Abstract
Acute hypoxia elicits complex time-dependent responses including rapid augmentation of inspiratory drive, shortening of inspiratory and expiratory durations (TI, TE), and short-term potentiation and depression. The central pathways mediating these varied effects are largely unknown. Here, we show that the lateral parabrachial nucleus (LPBN) of the dorsolateral pons specifically mediates TE-shortening during hypoxia and not other hypoxic response components. Twelve urethane-anesthetized and vagotomized adult Sprague-Dawley rats were exposed to 1-min poikilocapnic hypoxia before and after unilateral kainic acid or bilateral electrolytic lesioning of the LPBN. Bilateral lesions resulted in a significant increase in baseline TE under hyperoxia. After unilateral or bilateral lesions, the decrease in TE during hypoxia was markedly attenuated without appreciable changes in all other hypoxic response components. These findings add to the mounting evidence that the central processing of peripheral chemoafferent inputs is segregated into parallel integrator and differentiator (low-pass and high-pass filter) pathways that separately modulate inspiratory drive, TI, TE and resultant short-term potentiation and depression.
Keywords: Lateral parabrachial nucleus, hypoxia, respiratory control
1. Introduction
Abrupt exposure to low oxygen elicits a fast (<1 min) peripheral chemoreceptors-mediated increase in respiratory ventilation to a higher plateau for ~5 min (phase I) followed by a slow (5–20 min) hypoxic ventilatory decline or “roll-off” of the initial response (phase II) that is mediated by central (and perhaps also peripheral) mechanisms (Bisgard et al. 1995). This biphasic pattern has been extensively documented in humans (Cross et al. 1952; Weil et al. 1976; Easton et al. 1986; Duffin 2007; Steinback et al. 2007) and in many mammalian species (Woodrum et al. 1981; Long et al. 1984; Melton et al. 1988; Gershan et al. 1996; Vizek et al. 1998). Upon return to normoxia or hyperoxia (phase III), ventilation decreases rapidly then more slowly (~2 min) toward baseline levels, in what is known as respiratory afterdischarge or decay of short-term potentiation (STP) in humans and animal models (Eldridge et al. 1986; Georgopoulos et al. 1990).
During hypoxic ventilatory decline, all breathing pattern components (tidal volume, VT; respiratory frequency, f; inspiratory time, TI; expiratory time, TE) exhibit similar time-dependent changes that collectively decreases ventilation regardless of whether the hypoxia is maintained isocapnic or poikilocapnic (Steinback et al. 2007). Also, these depression effects are not mirrored in phase III as post-hypoxia short-term memory (Fig. 1A) suggesting that they are gated to the hypoxic input. Both these observations support the notion (Fig. 1B) that hypoxic ventilatory decline in all ventilatory components are mediated by common mechanisms that habituate the hypoxic stimulus (Poon et al. 2000a; Poon et al. 2006) – such as sensory hypoxic decline in the carotid body (Cummings et al. 2005) or synaptic depression in the medial and commissural nucleus tractus solitarius (NTS) (Zhou et al. 1997; Poon et al. 2000c; Bantikyan et al. 2009), a brainstem region which provides the first central synaptic relay to carotid chemoafferent inputs (Gozal et al. 2000).
Fig. 1. Components of the hypoxic respiratory response and corresponding central modulatory pathways.
A: Idealized curves showing the time-dependent responses in respiratory frequency (f), expiratory duration (TE), inspiratory duration (TI), inspiratory amplitude (∫Phr.), and minute ventilation (V̇) to acute hypoxia. STP, short-term potentiation; STD, short-term depression. B: Hypothetical model showing parallel central pathways that mediate various components of the hypoxic respiratory response. CSN, carotid sinus nerve; NTS, nucleus tractus solitarius; ∫L, leaky neural integrator; vl-pons and dl-pons, ventrolateral and dorsolateral pons; central chemoaff., inputs from central chemoreceptors; E and I, expiratory and inspiratory rhythm generators; Phr.m/pre-m phrenic motoneuron/premotoneuron. Open and filled triangles indicate excitatory and inhibitory effects. Adapted from Young et al. (2003).
