Abstract
Background
Purinergic (P2Y) receptors play an important role in intracellular Ca2+ regulation in hepatocytes. Prevention of mitochondrial Ca2+ (mCa2+) overload during ischemic conditions prevents cellular cell death during the early reperfusion period. P2Y-antagonists are cytoprotective in other settings. We studied the effect of P2Y receptor antagonism on mitochondrial associated cell death during the period of cold storage.
Methods
HepG2 cells were stored in UW with or without 300 μM reactive blue 2 (RB2) or 10μM ruthenium red (RR) under either normoxic-hypothermic (NH) or hypoxic-hypothermic (HH) conditions. Cytoplasmic cytochrome c levels were studied by transfection of cytochrome c-GFP. Immunofluorescence determined the intracellular, spatio-temporal distribution of Bax, and TUNEL staining was used to evaluate cell death. Intracellular compartmental ATP levels were assayed by transfecting with luciferase vectors specific for cytoplasm (PcDNA3-luciferase-LL/V) and mitochondria (PcDNA3-COX8-luciferase).
Results
Bax translocation to the mitochondria occurred immediately following cold storage and was followed by cytochrome c-GFP redistribution to the cytosol during rewarming. RB2 treatment significantly attenuated Bax translocation, cytochrome c-GFP redistribution, and cell death following both storage conditions. Both RR and RB2 provided cytoprotection despite ongoing cytoplasmic ATP consumption during cold ischemia.
Conclusion
These data indicate that the cytoprotective effects of mCa2+ uptake inhibition and P2Y receptor antagonism are independent of cytoplasmic ATP levels during cold ischemia.
Introduction
Purines and pyrimidines are important signaling molecules that affect a diverse array of cellular processes such as proliferation, differentiation, and cell death by interacting with purinergic receptors1–3. These receptors include the ligand gated channel P2X receptors and the G-protein coupled P2Y receptors1. More specifically, the mammalian P2Y receptors are coupled to the hydrolysis of phosphatidylinositol 4,5-bisphosphate and subsequent inositol 1,4,5-triphosphate-mediated release of intracellular Ca2+ 1,4. Purinergic receptors play important roles in hepatocyte intracellular Ca2+ signaling, such as the response to osmotic shifts and cellular swelling5–9. Intracellular Ca2+ regulation is also the proposed mechanism underlying the P2 receptor mediation of glycogen metabolism and cellular proliferation, in hepatocytes10.
Altered intracellular and mitochondrial Ca2+ (mCa2+) regulation during periods of ischemia and reperfusion contribute to primary hepatocyte injury. Cold ischemia is known to induce an increase in cytosolic Ca2+ concentration via both release from intracellular stores and increased cellular uptake11–13. Depletion of cellular ATP during periods of ischemia acts synergistically with the elevated cytoplasmic Ca2+ concentration to cause activation of the mCa2+ uniporter14–19. Mitochondria were thought initially to serve as simple calcium buffers during conditions causing increased cytosolic calcium, such as ischemia. However, it is now known that mCa2+ uptake during these conditions serves to stimulate mitochondrial ATP production as part of a still poorly defined feedback mechanism20. Recently described P2Y receptors in the mitochondrial membrane, which play a role in the regulation of mCa2+ uptake, may play a role in this feedback mechanism6,20,21. Despite the initial increase of ATP production, as ischemia persists, electron transport is stopped and the continued mitochondrial accumulation of calcium compromises the mitochondrial membrane potential (ΔΨ). In an attempt to maintainΔΨ, F0F1 synthase reverses its activity, and the mitochondria becomes an ATP consumer via reversal of the adenine nucleotide transporter (ANT)22. This preserves ΔΨ for the short term, but also allows ongoing uptake of calcium and further depletion of cellular ATP. Ultimately, mCa2+ overload initiates mitochondrial associated apoptosis and cell death.
While little is known about the role of hepatocyte P2Y receptors in cold ischemia and reperfusion injury, available data suggests that adenosine nucleotides can act directly on P2Y receptors to induce cell death23–25. Stimulation of P2 receptors by ATP has been implicated in many models of apoptosis and neurotoxicity3,23,26–28, and P2Y antagonists have been demonstrated to provide neuroprotection during ischemia29–31. The P2Y antagonists Suramin and RB2 have been shown to provide neuroprotection following exposure to dequalinium by preserving ΔΨ32.
