Abstract
Embryonic stem cells (ESCs) are pluripotent cells capable of differentiating into all somatic and germ cell types. The intrinsic ability of pluripotent cells to generate a vast array of different cells makes ESCs a robust resource for a variety of cell transplantation and tissue engineering applications, however, efficient and controlled means of directing ESC differentiation is essential for the development of regenerative therapies. ESCs are commonly differentiated in vitro by spontaneously self-assembling in suspension culture into 3D cell aggregates called embryoid bodies (EBs), which mimic many of the hallmarks of early embryonic development, yet the 3D organization and structure of EBs also presents unique challenges to effectively direct the differentiation of the cells. ESC differentiation is strongly influenced by physical and chemical signals comprising the local extracellular microenvironment, thus current methods to engineer EB differentiation have focused primarily on spatially controlling EB size, adding soluble factors to the media, or culturing EBs on or within natural or synthetic extracellular matrices. While most such strategies aim to influence differentiation from the exterior of EBs, engineering the microenvironment directly within EBs enables new opportunities to efficiently direct the fate of the cells by locally controlling the presentation of morphogenic cues.
Keywords: embryoid body, embryonic stem cells, differentiation, microenvironment, morphogenesis
1. Introduction
1.1. Embryonic Stem Cells
Embryonic stem cells (ESCs) are capable of limitless self-renewal in vitro and differentiate into cells constituting all three primitive germ layers– mesoderm, ectoderm and endoderm, as well as germ cells (sperm and ova). ESCs, isolated from the inner cell mass of blastocyst stage embryos, were first derived from mouse embryos (1–3), followed by the derivation of primate (4,5) and human (6,7) ESC lines. Recently, an alternative method for deriving pluripotent cells by retroviral transduction of a combination of embryonic genes into somatic cells was reported, first by Yamanaka’s group, followed shortly thereafter by several other groups independently (8–12). The “induced” pluripotent stem (iPS) cells created from both mouse and human somatic cells appear similar to ESCs in terms of both self-renewal and differentiation capacity.
A functional test of pluripotency is whether introduction of the cells into a blastocyst stage embryo results in a chimera with ESCs (or iPS cells) contributing to all tissues of the organism (13,14). Similarly, ESCs injected into various tissue sites of adult organisms spontaneously form teratomas, benign tumors composed of a disorganized mix of cells from all three germ layers. Blastocyst injection and teratoma studies demonstrate that the environment into which pluripotent cells are introduced can influence differentiation, however, in vivo studies are limited in their ability to attain mechanistic insights into the effects of environmental factors on stem cell differentiation. In contrast, differentiation of ESCs in vitro, affords more controlled methods to present morphogenic cues in the stem cell microenvironment and directly assess differentiated cell phenotypes. Common formats to induce ESC differentiation in vitro include monolayer culture on defined matrices (15), co-culture with heterotypic cell types (16) and the formation of cell aggregates grown in suspension termed embryoid bodies (EBs) (3). Culture of ESCs in planar formats (i.e. monolayer, co-culture) attempt to provide a more defined substrate for ESC attachment and uniform exposure to soluble media components, while the 3D aggregates of ESCs formed by EB culture techniques more accurately recapitulate the complex assembly of cell adhesions and intercellular signaling of early embryogenesis.
1.2. Embryoid Body Development
The in vitro culture of ESCs as EBs affords opportunities to mechanistically study early differentiation events of 3D assemblies of pluripotent cells. One advantage of in vitro differentiation studies is that genetic manipulation of ES cells can be studied for gene mutations or knockouts that prove to be lethal during normal embryonic development in vivo (17–19). Although several phenotypic and functional differences between mouse and human ESCs have been determined (20–23), few studies have directly examined differences between mouse and human EB differentiation. One such study, however, identified shared signaling pathways active during mouse and human EB differentiation, suggesting that mechanisms regulating differentiation may be conserved between the species (24). EB differentiation begins with the formation of an aggregate of ESCs, the size of which is dependent on the number of cells which initially self-assemble via cell-cell adhesion receptors (25–27). Following cell aggregation, the first indication of differentiation is the spontaneous formation of a layer of primitive endoderm (PE) on the exterior surface of the EBs (23). While the specific cues responsible for stimulating PE differentiation remain unknown, the formation of a PE layer on the exterior of EBs appears to be dependent on fibroblast growth factor (FGF) signaling mediated by the PI 3-kinase pathway (28,29). The PE cells exhibit an epithelial morphology on the EB surface, further differentiate into visceral and parietal endoderm, and deposit a basement membrane rich in laminin and collagen IV (17). The basement membrane which separates the PE cell layer from the remaining mass of undifferentiated cells within the EB is generally thought to promote the survival of adjacent cells, whereas cells not in direct contact with the basement membrane undergo apoptosis, contributing to the formation of cystic cavities in most EBs (30–32).