Changes in breathing pattern during phase I and phase III of acute hypoxia are much more complex (Fig. 1A), part of which have been expounded in previous reviews (Powell et al. 1998; Poon et al. 2000a). In anesthetized animals, STP elicited by carotid sinus nerve stimulation (as a time-resolved proxy for the hypoxic stimulus) is manifested as an exponential (~seconds) augmentation of inspiratory drive in phase I comprised of both an increase in phrenic discharge amplitude (Wagner et al. 1991) and decrease of TI (Poon et al. 1999), with subsequent afterdischarge (off-transients of STP) for both variables in phase III. In contrast, hypoxia or carotid sinus nerve stimulation elicits a rapid increase in f followed by short-term depression (STD, ~1 min) in phase I that is mirrored by subsequent poststimulus rebound decrease and slow recovery (~minutes) of f in phase III (Hayashi et al. 1993; Dick et al. 2000). The latter (off-transients of STD) is known as posthypoxia frequency decline (PHFD) and is due to an increase in TE (Dick et al. 2000). Indeed, both the on- and off-transients of STD of respiratory frequency are ascribable to corresponding time-dependent changes in TE (Poon et al. 2000b). Similar STD of expiration (as measured by increases in TE) and STP of inspiration (as measured by increases in VT and decreases in TI) on timescales of minutes are also found in awake humans (Gardner 1980; Steinback et al. 2007).
Interestingly, it has been pointed out that the temporal patterns of respiratory STP and STD resemble those of a biphasic neural integrator and differentiator (in time domain), or equivalently, low-pass and high-pass filters (in frequency domain) that dynamically transform carotid chemoafferent inputs (Poon et al. 1999; Poon et al. 2000a; Poon et al. 2000b; Poon et al. 2006). In support of this notion, recent studies using time-resolved decomposition of the respiratory response to carotid sinus nerve stimulation revealed that the central relay of carotid chemoafferent traffic beyond the NTS is comprised of parallel integrator-differentiator pathways that separately modulate inspiratory drive, TI and TE (Young et al. 2003). Specifically, four parallel pathways projecting from NTS to the respiratory rhythm generator have been postulated to mediate the hypoxic respiratory response (Fig. 1B): 1) fast-slow integrator pathways that mediate the rapid and STP augmentation of inspiratory drive; 2) fast-slow integrator pathways that mediate the rapid and STP shortening of TI; 3) a slow differentiator pathway that mediates the STD prolongation of TE; and 4) a fast integrator pathway (time constant ~2 sec) that mediates the rapid shortening of TE. Similar integrator-differentiator pathways also account for the off-transients of all these response components (Fig. 1b).
There is evidence that the STD pathway (pathway 3) is mediated by certain expiratory neurons in the A5 region of the ventrolateral pons (Coles et al. 1996; Dick et al. 2000). Apart from this, the neural correlates of the remaining pathways are presently unclear. Here, we show that pathway 4 (mediating the rapid shortening of TE during hypoxia) involves the lateral parabrachial nucleus (LPBN), a subnucleus of the dorsolateral pons ‘pneumotaxic’ integrative region in the rat (Song et al. 2006). Specifically, we show that chemical or electrolytic lesions of the LPBN markedly attenuate the hypoxia-elicited rapid shortening of TE without appreciably affecting all other hypoxic response components in anesthetized, vagotomized rats. Our results lend further support for the proposed carotid chemoafferent parallel integrator-differentiator model (Fig. 1B) and suggest a new role for the LPBN in respiratory control.
2. Methods
Animal preparation
Experiments were done on 12 male adult Sprague-Dawley rats (330–380 g, Charles River Laboratories). All experimental protocols had been reviewed and approved by the M.I.T. Committee on Animal Care in accordance with published guidelines. After injecting atropine sulphate (0.025 mg, s.c.), the rat was anesthetized with urethane (Sigma, 1.5 g/kg, i.p.). Trachea was intubated for artificial ventilation. The femoral vein and artery were cannulated for infusing solution (Lactated Ringer’s solution, 0.05–0.1 ml/min) or monitoring arterial blood pressure, respectively.
Rats were paralyzed with pancuronium bromide (Sigma, initial dose 0.5 mg, i.v., supplemented every hour at 0.1 mg, i.v.) and ventilated with hyperoxic medical air (40% O2 balance N2) by using a CWE AVS-1 ventilator. A respiratory gas analyzer (CWE Gemini) was used to monitor end-tidal O2 and CO2 levels (PETO2 and PETCO2). The latter was maintained at 5.0±0.2% (38±1.5 mmHg) or 5.5±0.2% (41.8±1.5 mmHg), which was the CO2-recruitment threshold (Boden et al. 1998) plus 1.0% or 1.5%. Heart rate was monitored by recording ECG with subcutaneous needle electrodes. Body temperature was kept at 36.5±0.2 ºC with a temperature controller (CWE, TC-831). During the experiment, the depth of anesthetization was checked regularly. Whenever a noxious stimulus (clamping the hind paw) caused changes in pupil size, respiration and heart rate or elicited a withdrawal reflex, a supplementary dose of urethane (1/10 original dosage) was given intravenously to maintain adequate anesthesia.