We have recently demonstrated that direct antagonism of the mCa2+ uniporter prevents a Bax-dependent apoptosis early during the reperfusion period33. Because of the ischemic neuroprotection afforded by P2Y antagonism in some models and the close relationship between purinergic receptor activation and intracellular calcium regulation, we investigated the role of reactive blue 2 (RB2), a non-competitive P2 inhibitor which does not discriminate between P2Y receptor subtypes1, in hepatocyte cold ischemia and reperfusion injury. We report that addition of RB2 to the cold storage solution attenuates Bax-dependent early reperfusion cell death, similar to that observed when the mCa2+ calcium uptake is inhibited by the well established mCa2+ uniporter inhibitor, ruthenium red (RR). In addition, both RB2 and RR provide their cytoprotection despite ongoing cellular ATP consumption.
Materials and Methods
Cell culture and cold ischemic conditions
The human HepG2 hepatoblastoma cell line (HepG2, ATCC, Rockville, MD, Catalog No. HB-8065) was chosen because of its stability and predictable growth as well as retained characteristics of primary hepatocytes34–37. Cells were grown at 37°C, 5% CO2 in MEM (ATCC, Manassas, VA), with 10% fetal bovine serum (Cascade Biologicals, Winchester, MA), and 1% penicillin/streptomycin (Gibco, Grand Island, NY) to 80% confluence. The media was replaced with Belzer solution (UW), and cells were incubated at 4°C in either normoxic or hypoxic conditions for 6 hours. Hypoxia was achieved by placing the cells in an airtight incubator (Forma Scientifica, Marietta, OH) which was flushed with 5% CO2 and 95% N2 until the oxygen content in the container reached < 0.1% as verified using a dissolved O2 meter (Model 4000, VWR Scientific Products, Suwannee, GA). To render the storage solution hypoxic before experiments were carried out, UW was pre-incubated in the hypoxic chamber in an open sterile container for 8 hr before experiments were carried out. This resulted in a final O2 concentration of < 0.1% as measured with the dissolved O2 meter. Reactive Blue 2 (RB2) (Sigma, St Louis, MO; 300 μM) or Ruthenium Red (RR) (Sigma, 30 μM) was added to the UW solution of selected samples before storage of cells.
For experiments requiring “reperfusion”, the UW was replaced with warm, oxygenated MEM following storage, and the cells were incubated at 37°C to simulate reperfusion.
TUNEL staining, Immunofluorescence, and fluorescence microscopy
TUNEL (TdT-mediated dUTP Nick-End Labeling) staining was performed after 180 minutes of reperfusion as described previously33. The percentage of TUNEL positive cells per high powered field (HPF) were recorded. Four HPF for each of 3 experiments are reported.
Immunofluorescence for Bax was performed immediately following cold storage (no reperfusion) using the methods previously described33. The percentage of cells demonstrating Bax translocation per high powered field (HPF) were recorded. Four HPF for each of 3 experiments are reported.
To obtain a qualitative measure of mitochondrial cytochrome c release as a marker of apoptosis, HepG2 cells were transiently transfected with cytochrome c-GFP (gift from Dr. Nieminen) as described by Nieminen 38. The transfected cells were grown, fixed, and studied as previously described33.
Western analysis
Western analysis was carried out using methods previously described33. The antibodies used were as follows: anti P44/42 MAPK and anti-phosphorylated P44/42 MAPK antibodies (1:1000, Cell Signaling Technology).
Determination of Intracellular ATP
Luciferase vectors, PcDNA3-luciferase-LL/V (cytoplasmic luciferase) and PcDNA3-COX8-luciferase (mitochondrial luciferase) were a gift of Dr. Manfredi39. HepG2 cells were transfected with the selected PcDNA containing the engineered luciferase gene using FuGENE6 (Roche Applied Science, Indianapolis, IN) as described by the manufacturer. Cells were grown for 48 hours and then subjected to the storage conditions described above [with or without RR (30μM) or RB2 (300 μM)]. Following storage, cells were counted using the trypan blue exclusion method to insure no difference in the rate of cell death during the storage period. 2 × 105 cells were resuspended in tricine buffer with 10 μL beetle luciferin. Luminescence was measured (BioOrbit Model 1251) for 30 seconds and peak values were used for analysis (N=6 for each group)40.