As EB development progresses, differentiated cell phenotypes of all three germ lineages begin to arise (33). For example, evidence of hematopoietic differentiation of EBs is supported by the appearance of yolk sac-like blood islands and spontaneously contractile foci of cells within EBs, indicative of cardiomyogenic differentiation, are readily apparent under low magnification (3,34). Upon plating onto an adherent substrate, elongated cell projections resembling neurite extensions emanate out from EBs and morphological evidence of endothelial cells, fibroblasts and other cell types can be readily observed. Global DNA microarray analysis indicates that EBs temporally express genes in a manner that recapitulates the sequence of normal development from primitive ectoderm formation, to gastrulation, and eventual early cell specification prior to organogenesis (35). Expression of phenotypic markers of endoderm (such as Foxa2, Sox17, GATA 4/6, α-fetoprotein, and albumin), mesoderm (such as Brachyury-T, Msp1/2, Isl-1, α-actin, ζ-globin, and Runx2), and ectoderm (such as Sox1, Nestin, Pax6, GFAP, Olig2, neurofilament, and β–III Tubulin) definitively demonstrate the ability of EBs to generate cells from all three germ layers (36). However, the typical heterogeneous differentiation of EBs is a significant challenge for the efficient production of defined cell types and can be influenced by EB formation and culture methods.
1.3. Embryoid Body Culture Methods
The term ‘embryoid body’ has been broadly applied to describe pluripotent cell aggregates induced to differentiate using a variety of different formation and culture methods. Generally speaking, an aggregate of pluripotent stem cells, cultured in suspension, and capable of forming derivatives of all three germ lineages is regarded as an EB. Although no universally accepted benchmarks currently exist for EB formation, characteristics such as EB size, shape and homogeneity are typically used as points of reference for comparison. Common EB culture practices, such as hanging drop and static suspension culture were adopted from in vitro differentiation methods originally used for embryonic carcinoma (EC) cells, pluripotent precursors to the ESCs themselves (37). A comprehensive review describing several of the most common EB culture methods has recently been published (38).
The hanging drop method of EB formation produces homogeneous cell aggregates by dispensing a defined number of ESCs in physically separated droplets of media suspended from the lid of a Petri dish (39,40). Individual EBs form within each drop via gravity-induced aggregation of the cells and although EBs created by the hanging drop method can be subsequently introduced to suspension batch culture, the technique is not easily amenable to scale up for production of large numbers of EBs. An additional limitation of hanging drop culture is the difficulty in exchanging or manipulating the small volume of medium (typically 10–20 µl) without disturbing the EBs, thus the composition of the media cannot be easily controlled or assayed during the period of hanging drop suspension.
In contrast to hanging drop methods, static suspension culture is performed by simply adding a suspension of ESCs to a bacteriological grade Petri dish or similar vessel that inhibits cell adhesion (i.e. agar- or other hydrophilic polymer-coated substrate), thereby allowing the cells to spontaneously aggregate via cell-cell adhesions (3,41,42). Static suspension cultures produce a large number of EBs rather simply, but the size and shape of the resulting EBs are highly variable due to the tendency of EBs in static suspension to agglomerate after initial formation, often producing large, irregularly shaped masses of cells. Often times, depending on the surface chemistry of the culture vessel, EBs may prematurely attach to the substrate, leading to greater heterogeneity and loss of EBs from suspension culture.
Entrapment of a single cell suspension or small clusters of ESCs in hydrogels, such as methylcellulose (34,43,44), fibrin (45) or hyaluronic acid (46), represents a compromise between hanging drop and static suspension approaches to attain physically separated EBs in a bulk semi-solid suspension media. Entrapment in methylcellulose, a temperature sensitive hydrogel, yields EBs of clonal origin, thereby improving the overall synchrony and reproducibility of EB differentiation; however, the efficiency of EB formation from individual ESCs can be rather low and soluble factor treatments and retrieval of differentiated cells may be complicated by the presence of the hydrogel material (43).
Alternative techniques for EB formation and culture have also been recently developed using multi-well and microfabrication technologies, as well as stirred and mixed suspension culture systems. Centrifugation of ESCs within round-bottomed 96-well plates induces aggregation more rapidly than hanging drops, but still requires individual processing and manipulation of the resulting EBs (47). Microwells fabricated by lithographic techniques yield EBs in parallel at a much higher density than other physical separation methods and the ability to form EBs within microwells in a continuous volume of medium permits batch processing, therefore significantly improving the throughput of EB formation (48–50). Likewise, batches of EBs can be formed in microfluidic chambers, separated from the flowing culture medium by a semi-permeable membrane, which allows for temporal control of the molecular makeup of the medium (51).