The right phrenic nerve and both vagus nerves were isolated and severed at the cervical level from ventral approach. The head of the rat was then fixed in a stereotaxic frame (KOPF 1430, David Kopf Instruments, Tujunga, CA) in a tilted position (with Bregma 1.5 mm higher than Lambda) with the dorsolateral pons being readily accessible from a vertical dorsal approach. A craniotomy (diameter 0.5 cm) was performed at interaural level. Dura and pia were carefully removed. The exposed brain surface was covered with petroleum jelly.
Recording of phrenic discharge
To record phrenic discharge, the separated right phrenic nerve was exposed from dorsal approach and mounted on a bipolar platinum wire electrode (FHC). The raw phrenic discharge signal (Phr) was amplified (CyberAmp 380, Axon Instruments, Union City) and sampled (at 10 KHz) into a Dell PC with LabView (National Instruments, Austin, TX). In most experiments, the Phr signal was integrated with a Paynter filter (time constant 15 ms).
Pontine lesions
The LPBN was lesioned either chemically or electrolytically. The stereotaxic coordinates of the LPBN were −0.20 (caudal) −+0.2 mm (rostral) to the level of lambda, 2.3 – 2.4 mm lateral to midline, and 7.7 – 8.2 mm below lambda surface. Chemical lesion was made unilaterally to one side using a glass micropipette (tip diameter 15–30 μm) filled with kainic acid solution (KA, from Sigma; concentration at 1μg/μl in ACSF). The pipette was connected to a BH2 microinjector (Harvard Apparatus, Holliston, MA). A total volume of 50 nl was injected by giving multiple pressure pulses to the micropipette. At the end of the experiment, the injection site was marked by passing anodal D.C. (100 μA for 60 sec) through the injection pipette. Electrolytic lesions were made bilaterally to both sides with a tungsten microelectrode (tip diameter 1–2 μm, impedance 0.5–1 MΩ; Micro Probes, Gaithersburg, MD). The lesion current was 100 μA, anodal D.C., lasting 30 sec.
Hypoxia test
Only brief poikilocapnic hypoxia (1 min) was used in this study in order to minimize the effects of hypoxic ventilatory decline and decreases in PETCO2 (as a result of decreased body metabolism) and in arterial blood pressure during hypoxia. Each hypoxia test lasted 7–10 min: 1 min of pre-hypoxia baseline, 1 min of hypoxia, and 5–8 min of posthypoxia recovery. Hypoxia was applied by switching the hyperoxic ventilation gas to 8% O2 (balance N2). A control hypoxia test was performed right before KA or electrolytic lesions of the LPBN. After lesions, the animals were allowed to stabilize for 30–45 min before another hypoxia test was given.
In animals that received unilateral KA lesion, separate hypercapnia tests were also performed (see companion report (Song et al. 2009)). In this event the hypoxia tests were performed at 15 min after each hypercapnia test, when the animal’s PETO2, PETCO2 and blood pressure had returned to corresponding pre-test baseline levels.
Data analysis
TI, TE and f were measured for each respiratory cycle from Phr. Amplitude of inspiratory motor output (∫Phr) was measured as the peak of the integrated Phr signal. Inspiratory drive was calculated as the ratio ∫Phr/TI. These values were also normalized against their corresponding average (1 min) pre-hypoxia baseline values in control or lesioned conditions. Each animal served as its own control for all statistical analyses.
Histology
At the end of the experiment, the animal was killed with an overdose of urethane (2 g/kg, i.v.) and immediately perfused transcardially with 300 ml of heparinized saline followed by another 300 ml of chilled paraformaldehyde solution (4% in 0.05 M PBS). The brain was removed, post-fixed and cut into 100-μm coronal sections on a vibratome. Sections were then stained with cresyl violet and checked microscopically for the loci of lesions or injections.