Untreated (MEM 37°C storage) cells served as a control. Standard ATP curves were performed in the presence of varying concentrations of RR and RB2 to control for interference from these compounds. Six experiments are reported for each condition.
Statistical Analysis
Statistical significance was determined using a two-tailed-homoscedastic student t-test. A p value of ≤ 0.05 was considered significant.
Results
Reactive Blue 2 attenuates cell death following hypothermic storage
Following 6 hours of normoxic-hypothermic (NH) or hypoxic-hypothermic (HH) storage and 180 min of rewarming, TUNEL staining was performed. Figure 1 summarizes the results of these experiments. NH stored HepG2 cells demonstrate approximately 27.5% TUNEL positive nuclei per high power field after 180 min of rewarming. The percentage of apoptotic cells increases significantly to 45% (p=0.034) when cells are rewarmed for 180 min following HH storage. Addition of RB2 to the UW storage solution decreases the rate of cellular cell death following NH storage to 2.5% (p=0.001), while addition of RB2 to HH stored cells decreased cell death rates to 21% (p=0.001). These results are similar to those found when ruthenium red (RR) is added to the storage solution33.
Figure 1.
TUNEL results following hypothermic storage with RB2 reported as percentage of cells TUNEL positive per high power field. Following NH storage, 27.5% of cells are TUNEL positive after 180 min of rewarming. Following HH storage, the percentage of apoptotic cells increases significantly to 45% (@; p=0.034). Addition of RB2 to the UW storage solution decreases the rate of cellular apoptosis to 2.5% (#, p=0.001) and 21% ($, p=0.001) following NH and HH storage respectively.
RB2 attenuation of cell death is not mediated via a ERK 1, 2-mechanism
P2 agonists have been demonstrated to promote apoptosis via pathways resulting in activation of ERK 1, 2, and P2 antagonists have been demonstrated to prevent this activation41–43. We studied the expression levels and phosphorylation states of ERK 1, 2 following our storage conditions by Western analysis. Figure 2 demonstrates that neither ERK 1, 2 expression levels nor phosphorylation states were altered by our experimental conditions, indicating that any effect of RB2 is independent of this pathway.
Figure 2.
Western analysis demostrating ERK 1,2 epression levels and ERK 1,2 posphorylation states. Neither ERK 1, 2 expression levels nor phosphorylation states were altered by our experimental conditions. Positive and negative controls are shown.
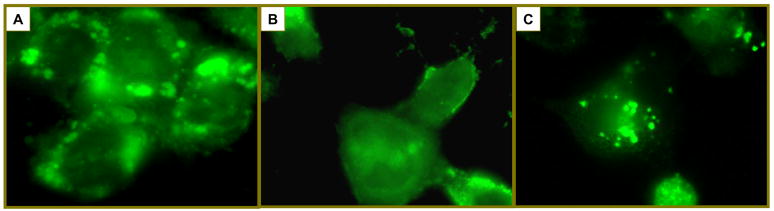
Reactive Blue 2 attenuates hypothermic storage-induced Bax translocation from cytosol to mitochondria
We have previously demonstrated that Bax translocation to the mitochondria plays a major role in early reperfusion apoptosis following hypothermic storage in HepG2 cells33. Inhibition of mCa2+ uptake by RR attenuated this occurrence. During the current experiments, RB2 was added to the storage solution. Figure 3 demonstrates representative examples of Bax immunofluorescence following hypothermic storage with and without RB2 treatment. As shown, the Bax staining pattern was diffuse in untreated cells indicating a equal distribution throughout the cytoplasm. Following cold storage (NH or HH), the Bax staining pattern became punctate, indicating translocation to mitochondria. When RB2 is added to the UW during storage, attenuation of the punctate Bax staining pattern was observed. When quantified (Figure 3), punctate Bax staining occurred in approximately 35% of cells per high power field following NH storage and increased significantly to 45% following HH storage. Addition of RB2 to the UW storage solution significantly decreased the rate of punctate Bax staining to 12% (p=0.001) following NH storage and 22% (p=0.009) following HH storage.