Formation of EBs in hydrodynamic conditions created by rotary orbital culture, stirred/rotating culture vessels, or spinner flasks generally enhances ESC aggregation, forming EBs faster and more uniformly than static bulk cultures (25,52–57). Hence, hydrodynamic conditions can generate large populations of EBs at a relatively high density, while at the same time, controlling the extent of EB agglomeration and subsequent differentiation of the cells (53,55,57,58). The mixing environment also distributes media components more homogenously throughout the culture volume so that the population of EBs is continuously exposed to a more uniform concentration of soluble factors and environmental conditions (i.e. pH, oxygen, etc.).
An inherent trade-off in most of the current systems available for EB formation is that batch-based suspension methods produce large numbers of EBs rather simply, but generally lack the fidelity of physical separation methods (hanging drop, microwell), thus yielding more heterogeneous populations of EBs. On the other hand, physical separation methods capable of generating homogeneous EB populations are often not capable of being directly scaled up to produce the yields of ESC derivatives thought to be necessary for therapeutic or diagnostic applications.
2. Engineering Embryoid Body Cues
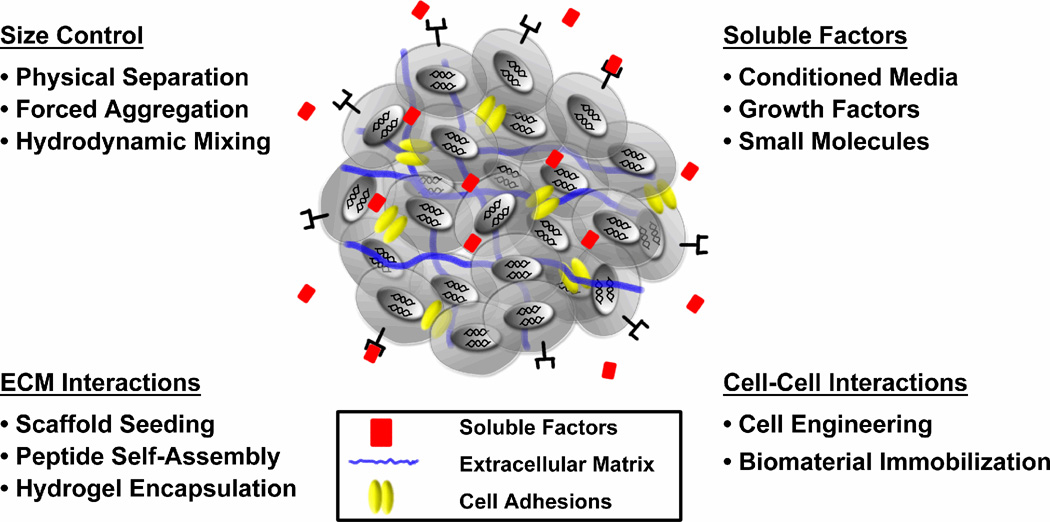
Differentiation of cells within EBs is directed by morphogenic cues comprising the intercellular and surrounding extracellular microenvironment, including exogenously administered molecules and endogenous factors produced by the ESCs. Individual aspects of the microenvironment can be studied rather simply in planar culture formats, but similar to a developing embryo, the 3D organization of an EB is inherently comprised of a complex milieu of integrated signals that synergistically affect cell differentiation. Although the 3D assembly of cells to form EBs presents unique challenges for regulating the homogeneity of stem cell differentiation, attempts to control EB size, soluble factor delivery, extracellular matrix (ECM) interactions and cell-cell adhesions within EBs may influence differentiated cell phenotypes (Fig. 1).
Figure 1.
2.1. Size Control
The size of EBs, typically in the range of 100–400 µm, is thought to be a simple, yet important physical parameter capable of influencing the proportion of cells differentiating toward different lineages. EB size, which is primarily a function of the number of ESCs constituting each cell aggregate, impacts other environmental parameters affecting differentiation, such as the diffusion of soluble molecules and the extent of ECM-cell and cell-cell adhesive interactions. Recent developments in EB formation techniques have enabled more controlled systems capable of modulating EB size in order to begin to determine the effects on subsequent differentiation of the cells.