3. Results
Loci of chemical or electrolytic lesions in the LPBN
In the six animals that received unilateral KA injection, the injection sites as marked by post-injection electrolytic lesions were verified to lie at the central or external-lateral subnucleus of the LPBN (Fig. 2). In the other six animals that received bilateral electrolytic lesions, the lesioned area comprised a hole (400–800 μm in diameter) surrounded by a layer of dead neurons. The epicenters of such lesions were at the central or external-lateral subnucleus (Fig. 2). In both cases, surrounding structures such as the Kölliker-Fuse nucleus, superior cerebellum peduncle, and even medial parabrachial nucleus were occasionally invaded. We did not see any changes in respiratory pattern when the superior cerebellum peduncle was partially lesioned. However, apneusis was always observed when the Kölliker-Fuse or medial parabrachial nucleus was encroached upon as with previous studies (Denavit-Saubie et al. 1980); such animals were excluded from analysis.
Fig. 2. Histological sections showing LPBN lesions in two representative animals.
Left column: Montage photomicrographs showing the kainic acid injection site. The center of injection (arrowhead) was at the external-lateral LPBN. Right column: Photomicrographs and camera-lucida drawings on standard plates (Plate 51–53 of (Paxinos et al. 1986)) showing bilateral electrolytic lesions. The lesioned area (shaded) had dimensions of about 700 × 700 × 700 μm in the left side, and about 500 × 500 × 500 μm in the right side. The centers of lesions (*) on both sides were at the central LPBN. In both sides, Kölliker-Fuse nucleus (KF) was not affected. 5, trigeminal motor nucleus; scp, superior cerebellum peduncle. Numbers indicate the distances (in μm) of each section from the centers of microinjection (left column) or lesions (right column). See also (Song et al. 2009).
Expiratory phase is prolonged after bilateral lesions at LPBN
In animals with unilateral KA injection, all respiratory rhythm variables (f, TE, TI) at 30 min post-injection were not significantly different from the corresponding pre-injection baseline values under hyperoxia (P>0.1, two-tailed paired t test; Table 1). In animals with bilateral electrolytic lesions, TI after lesions was also not significantly different from control (P>0.1) but f was significantly decreased from a baseline value of 44.2±1.5 to 35.8±1.7 min−1 (P<0.01), primarily due to a prolongation of TE (from 0.80±0.05 to 1.14±0.06 sec, P<0.01; Table 1).
Table 1.
Effects of LPBN lesions on baseline respiratory patterns.
| Before | After | ||
|---|---|---|---|
| Unilateral Lesion (n=6) | f (min−1) | 39.2±1.8 | 43.0±3.3 |
| TE (sec) | 1.15±0.06 | 1.05±0.12 | |
| TI (sec) | 0.40±0.03 | 0.42±0.03 | |
| Bilateral Lesions (n=6) | f (min−1) | 44.2±1.5 | 35.8±1.7* |
| TE (sec) | 0.80±0.05 | 1.14±0.06* | |
| TI (sec) | 0.52±0.02 | 0.55±0.03 |
Data are means±SE of 1-min baseline recordings before or after LPBN lesions. f, respiratory frequency; TE and TI, expiratory and inspiratory durations.
P<0.01 (two-tailed paired t test).
Lesions at LPBN markedly attenuate the shortening of expiration during hypoxia
Before LPBN lesions, hypoxia elicited rapid increases in f that peaked within 30 sec before declining toward baseline (STD). Upon resumption of hyperoxia, f first dropped abruptly below baseline and then recovered gradually, evidencing PHFD in the off-transients of STD. (Fig. 3)
Fig. 3. Examples of poikilocapnic hypoxia test before and after LPBN lesion.
Representative recordings of raw (Phr.) and integrated phrenic discharge (∫Phr.); expired CO2 level (ECO2, expiration upward); and femoral artery blood pressure (BP). Shaded section indicates hypoxia. A: Control hypoxia test performed before lesion. B: Hypoxia test performed at 30 min after microinjection of KA. Note the almost identical changes in PETCO2 and blood pressure during poikilocapnic hypoxia before and after lesion.