Figure 3.
Representative examples of Bax immunofluorescence following hypothermic storage with and without RB2 treatment. (A) In the untreated cell, Bax staining occurs diffusely throughout the cytoplasm. (B) Following cold storage, Bax translocation to the mitochondria results in a punctate pattern of immunofluorescence staining. This photo is representative of the similar patterns were observed for both NH and HH storage. (C) Cold storage (NH or HH) in UW containing RB2 attenuates this mitochondrial translocation and preserves the diffuse cytoplasmic staining pattern. The graft reports percentage of cells per high power field demonstrating punctate Bax staining. Following NH storage, approximately 35% of cells demonstrate punctate staining. This increased significantly to 45% following HH storage. Addition of RB2 to the UW storage solution significantly decreased the rate of punctate Bax staining to 12% (@, p=0.001) following NH storage and 22% (#, p=0.009) following HH storage.
RB2 treatment prevents cytochrome c release following Bax translocation
Cytochrome c release from the mitochondria is an important downstream occurrence following Bax translocation, indicating that the cell is committed to apoptosis. To insure that Bax translocation in our model signified commitment of the cell to apoptosis, we qualitatively studied cytochrome c release. In order to visualize the spatio temporal intracellular distribution of cytochrome c during our study, HepG2 cells were transfected with GFP tagged cytochrome c and subjected to the storage and rewarming conditions. Figure 4 demonstrates representative photomicrographs. In untreated cells, cytochrome c-GFP is visualized in punctate grouping in the cytoplasm, indicating association with mitochondria. Cold storage in UW (both NH and HH) alone (without rewarming) resulted in very little diffusion of the GFP. In contrast, the GFP staining pattern is very diffuse following 6 hr of cold storage and 3hr of reperfusion. Addition of RB2 to UW attenuates this occurrence. These studies indicate that the Bax translocation precedes cytochrome c release in our model of cold storage and reperfusion. Similar to the observation when cells were treated with RR, RB2 treatment of cells during cold storage attenuates cell death of cells during reperfusion33.
Figure 4.
Representative photomicrographs showing cytochrome c-GFP distribution. A: In untreated cells, cytochrome c-GFP is visualized in punctate groupings in the cytoplasm, indicating association with mitochondria. B: Following 6 hr of cold storage and 3hr of reperfusion, GFP is widely distributed in the cytoplasm. C: RB2 treatment attenuates this occurrence.
Hypothermic storage depletes cytosolic but not mitochondrial ATP concentrations
HepG2 cells were transfected with a PcDNA3-luciferase-LL/V (LL/V) cDNA construct to report cytosolic ATP levels following cold storage. Following storage, the stored transfected cells were counted using the trypan blue exclusion method. There was no difference in the number of necrotic cells immediately following NH storage when cells subjected to cold storage in UW alone were compared to cells stored in UW with RB2 (15% vs 20%; p = 0.06) or RR (15% vs 18%; p = 0.30). There was also no difference in cell death immediately following HH storage (storage alone, 15%; RB2, 20%, p = 0.13; RR, 15%, p = 0.89). In addition, there was no difference in cell death during storage when the UW alone groups (NH and HH) were compared with each other (15% vs 15%; p = 0.31). Therefore differences detected in ATP levels between groups are not due to differences in cell death during the storage period. As an additional quality assurance measure, standardized ATP curves were performed in the presence of varying concentrations of RR and RB2. Neither RR nor RB2 caused absorbance of light which interfered with the luminescence assay.
For each group, 2 × 105 total cells of each group were resuspended in tricine buffer with 10 μL beetle luciferin. Luminescence was then measured. As demonstrated in Figure 5, cytosolic ATP levels were significantly reduced by both NH and HH storage conditions. When compared to untreated controls, NH storage reduced cytoplasmic ATP levels by 46% (p = 0.0002) and HH storage reduced cytoplasmic ATP levels by 61% (p=0.0005). In addition, HH storage resulted in a further decrease in cytoplasmic ATP levels when compared to NH (p=0.05).
Figure 5.