As described above, forced aggregation of ESCs using multi-well round-bottomed plates or microtechnologies provides a very direct manner to precisely control the number of cells in individual cell aggregates. For example, the number of cells used to form hanging drops can influence the chondrogenic differentiation potential of EBs (59). Likewise, forced centrifugation studies examining hematopoietic differentiation of human ESCs of varying sizes indicated that a minimum EB starting size (500 cells/EB) was required for myeloid differentiation to occur in over 90% of EBs and that an intermediate size range (1000 cells/EB) promoted erythroid cell differentiation (47). The initial size of EBs can also be controlled through the geometric size of microwell or micropattern features in order to spatially define the number of ESCs within individual aggregates (49,50,60). Micropatterned control of ESC colonies can dictate both the size of EBs and the phenotype(s) of the starting cell population used to form EBs, which can affect the differentiation of the cells to particular germ lineages (60). Recently, microfabricated cell culture inserts compatible with standard multi-well culture plates were reported which significantly enhance the yield of EBs formed using forced aggregation (48). The size of the resulting EBs can be controlled by the concentration of the cells inoculated into the well and after 24 hours, EBs can be extracted from the microwells with gentle pipetting and transferred to suspension culture. Depending on the dimensions of the microwells, the poly(dimethylsiloxane) inserts contain between 104 to 106 microwells per 100 cm2 of surface area - a dramatic increase over the capabilities of round-bottomed 96-well plates (48).
In addition to forced aggregation methods, hydrodynamic culture conditions can be used not only to prevent EB agglomeration, but also regulate the size of EBs formed from single cell suspensions (52–54). For EBs in horizontal rotary culture and stirred bioreactor culture, an inverse relationship exists between mixing speed and EB size, with decreasing EB size achieved by faster mixing conditions; thus EB size in bulk suspension can be modulated by hydrodynamic mixing conditions (53,57). EB size can also be controlled by encapsulating suspensions of individual ESCs or primitive EBs into hydrogel microbeads of controlled volumes. For example, agarose (25), alginate (61–63), and dextran (64) have all been used successfully to encapsulate ESCs, either as single cells or small clumps of cells, to form EBs within microgels. The diameter of the microgels laden with ESCs can vary greatly from 100 µm agarose beads (25) to 2.3 mm diameter alginate beads (63). One problem with increasing microgel size, however, is that encapsulated ESCs may have a tendency to form multiple EBs within individual beads, limiting the ability to accurately control EB size.
Depending on the different culture methods used, the kinetics of EB formation vary dramatically from minutes (forced aggregation) to hours (hydrodynamic mixing) to days (cell encapsulation),. Despite such differences, the consequences of the time scale for EB formation on cell fate and lineage determination has not been directly examined independently of EB size. In addition, although different methods to control initial EB size have been developed, the mechanisms regulating the causal relationship between the size of individual EBs and their propensity to differentiate into different cell phenotypes has yet to be fully elucidated.
2.2. Soluble Factors
Controlling the molecular composition of culture media to direct ESC differentiation has been studied extensively in a variety of systems and the effects of specific soluble factors and signaling pathways on ESC differentiation have been thoroughly discussed previously (65–67). Small molecules such as ascorbic acid (68), retinoic acid (69) and dexamethasone (70), as well as larger growth factors such as fibroblast growth factors, bone morphogenic proteins and transforming growth factors (66,71), are examples of soluble factors which have been shown to affect ESC differentiation. Presentation of soluble signaling molecules to ESCs in monolayer culture has been the primary method to screen the ability of libraries of chemical compounds and biomolecules to induce ESC differentiation into specific cell types (15,68,72). In lieu of direct co-culture, complex, yet poorly defined media conditioned by secondary cell types has been applied to stem cells in order to direct differentiation (73,74). On the other hand, defined soluble media comprised of known amounts of different factors has also been used successfully to generate relatively homogeneous populations of cells, particularly for neural progenitors or neurogenic cell fates (15,75,76).
