entamoeba invadens. Molecular and biochemical parasitology. 1994;67:125–135. doi: 10.1016/0166-6851(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Silberman JD, Clark CG, Diamond LS, Sogin ML. Phylogeny of the genera Entamoeba and Endolimax as deduced from small-subunit ribosomal RNA sequences. Molecular biology and evolution. 1999;16:1740–1751. doi: 10.1093/oxfordjournals.molbev.a026086. [DOI] [PubMed] [Google Scholar]
- Van Dellen K, Ghosh SK, Robbins PW, Loftus B, Samuelson J. Entamoeba histolytica lectins contain unique 6-Cys or 8-Cys chitin-binding domains. Infection and immunity. 2002;70:3259–3263. doi: 10.1128/IAI.70.6.3259-3263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquezdelara-Cisneros LG, Arroyo-Begovich A. Induction of encystation of Entamoeba invadens by removal of glucose from the culture medium. Journal of parasitology. 1984;70:629–633. [PubMed] [Google Scholar]
- Vines RR, Ramakrishnan G, Rogers JB, Lockhart LA, Mann BJ, Petri WA., Jr Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a beta2 integrin motif. Molecular biology of the cell. 1998;9:2069–2079. doi: 10.1091/mbc.9.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]