Cellular compartmental ATP levels following hypothermic storage. (A) When compared to untreated controls, NH storage reduced cytoplasmic ATP levels by 46% (#, p = 0.0002) and HH storage reduced cytoplasmic ATP levels by 61% (@, p=0.0005). In addition, HH storage resulted in a further decrease in cytoplasmic ATP levels when compared to NH ($, p=0.05). (B) Mitochondrial ATP levels were slightly lower following NH and HH storage. However, there was no statistically significant difference compared to untreated controls.
When the cells were transfected with PcDNA3-COX8-luciferase (COX-8), NH and HH had slightly lower mitochondrial ATP levels than untreated controls. However, there was no statistically significant difference. This is also illustrated in Figure 5.
RB2 and RR treatment results in further cytoplasmic ATP depletion during both NH and HH storage
When compared to cells undergoing NH or HH storage alone, the addition of RB2 to the storage solution resulted in lower cytoplasmic ATP levels following the same period of storage (Figure 6). Cells undergoing NH storage with RB2 had 28% less cytosolic ATP than did cells undergoing NH storage alone (p=0.003). Similarly, cells undergoing HH storage with RB2 had 25% less cytosolic ATP than cells undergoing HH storage alone (p=0.005). In a similar manner, NH and HH storage with RR resulted in lower cytoplasmic ATP levels (16%, p=0.028; 17%, p=0.032) than cells subjected to cold storage alone (Figure 6). There was no significant difference in the cytoplasmic ATP levels between the RR and RB2 groups following either storage method.
Figure 6.
Storage with RB2 or RR results in further decreases in cytoplasmic ATP levels. (A) Addition of RB2 to the storage solution results in 28% less cytosolic ATP when compared to cells undergoing NH storage alone (@, p=0.003). Similarly, cells undergoing HH storage with RB2 had 25% less cytosolic ATP than cells undergoing HH storage alone (#, p=0.005). NH and HH storage with RR also resulted in lower cytoplasmic ATP levels [($, 16%, p=0.028);(&, 17%, p=0.032)] than cells in stored without RR treatment. (B) Neither addition of RB2 nor RR to the cold storage solution had a significant effect on mitochondrial ATP levels.
Neither addition of RB2 nor RR to the cold storage solution had a significant effect on mitochondrial ATP levels.
Discussion
The experiments presented in this manuscript were designed to determine if P2 receptor antagonism had a cytoprotective role in cold ischemia and reperfusion and to elucidate potential mechanisms of these cytoprotective effects. Our results show that RB2, when added to the cold storage solution, attenuates a Bax-dependent cell death during the early rewarming period following cold storage. We have previously described that inhibition of mCa2+ uptake during cold ischemic storage results in cytoprotection via the same mechanism33. The cytoprotection afforded by RB2 in our model occurs in a similar time frame and to an almost identical extent as that provided by RR33.
Neither RR nor RB2 had significant effects on mitochondrial ATP levels during cold storage when compared to untreated cells undergoing cold storage alone. We expected that by preventing mCa2+ uptake during the cold storage period RR would decrease the calcium driven stimulus for the mitochondria to increase ATP production, resulting in a more rapid depletion of the existing cytosolic ATP. As predicted, inhibition of mCa+2 uptake with RR resulted in decreased level of cytosolic ATP compared to cells undergoing storage alone. We observed an identical effect from purinergic inhibition with RB2 during the period of cold storage. The decreased cytoplasmic ATP levels along with the unchanged mitochondrial ATP levels suggests that cells stored with RR or RB2 have higher rates of total cellular ATP consumption than do cells subjected to cold storage alone. In cells subjected to cold storage in UW alone, the rate of cell death was higher than that of cells cold stored in UW with RR or RB2. Therefore, as cells die, the total ATP consumption of the population of cells studied decreases resulting in higher detectable ATP levels. For cells protected from apoptosis, hydrolysis of mitochondrial ATP is required at significant levels in order to maintain important cellular processes, such as preservation of ΔΨ. The ATP is supplied to the mitochondria at the expense of cytoplasmic ATP stores via reversal of the ANT22. Our observation suggests that more energy in the form of ATP is required to sustain cellular processes during ischemia than to undergo cell death.