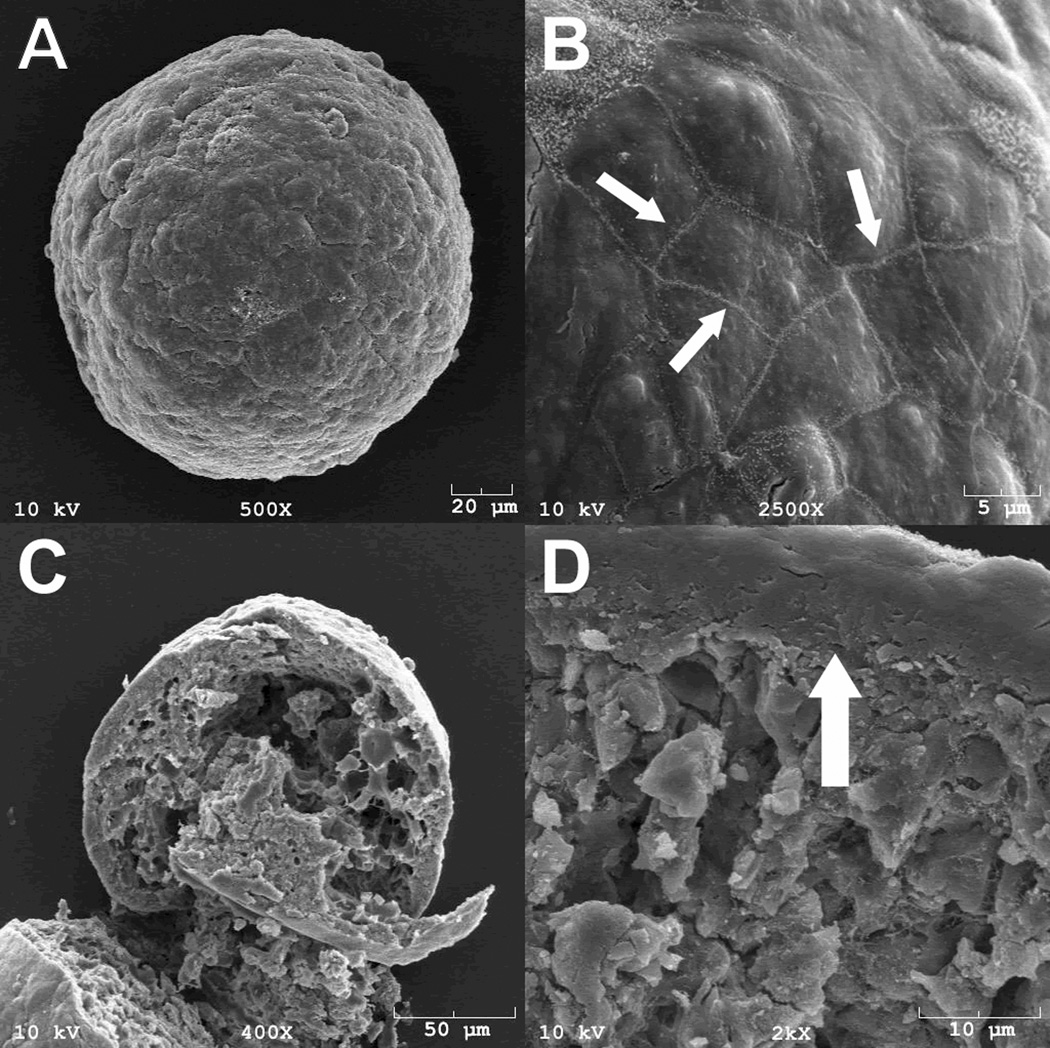
In stark contrast to 2D planar culture formats, only the cells on the exterior of 3D EBs are in direct contact with soluble factors present in the culture medium. Soluble factors must diffuse through this multi-layered cell environment and barriers to transport, which likely vary as a function of stages of EB differentiation, contribute to the formation of concentration gradients which comprise the cell microenvironment. Even the diffusion of small molecules (<1000 Da), may have a limited ability to pass through the peripheral cells of EBs (77). High-powered SEM microscopy analysis of EBs indicates that the surface layer of epithelial-like cells (Fig. 2A) exhibit tight cell-cell junctions (Fig. 2B) and cross-sectional analysis of EBs (Fig. 2C) indicates that EBs tend to form a relatively dense layer of ECM and cells at the periphery of EBs (Fig. 2D), compared to the rest of the interior cellular morphology. Therefore, steric barriers to diffusion posed by EB structure make it unlikely that homogenous concentrations of molecules can be attained uniformly throughout the interior of EBs and limit the efficacy of differentiation strategies relying solely on the addition of soluble factors to the culture medium.
Figure 2.
2.3. Extracellular Matrix Interactions
The ECM can be a potent mitigator of cell fate decisions by providing a complex assembly of morphogenic cues to stem cells. The ECM is a structural framework of secreted macromolecules consisting primarily of glycosaminoglycans and fibrous proteins which provide mechanical support, adhesive interactions and sequestration of growth factors. Native ECM components direct cell differentiation through integrin-mediated signaling events with adhesive proteins, as well as proteolytic release of affinity-bound growth factors during matrix remodeling (67,78). Integrin ligation and growth factor binding to receptors initiate intracellular signaling cascades that ultimately culminate in gene expression changes that modulate cell phenotype (79).
The effects of ECM molecules on EB differentiation have largely been examined by seeding ESCs or pre-formed EBs directly within natural ECM hydrogel materials (45,46,71,80). EBs differentiated in collagen scaffolds consisting of variable amounts of fibronectin and laminin demonstrated that varying the composition of the ECM could differentially direct EB differentiation. EBs in collagen scaffolds with high laminin content adopted a cardiomyocyte phenotype more frequently, whereas EBs were directed towards more epithelial and vascular cell fates in hydrogels with high fibronectin content, and EB cavitation and differentiation appeared to be inhibited in hydrogels with increasing collagen content (80). ECM signaling peptides can also be incorporated into non-bioactive hydrogels used to encapsulate EBs, such as RGD modified dextran (64), to examine the effects of ECM on ESC differentiation. In addition to changes in the specific biochemical constituents of the ECM, differences in the elasticity of the ECM may also provide mechanotransductive cues capable of affecting stem cell differentiation (81).