After lesions, the increase in f during hypoxia became much smaller in both absolute and relative magnitudes (Fig. 4A). In the unilateral lesion group, the peak increase in f (over pre-hypoxia baseline) was 40.7±3.4% before and 10.9±1.5% after lesion (73% attenuation). The blunting of the respiratory frequency response to hypoxia was caused mainly by a weakening of the hypoxic response in TE. Before lesion, the maximal shortening of TE was −37.3±2.0% (below pre-hypoxia baseline), coincident with the maximal increase in f. After lesion, the maximal TE shortening was only −13.0±1.7% (65% attenuation). In the bilateral lesions group (Fig. 4B), the peak increase in f was 28.1±3.7% before and 5.8±3.6% after lesions (79% attenuation). Again, this was largely due to a much smaller shortening of TE (maximal shortening −25.7±3.3% vs. −1.0±6.4%; 96% attenuation).
Fig. 4. Unilateral or bilateral lesions at the LPBN attenuated the increase in respiratory frequency and shortening of expiration during hypoxia without affecting PHFD or STD.
Left column: Hypoxic responses in normalized respiratory frequency (f) and expiratory duration (TE). Data are means±SE (n=6). The increases of f and shortenings of TE during hypoxia (shaded areas) became much smaller after unilateral (A) or bilateral lesions (B) at the LPBN. However, at time 80 sec (20 sec post-hypoxia), the two curves again converged and differences were insignificant at this point (P>0.1, two-tailed paired t test). Right column: Responses in f (cycles/min.) and TE (sec) shown in absolute values.
Posthypoxia frequency decline is not affected by LPBN lesions
In the unilateral lesion group, the average PHFD at 20 sec post-hypoxia was −17.9±5.7% before and −13.6±4.3% after lesion. In the bilateral lesions group, the corresponding values were −29.9±4.4% and −28.8±5.3%. In both cases, the differences in PHFD between the control and lesioned conditions were not statistically significant (P>0.1, two-tailed paired t test) (Fig. 4).
Inspiratory components of the hypoxic response are not affected by LPBN lesions
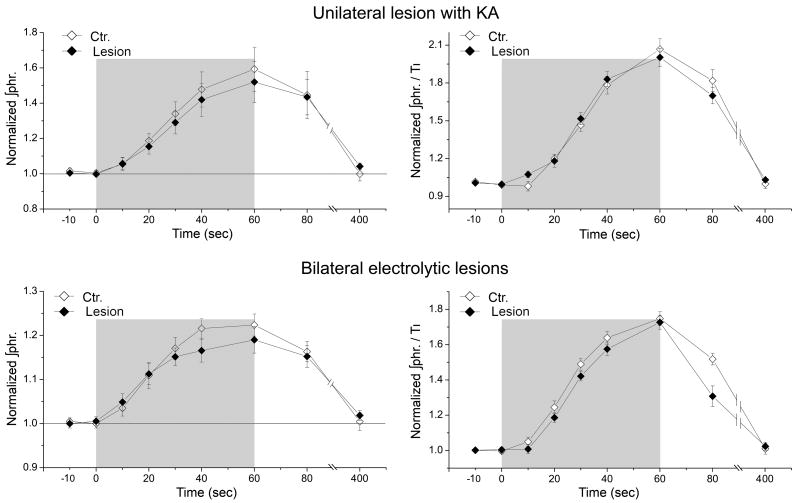
In either control or lesioned conditions, hypoxia caused similar progressive shortening of TI (Fig. 5) and progressive increases of ∫Phr and ∫Phr/TI (Fig. 6). All these changes reached peak values at the end of the 1-min hypoxic challenge indicating gradual development of STP in these variables. Upon resumption of hyperoxia, all these variables returned gradually to the corresponding baseline levels, evidencing afterdischarge. The response in TI during and after hypoxia were not significantly different between the control and both lesioned conditions (P>0.1, two-way ANOVA with repeated measures), whether in normalized or absolute values. The normalized values of ∫Phr and ∫Phr/TI during and after hypoxia were also not significantly different between the control and both lesioned conditions (P>0.1, two-way ANOVA with repeated measures).
Fig. 5. Shortening of inspiration and STP during and after hypoxia were not affected by unilateral or bilateral lesions at LPBN.
Response curves are expressed in normalized (left panels) or absolute values (right panels) for unilateral lesion group and bilateral lesions group. None of the response curves are significantly different between control (Ctr.) and lesioned conditions (P>0.1, two-way ANOVA with repeated measures).
Fig. 6. Increases of phrenic discharge amplitude and inspirtory drive and their STP during and after hypoxia were not affected by unilateral or bilateral lesions at LPBN.