A potential explanation for the similar results observed with RR and RB2 may be the recently documented presence of P2Y receptors in the mitochondrial membrane of hepatocytes6,21. Similar to the P2 receptors in the cell membrane, the mitochondrial receptor analogs play a role in the regulation of mCa2+ uptake, potentially via the hydrolysis of phosphatidylinositol 4,5-bisphosphate6,44. The existence of these receptors suggests a mechanism by which ATP and/or ADP may participate in an ATP feedback mechanism by regulating mCa2+ uptake6,20,21. If these P2Y-like receptors play a major role in the regulation of mCa2+ uptake, P2Y antagonists should convey a cytoprotection similar to that which has been demonstrated with RR. Together these data suggest, but do not prove, that in our cold ischemic model, RB2 is conveying cytoprotection in a manner similar to RR -- inhibition of mCa2+ uptake. To further strengthen this hypothesis, the failure of our model to stimulate ERK 1, 2 activation rules out suppression of ERK 1,2 activation as the mechanism of cytoprotection.
Recently, a non-purinergic mechanism of RB2 has been reported. RB2 has been observed to inhibit phosphatidylinositol 3-kinase (PI 3-K) in rat glioma cells 45. The PI 3-K/AKT pathway is crucial to many aspects of cell growth and survival. Inhibition of PI 3-K leads to an increase of apoptosis, and this has been exploited as a potential target for cancer therapeutics 46,47. Thus, if RB2 were affecting our cells via this mechanism, we would have expected an increase in cell death in RB2 treated cells rather than the decrease which was actually observed.
A final consideration regarding our observations is the argument that the findings of the TUNEL staining and ATP measurements presented in this manuscript can be explained by an increased rate of necrotic cell death during cold storage in the presence of RR or RB2. However, the trypan blue exclusion method was used to count cells for the ATP studies. This counting method revealed that neither RR nor RB2 storage resulted in significantly greater necrotic cell death than did storage alone. In our model of cold ischemic storage, Bax translocation to the mitochondria occurs during cold storage resulting in cytochrome c release and cellular apoptosis during the reperfusion period. Bax translocation and cellular apoptosis occurred at similar rates suggesting that the cell death noted in our study is Bax translocation dependent. The cell death induced in this study is attenuated at similar rates by RR and RB2. In addition, the cytoprotection afforded by RR and RB2 occurs despite cytosolic ATP consumption beyond that of untreated control cells.
In summary, the data presented here demonstrate that neither purinergic antagonism nor inhibition of mCa2+ uptake during the cold storage period maintains cytoplasmic ATP levels. While it is clear that cold storage with RR or RB2 results in an increase in total cellular utilization of ATP during the storage period, at this point it remains unclear if inhibition of mCa2+ uptake allows the cell to use ATP more efficiently or if mitochondrial calcium uptake itself is a specific trigger for cell death independent of ATP levels.
Acknowledgments
The authors would like to express our appreciation to Dr. Niemanen and Dr. Manfedi for their kind gifts (cytochrome C-GFP and luciferase vectors, respectively). This work was supported in part by National Institute of Health Ruth L. Kirschstein National Research Service Award T32 DK07673 (CDA), Association for Academic Surgery Research Fellowship (CMJ), National Institute of Health Grants DK59390-1 (RSC) and DK064669 (RSC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ralevic V, Burnstock G. Receptors for Purines and Pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- 2.Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira SC, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–G1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- 3.Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- 4.Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- 5.Roe MW, Moore AL, Lidofsky SD. Purinergic-independent calcium signaling mediates recovery from hepatocellular swelling: implications for volume regulation. J Biol Chem. 2001;276:30871–30877. doi: 10.1074/jbc.M102362200. [DOI] [PubMed] [Google Scholar]