Encapsulation of EBs within ECM matrices limits the interactions between ESCs and the ECM to the exterior surface of the ESC aggregates. Therefore, in an attempt to directly manipulate the composition of the ECM within the EB microenvironment, individual matrix molecules like collagen and laminin have been added solubly to suspensions of ESCs during EB formation (17,82,83). Similarly, the addition of soluble complex, tissue-derived matrices, such as Matrigel or Cartigel, to EB culture media has been used to promote the formation of glandular and tubular-like structures or cartilage development, respectively (82). Although soluble addition of ECM molecules to ESC suspensions may favor incorporation within EBs, soluble ECM molecules alone do not necessarily assemble to form a functional matrix. Self-assembling peptide-based matrices, on the other hand, can rapidly form within developing cell aggregates to form a hydrogel network of nanofibers presenting different signaling epitopes (84,85). Utilizing this strategy, neural progenitor cells encapsulated as neurospheres in a self-assembling IKVAV (laminin epitope) amphiphile solution differentiated rapidly into neurons, while astrocyte differentiation was attenuated (85). Interestingly, the density of the peptide epitope within the cell microenvironment, a material characteristic which can be controlled by matrix formulation conditions, could modulate the differentiation of the cells. Applying a similar principle to EBs, self-assembling matrices could provide a novel route to control the composition and spatial distribution of extracellular signaling motifs present within aggregates of ESCs undergoing differentiation.
2.4. Cell-Cell Interactions
EBs are initially formed via cell-cell adhesive interactions, but intercellular adhesions can also serve an important role in cell signaling throughout EB differentiation. Cell-cell interactions are mediated primarily by cadherins, a family of Ca2+ dependent transmembrane adhesion receptors that play important roles in cell differentiation during embryogenesis (86). Homophilic cadherin receptor binding triggers intracellular signaling pathways mediated by cytoplasmic catenin proteins, such as β-catenin, which is linked to the Wnt pathway, a potent regulator of cell morphogenesis and differentiation (86). Undifferentiated ESCs express epithelial-cadherin (E-cadherin), which is the primary molecular mediator of EB formation, but sustained E-cadherin expression can also be responsible for the agglomeration of EBs at later stages of differentiation (25). Inhibition of E-cadherin mediated adhesion, either by the use of E-cadherin binding antibodies or E-cadherin null ESCs, prevents normal EB formation and subsequent differentiation (25,26,54). Differential cadherin expression, associated with different cell phenotypes, is temporally regulated during the course of EB differentiation and can directly influence cell fate specification. For example, ESCs constitutively expressing E-cadherin are prone to more epithelial differentiation, while ESCs constitutively expressing N-cadherin differentiate more readily into cartilage and neuroepithelium (27). Although it has not been systematically investigated, the use of cadherin signaling to control EB differentiation either through integration of a genetically modified cell line over-expressing a particular cadherin or through the presentation of cadherins on biomaterial surfaces integrated within EBs to mimic cell-cell interactions is a promising area for control of EB differentiation.
Strategies to control other types of cell-cell interactions, including transmembrane receptors and ligands not anchored to the cytoskeleton, have also been explored in stem cells. For example, the Notch pathway is involved in a variety of cell fate decision processes through development and adult tissue morphogenesis (87,88). ESCs can express multiple Notch receptors and Notch signaling has been implicated both in stem cell self-renewal and differentiation towards different phenotypes, such as neuronal cells (89–91). In general, Notch signaling requires immobilized ligand presentation from a surface or cell membrane in order to achieve optimal bioactivity (92). Jagged-1, a Notch ligand, immobilized to polystyrene or polyHEMA surfaces promoted early and late stage differentiation of cultured epithelial stem cells (93). Comparable methods of presenting Notch ligands to cells uniformly within EBs would require that engineered biomaterials be integrated directly within the interior of the ESC aggregate.
3. Future Opportunities
In general, most of the strategies attempted thus far to direct EB differentiation have relied on an ‘outside-in’ approach to control aspects of the microenvironment. From the instant an aggregate of ESCs begins to form an EB, access to the interior intercellular environment and molecular composition of an EB becomes progressively restricted and ‘outside-in’ manipulation of cell fate within an EB may become limited. Although 2D differentiation of ESCs has been successfully used to spatially and temporally control the presentation of molecules for the differentiation of several cell phenotypes, the differentiation of some cells may require the synergistic effects of cell-cell and cell-ECM interactions provided within the context of EBs in 3D. Therefore, in order to efficiently control the 3D microenvironment of differentiating ESCs within EBs, further development of engineering technologies capable of directly influencing cell fates within EBs are needed.