∫Phr., phrenic discharge amplitude; ∫Phr./TI, inspiratory drive. None of the response curves are significantly different between control (Ctr.) and lesioned conditions (P>0.1, two-way ANOVA with repeated measures).
4. Discussion
The most important finding of this study is that lesions at the LPBN markedly and selectively attenuate the rapid shortening of TE (and resultant increase in f) during hypoxia but have no appreciable effects on other response components in anesthetized, vagotomized rats. Early studies of STP and STD in anesthetized and vagotomized rats simulated isocapnic hypoxia by keeping PETCO2 constant (Hayashi et al. 1993). However, changes in PETCO2 may not necessarily indicate similar changes in arterial PCO2 since alveolar dead space [hence arterial-alveolar PCO2 difference (Severinghaus et al. 1957)] may increase during hypoxia (Olson 1994). Subsequent studies show that poikilocapnic hypoxia elicits similar (albeit smaller) STP and STD effects in anesthetized or awake rodents (Coles et al. 1996; Kline et al. 2002) and awake humans (Steinback et al. 2007). In the present study, we employed brief poikilocapnic hypoxia in a consistent manner (with similar corresponding decreases in PETCO2) before and after LPBN lesioning to elicit similar STP and STD without causing significant hypoxic ventilatory decline in both conditions. We found that the rapid shortening of TE and corresponding increase in f were the only hypoxic response component that was significantly affected after unilateral or bilateral LPBN lesions.
In this study, lesions were made with either KA or electrolysis and were histologically identified to be at the central or external-lateral subnucleus of the LPBN. Electrolysis destroys neurons, glia, and axons of passage at the lesioned area, whereas KA selectively kills neurons that express glutamate kainate receptors at the injection site (Coyle et al. 1978). Considering the abundant expression of glutamate receptors in the dorsolateral pons (Takayama et al. 1993; Chamberlin et al. 1994), KA would cause significant neuronal death when injected into this region. In an early study performed in adult rats, injection of 0.1 μl of KA solution (concentration at 0.36 μg/μl) into dorsolateral pons resulted in neuronal loss in an area with diameter of ~0.5 mm (Mizusawa et al. 1995). Accordingly, we estimate that the diameter of affected area for the present dosage (0.05 μl at concentration of 1 μg/μl) should be between 0.5–1.0 mm, thus comparable to the size of electrolytic lesions both of which covered a major part of the LPBN but not all. The consistency between the effects of KA and electrolytic lesions made either unilaterally or bilaterally confirms that the LPBN was the specific site that mediated the rapid shortening of TE during hypoxia.
Another important finding of this study is that bilateral LPBN lesions resulted in a significant increase in baseline TE (and corresponding decrease in f ) under hyperoxia without appreciable changes in TI. This observation corroborates the notion that the LPBN also mediates TE-shortening by tonic factors beside peripheral chemoreceptor inputs, such as central chemoreceptor inputs (Song et al. 2009). The relative stability of TE and f after unilateral lesion compared to bilateral lesions indicate possible compensatory (plasticity) effects in the contralateral (intact) pathway after unilateral lesion. We did not compare the changes in ∫Phr and ∫Phr/TI before and after lesions because the absolute values of phrenic recordings (in arbitrary units) might drift in the course of a lengthy experimental procedure (lesioning and subsequent stabilization). Thus, only the relative changes in ∫Phr and ∫Phr/TI during and after brief hypoxia are presented.
The LPBN constitutes a subdivision of the dorsolateral pons ‘pneumotaxic center’ in the rat (Song et al. 2006). Chemical or electrical stimulations at this structure in cats or rats evoke predominantly respiratory facilitation, as opposed to respiratory inhibition for the more ventrally located Kölliker-Fuse nucleus or medial parabrachial nucleus (Cohen 1971; Takayama et al. 1993; Chamberlin et al. 1994; Lara et al. 1994; Dawid Milner et al. 2003). It has been suggested that the LPBN may play an important role in integrating respiratory-related afferents with other afferents such as nociceptive, thermosensitive, somatic proprioceptive and cardiovascular-related afferents (Jiang et al. 2004; Potts et al. 2005; Song et al. 2006; Morrison et al. 2008). In addition, several lines of evidence point to a possible involvement of the LPBN in mediating hypoxic respiratory response. For example, chronic lesion of a dorsolateral pontine region that included the LPBN resulted in decreased ventilatory response to hypoxia and hypercapnia in awake rats (Mizusawa et al. 1995). Anatomical data show that the LPBN has rich projections from chemoafferent- or chemoreception-related medullary structures that receive peripheral chemoreceptor inputs, such as commissural NTS and retrotrapezoid nucleus (Song et al. 2004; Rosin et al. 2006; Takakura et al. 2006). Neurons in LPBN express c-Fos protein in response to hypoxia challenge or carotid sinus nerve stimulation (Berquin et al. 2000; Bodineau et al. 2001) and their discharges are excited by carotid body stimulation (Hayward et al. 1995).