- 6.Belous A, Wakata A, Knox CD, Nicoud IB, Pierce J, Anderson CD, Pinson CW, Chari RS. Mitochondrial P2Y-Like receptors link cytosolic adenosine nucleotides to mitochondrial calcium uptake. J Cell Biochem. 2004;92:1062–1073. doi: 10.1002/jcb.20144. [DOI] [PubMed] [Google Scholar]
- 7.Schofl C, Ponczek M, Mader T, Waring M, Benecke H, von zur MA, Mix H, Cornberg M, Boker KH, Manns MP, Wagner S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164–G172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- 8.Feranchak AP, Fitz JG, Roman RM. Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol. 2000;33:174–182. doi: 10.1016/s0168-8278(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 9.Junankar PR, Karjalainen A, Kirk K. The role of P2Y1 purinergic receptors and cytosolic Ca2+ in hypotonically activated osmolyte efflux from a rat hepatoma cell line. J Biol Chem. 2002;277:40324–40334. doi: 10.1074/jbc.M204712200. [DOI] [PubMed] [Google Scholar]
- 10.Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther. 2005;313:1305–1313. doi: 10.1124/jpet.104.082743. [DOI] [PubMed] [Google Scholar]
- 11.Farber JL. The role of calcium in lethal cell injury. Chem Res Toxicol. 1990;3:503–508. doi: 10.1021/tx00018a003. [DOI] [PubMed] [Google Scholar]
- 12.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber JL. The role of calcium ions in toxic cell injury. Environ Health Perspect. 1990;84:107–111. doi: 10.1289/ehp.9084107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 15.Belous A, Knox C, Nicoud IB, Pierce J, Anderson C, Pinson CW, Chari RS. Altered ATP-dependent mitochondrial Ca2+ uptake in cold ischemia is attenuated by ruthenium red. J Surg Res. 2003;111:284–289. doi: 10.1016/s0022-4804(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Southard JH. Alteration in cellular calcium and mitochondrial functions in the rat liver during cold preservation. Transplantation. 1998;65:369–375. doi: 10.1097/00007890-199802150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341 (Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh AB. Mitochondrial regulation of intracellular Ca2+ signaling: more than just simple Ca2+ buffers. News Physiol Sci. 2003;18:252–256. doi: 10.1152/nips.01458.2003. [DOI] [PubMed] [Google Scholar]
- 21.Belous AE, Jones CM, Wakata A, Knox CD, Nicoud IB, Pierce J, Chari RS. Mitochondrial calcium transport is regulated by P2Y(1)- and P2Y(2)-like mitochondrial receptors. J Cell Biochem. 2006;99:1165–1174. doi: 10.1002/jcb.20985. [DOI] [PubMed] [Google Scholar]
- 22.Belous A, Knox C, Nicoud IB, Pierce J, Anderson C, Pinson CW, Chari RS. Reversed activity of mitochondrial adenine nucleotide translocator in ischemia-reperfusion. Transplantation. 2003;75:1717–1723. doi: 10.1097/01.TP.0000063829.35871.CE. [DOI] [PubMed] [Google Scholar]
- 23.Ryu JK, Kim J, Choi SH, Oh YJ, Lee YB, Kim SU, Jin BK. ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport. 2002;13:1611–1615. doi: 10.1097/00001756-200209160-00008. [DOI] [PubMed] [Google Scholar]
- 24.Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem. 2001;276:16379–16390. doi: 10.1074/jbc.M006617200. [DOI] [PubMed] [Google Scholar]
- 25.Hopfner M, Maaser K, Barthel B, von Lampe B, Hanski C, Riecken EO, Zeitz M, Scherubl H. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: involvement of intracellular calcium and cyclic adenosine monophosphate. Int J Colorectal Dis. 2001;16:154–166. doi: 10.1007/s003840100302. [DOI] [PubMed] [Google Scholar]
- 26.Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sylte MJ, Kuckleburg CJ, Inzana TJ, Bertics PJ, Czuprynski CJ. Stimulation of P2X receptors enhances lipooligosaccharide-mediated apoptosis of endothelial cells. J Leukoc Biol. 2005;77:958–965. doi: 10.1189/jlb.1004597. [DOI] [PubMed] [Google Scholar]