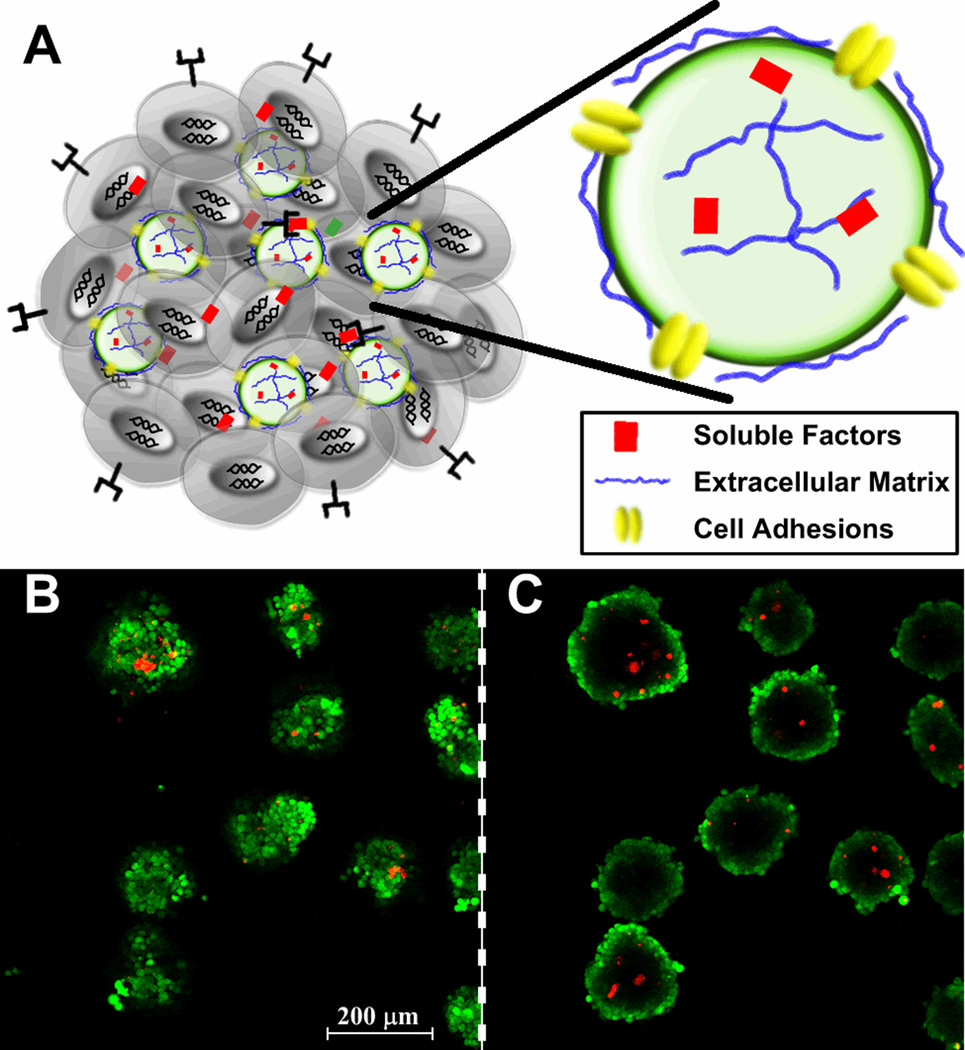
Future methods to enhance the directed differentiation of cells within EBs could be engineered by utilizing an ‘inside-out’ approach. Integration of micro- or nano-scale biomolecule delivery technologies directly within EBs could create a more homogeneous and defined microenvironment for cells constituting EBs. One such approach is the use of particles engineered to mimic elements of the natural microenvironment to control differentiation signals. Microparticles have been previously used to create a synthetic microenvironment, consisting of encapsulated growth factors and surface-bound ECM, in spheroids of fetal rat brain cells (94) and recently PLGA microparticles delivering growth factors were incorporated into EBs (95). Microparticles provide a versatile platform to deliver molecular cues via surface engineering and controlled release approaches used in the design of the particles (Fig. 3A). Similarly, adhesive microparticles can be readily incorporated within EBs during initial formation when the particles are adequately mixed with ESCs in suspension (Fig. 3B). Synthetic polymeric microparticles can be engineered to release specific amounts of soluble factors with controlled kinetics, and cell adhesion ligands and receptors can be coupled to the surface of microparticles to activate integrin and cell-cell adhesion receptor signaling pathways. Molecules physically adsorbed or immobilized to the surface of microparticles may not only promote efficient incorporation into EBs as they form, but also subsequently influence extracellular signaling events directing cell differentiation and morphogenesis. Even if small molecules are capable of diffusing within small cell aggregates, delivery of larger molecules, such as ECM proteins or growth factors is likely to be limited by steric diffusion barriers presented by the 3D structure of EBs. Engineering the molecular composition of the EB microenvironment from the “inside-out” represents a novel approach to control the presentation of morphogenic cues to ESCs in order to enhance the directed differentiation of the cells in a 3D spheroid configuration.