Extending these previous findings, the present study revealed that the LPBN specifically mediated the rapid shortening of TE (hence rapid increase in f) during hypoxia and not any other hypoxic response components. Furthermore, the present study provided evidence indicating that the LPBN also mediated TE-shortening by certain tonic inputs, such as central chemoreceptor inputs (Song et al. 2009). Taken together, these findings raise the possibility that the LPBN may be the site of the putative fast integrator whereby central chemoreceptor inputs are potentiated by peripheral chemoreceptor inputs to shorten TE (Fig. 1B). This observation, together with earlier suggestion that PHFD is mediated by a subdivision of the pneumotaxic center in the ventrolateral pons (Coles et al. 1996), corroborate the notion (Fig. 1B) that the central processing of peripheral chemoafferent inputs is segregated into parallel integrator-differentiator pathways that separately modulate inspiratory drive, TI, TE and resultant STP and STD (Young et al. 2003).
Acknowledgments
We thank A.S. Lo for assistance with data analysis. This work was supported by National Institutes of Health grants HL067966, HL072849 and HL079503.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bantikyan A, Song G, Feinberg-Zadek P, Poon C-S. Intrinsic and Synaptic Long-Term Depression of NTS Relay of Nociceptin-Sensitive and Capsaicin-Sensitive Cardiopulmonary Afferents Hyperactivity. Pflugers Arch (European Journal of Physiology) 2009 Mar;457(5):1147–59. doi: 10.1007/s00424-008-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Vol. 79. Marcel Dekker; New York: 1995. pp. 617–668. [Google Scholar]
- Boden AG, Harris MC, Parkes MJ. Apneic threshold for CO2 in the anesthetized rat: fundamental properties under steady-state conditions. J Appl Physiol. 1998;85:898–907. doi: 10.1152/jappl.1998.85.3.898. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Larnicol N. Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience. 2001;108:643–653. doi: 10.1016/s0306-4522(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol. 1971;217:133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J Physiol. 1996;497( Pt 1):79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME, Kuhar MJ. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J Comp Neurol. 1978;180:301–323. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Cross KW, Oppe TE. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. J Physiol. 1952;117:38–55. [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Wilson RJ. Time-dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1571–1580. doi: 10.1152/ajpregu.00788.2004. [DOI] [PubMed] [Google Scholar]
- Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S. A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res. 2003;982:108–118. doi: 10.1016/s0006-8993(03)03005-1. [DOI] [PubMed] [Google Scholar]
- Denavit-Saubie M, Riche D, Champagnat J, Velluti JC. Functional and morphological consequences of kainic acid microinjections into a pontine respiratory area of the cat. Neuroscience. 1980;5:1609–1620. doi: 10.1016/0306-4522(80)90025-1. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Duffin J. Measuring the ventilatory response to hypoxia. J Physiol. 2007;584:285–293. doi: 10.1113/jphysiol.2007.138883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol. 1986;61:906–911. doi: 10.1152/jappl.1986.61.3.906. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE. Oscillation, gating, and memory in the respiratory control system. In: Fishman AP, Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, Section 3: The Respiratory System, Vol. II: Control of Breathing, part 1. American Physiological Society; Bethesda, MD: 1986. pp. 93–114. [Google Scholar]
- Gardner WN. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos D, Bshouty Z, Younes M, Anthonisen NR. Hypoxic exposure and activation of the afterdischarge mechanism in conscious humans. J Appl Physiol. 1990;69:1159–1164. doi: 10.1152/jappl.1990.69.3.1159. [DOI] [PubMed] [Google Scholar]
- Gershan WM, Forster HV, Lowry TF, Garber AK. Effect of theophylline on ventilatory roll-off during hypoxia in goats. Respir Physiol. 1996;103:157–164. doi: 10.1016/0034-5687(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol. 2000;121:209–221. doi: 10.1016/s0034-5687(00)00129-8. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Felder RB. Peripheral chemoreceptor inputs to the parabrachial nucleus of the rat. Am J Physiol. 1995;268:R707–714. doi: 10.1152/ajpregu.1995.268.3.R707. [DOI] [PubMed] [Google Scholar]
- Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol. 2002;539:309–315. doi: 10.1113/jphysiol.2001.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol. 1994;477( Pt 2):321–329. doi: 10.1113/jphysiol.1994.sp020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long WA, Lawson EE. Neurotransmitters and biphasic respiratory response to hypoxia. J Appl Physiol. 1984;57:213–222. doi: 10.1152/jappl.1984.57.1.213. [DOI] [PubMed] [Google Scholar]
- Melton JE, Neubauer JA, Edelman NH. CO2 sensitivity of cat phrenic neurogram during hypoxic respiratory depression. J Appl Physiol. 1988;65:736–743. doi: 10.1152/jappl.1988.65.2.736. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489( Pt 3):877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB., Jr Physiological dead space increases during initial hours of chronic hypoxemia with or without hypocapnia. J Appl Physiol. 1994;77:1526–1531. doi: 10.1152/jappl.1994.77.3.1526. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS, Young DL, Eldridge FL. Short-term potentiation of carotid chemoreflex: An NMDAR-dependent neural integrator. NeuroReport. 1999;10:2261–2265. doi: 10.1097/00001756-199908020-00007. [DOI] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS. Plasticity of cardiorespiratory neural processing: classification and computational functions. Respir Physiol. 2000a;122:83–109. doi: 10.1016/s0034-5687(00)00152-3. [DOI] [PubMed] [Google Scholar]
- Poon CS, Young DL. Nonassociative learning as gated neural integrator and differentiator in stimulus-response pathways. Behav Brain Funct. 2006;2:29. doi: 10.1186/1744-9081-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CS, Young DL, Siniaia MS. High-pass filtering of carotid-vagal influences on expiration in rat: role of N-methyl-D-aspartate receptors. Neurosci Lett. 2000b;284:5–8. doi: 10.1016/s0304-3940(00)00993-9. [DOI] [PubMed] [Google Scholar]
- Poon CS, Zhou Z, Champagnat J. NMDA receptor activity in utero averts respiratory depression and anomalous long-term depression in newborn mice. J Neurosci. 2000c;20:RC73. doi: 10.1523/JNEUROSCI.20-09-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Stupfel M. Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol. 1957;10:335–348. doi: 10.1152/jappl.1957.10.3.335. [DOI] [PubMed] [Google Scholar]
- Song G, Poon C-S. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009 Jan 1;165(1):9–12. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback CD, Poulin MJ. Ventilatory responses to isocapnic and poikilocapnic hypoxia in humans. Respir Physiol Neurobiol. 2007;155:104–113. doi: 10.1016/j.resp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Miura M. Respiratory responses to microinjection of excitatory amino acid agonists in ventrolateral regions of the lateral parabrachial nucleus in the cat. Brain Res. 1993;604:217–223. doi: 10.1016/0006-8993(93)90372-t. [DOI] [PubMed] [Google Scholar]
- Vizek M, Bonora M. Diaphragmatic activity during biphasic ventilatory response to hypoxia in rats. Respir Physiol. 1998;111:153–162. doi: 10.1016/s0034-5687(97)00116-3. [DOI] [PubMed] [Google Scholar]
- Wagner PG, Eldridge FL. Development of short-term potentiation of respiration. Respir Physiol. 1991;83:129–139. doi: 10.1016/0034-5687(91)90098-4. [DOI] [PubMed] [Google Scholar]
- Weil JV, Zwillich CW. Assessment of ventilatory response to hypoxia: methods and interpretation. Chest. 1976;70:124–128. [PubMed] [Google Scholar]
- Woodrum DE, Standaert TA, Mayock DE, Guthrie RD. Hypoxic ventilatory response in the newborn monkey. Pediatr Res. 1981;15:367–370. doi: 10.1203/00006450-198104000-00016. [DOI] [PubMed] [Google Scholar]
- Young DL, Eldridge FL, Poon CS. Integration-differentiation and gating of carotid afferent traffic that shapes the respiratory pattern. J Appl Physiol. 2003;94:1213–1229. doi: 10.1152/japplphysiol.00639.2002. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Champagnat J, Poon C-S. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptor desensitization. J Neurosci. 1997;17:5349–5356. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]