- 28.Kim SG, Gao ZG, Soltysiak KA, Chang TS, Brodie C, Jacobson KA. P2Y6 nucleotide receptor activates PKC to protect 1321N1 astrocytoma cells against tumor necrosis factor-induced apoptosis. Cell Mol Neurobiol. 2003;23:401–418. doi: 10.1023/a:1023696806609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavaliere F, D’Ambrosi N, Sancesario G, Bernardi G, Volonte C. Hypoglycaemia-induced cell death: features of neuroprotection by the P2 receptor antagonist basilen blue. Neurochem Int. 2001;38:199–207. doi: 10.1016/s0197-0186(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 30.Cavaliere F, D’Ambrosi N, Ciotti MT, Mancino G, Sancesario G, Bernardi G, Volonte C. Glucose deprivation and chemical hypoxia: neuroprotection by P2 receptor antagonists. Neurochem Int. 2001;38:189–197. doi: 10.1016/s0197-0186(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 31.Kharlamov A, Jones SC, Kim DK. Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exp Brain Res. 2002;147:353–359. doi: 10.1007/s00221-002-1251-1. [DOI] [PubMed] [Google Scholar]
- 32.Chan CF, Lin-Shiau SY. Site of action of suramin and reactive blue 2 in preventing neuronal death induced by dequalinium. J Neurosci Res. 2000;62:692–699. doi: 10.1002/1097-4547(20001201)62:5<692::AID-JNR8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CD, Belous A, Pierce J, Nicoud IB, Knox C, Wakata A, Pinson CW, Chari RS. Mitochondrial calcium uptake regulates cold preservation-induced Bax translocation and early reperfusion apoptosis. Am J Transplant. 2004;4:352–362. doi: 10.1111/j.1600-6143.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim RD, Darling CE, Roth TP, Ricciardi R, Chari RS. Activator protein 1 activation following hypoosmotic stress in HepG2 cells is actin cytoskeleton dependent. J Surg Res. 2001;100:176–182. doi: 10.1006/jsre.2001.6225. [DOI] [PubMed] [Google Scholar]
- 35.Enosawa S, Miyashita T, Fujita Y, Suzuki S, Amemiya H, Omasa T, Hiramatsu S, Suga K, Matsumura T. In vivo estimation of bioartificial liver with recombinant HepG2 cells using pigs with ischemic liver failure. Cell Transplant. 2001;10:429–433. [PubMed] [Google Scholar]
- 36.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esumi H, Izuishi K, Kato K, Hashimoto K, Kurashima Y, Kishimoto A, Ogura T, Ozawa T. Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent manner. J Biol Chem. 2002;277:32791–32798. doi: 10.1074/jbc.M112270200. [DOI] [PubMed] [Google Scholar]
- 38.Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem. 1999;274:5654–5658. doi: 10.1074/jbc.274.9.5654. [DOI] [PubMed] [Google Scholar]
- 39.Manfredi G, Yang L, Gajewski CD, Mattiazzi M. Measurements of ATP in mammalian cells. Methods. 2002;26:317–326. doi: 10.1016/S1046-2023(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 40.Chari RS, Schutz SM, Haebig JE, Shimokura GH, Cotton PB, Fitz JG, Meyers WC. Adenosine nucleotides in bile. Am J Physiol. 1996;270:G246–G252. doi: 10.1152/ajpgi.1996.270.2.G246. [DOI] [PubMed] [Google Scholar]
- 41.Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, Adam D, Bulfone-Paus S. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- 42.Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, Bulfone-Paus S. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- 43.Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem. 2001;276:16379–16390. doi: 10.1074/jbc.M006617200. [DOI] [PubMed] [Google Scholar]
- 44.Knox CD, Belous AE, Pierce JM, Wakata A, Nicoud IB, Anderson CD, Pinson CW, Chari RS. Novel role of phospholipase C-delta1: regulation of liver mitochondrial Ca2+ uptake. Am J Physiol Gastrointest Liver Physiol. 2004;287:G533–G540. doi: 10.1152/ajpgi.00050.2004. [DOI] [PubMed] [Google Scholar]
- 45.Claes P, Van Kolen K, Roymans D, Blero D, Vissenberg K, Erneux C, Verbelen JP, Esmans EL, Slegers H. Reactive blue 2 inhibition of cyclic AMP-dependent differentiation of rat C6 glioma cells by purinergic receptor-independent inactivation of phosphatidylinositol 3-kinase. Biochem Pharmacol. 2004;67:1489–1498. doi: 10.1016/j.bcp.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 47.Blanco-Aparicio C, Pequeno B, Moneo V, Romero L, Leal JF, Velasco J, Fominaya J, Carnero A. Inhibition of phosphatidylinositol-3-kinase synergizes with gemcitabine in low-passage tumor cell lines correlating with Bax translocation to the mitochondria. Anticancer Drugs. 2005;16:977–987. doi: 10.1097/01.cad.0000180117.93535.cf. [DOI] [PubMed] [Google Scholar]