Figure 3.
4. Conclusions
The formation of EBs is a reliable and commonly used method to induce the differentiation of ESCs into various somatic cell types. Hence, EB differentiation permits mechanistic studies of embryological development in vitro, including the examination of the effects of morphogenic cues on cell fate determination. Previous efforts to engineer the EB microenvironment have focused primarily on the regulation of EB size and cell-cell interactions, as well as addition of soluble factors, and ECM-molecules to EB cultures. The inherent 3D organization of EBs limits the effectiveness of ‘outside-in’ approaches which aim to affect differentiation of cells on the EB interior by controlling elements of the exterior EB environment. An alternative strategy to improve the control of the EB microenvironment in order to better direct ESC differentiation may be to use an ‘inside-out’ approach, such as integrating engineered biomaterials within the assembly of ESCs during EB formation. Engineering the interior of the EB microenvironment via molecularly engineered biomaterials to enhance the directed differentiation of ESCs could facilitate the production of large numbers of homogeneous cell populations useful to the development of regenerative cellular therapies and diagnostic cell-based technologies.
Acknowledgements
This work is supported by funding from the National Science Foundation (CBET 0651739) and additional funding is provided to TM by the National Institutes of Health (R21 EB007316) and the American Heart Association (0665265B). A.B.L. is currently supported by an NIH Training Grant (GM008433), as well as funding from the Goizueta Foundation, and was previously supported by a fellowship from the Georgia Tech/Emory Center for Engineering of Living Tissues (NSF EEC 9731463). R.L.C. was supported by a GAANN fellowship from the Center for Drug Design, Development and Delivery.
References
- 1.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 4.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92(17):7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55(2):254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 6.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnology. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 11.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 12.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 13.Gossler A, Doetschman T, Korn R, Serfling E, Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson E, Bradley A, Kuehn M, Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 15.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature Biotechnology. 2003;21(2):183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 16.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265(5175):1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 2001;153(4):811–822. doi: 10.1083/jcb.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komura H, Ogita H, Ikeda W, Mizoguchi A, Miyoshi J, Takai Y. Establishment of cell polarity by afadin during the formation of embryoid bodies. Genes Cells. 2008;13(1):79–90. doi: 10.1111/j.1365-2443.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, Brakebusch C. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn. 2007;236(10):2767–2778. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260(2):404–413. doi: 10.1016/s0012-1606(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 21.Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269(2):360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY, Gupta S, Stanton L, Luo Y, Schmitt J, Thies S, Wang W, Khrebtukova I, Zhou D, Liu ET, Ruan YJ, Rao M, Lim B. Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells. 2005;23(2):166–185. doi: 10.1634/stemcells.2004-0162. [DOI] [PubMed] [Google Scholar]
- 23.Maurer J, Nelson B, Cecena G, Bajpai R, Mercola M, Terskikh A, Oshima RG. Contrasting expression of keratins in mouse and human embryonic stem cells. PLoS ONE. 2008;3(10):e3451. doi: 10.1371/journal.pone.0003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Li H, Liu Y, Mattson MP, Rao MS, Zhan M. Evolutionarily conserved transcriptional co-expression guiding embryonic stem cell differentiation. PLoS ONE. 2008;3(10):e3406. doi: 10.1371/journal.pone.0003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22(3):275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta A, Hughey R, Lancin P, Larue L, Moghe PV. E-cadherin synergistically induces hepatospecific phenotype and maturation of embryonic stem cells in conjunction with hepatotrophic factors. Biotechnol Bioeng. 2005;92(3):257–266. doi: 10.1002/bit.20676. [DOI] [PubMed] [Google Scholar]
- 27.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R. A role for cadherins in tissue formation. Development. 1996;122(10):3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000;19(33):3750–3756. doi: 10.1038/sj.onc.1203726. [DOI] [PubMed] [Google Scholar]
- 29.Esner M, Pachernik J, Hampl A, Dvorak P. Targeted disruption of fibroblast growth factor receptor-1 blocks maturation of visceral endoderm and cavitation in mouse embryoid bodies. Int J Dev Biol. 2002;46(6):817–825. [PubMed] [Google Scholar]
- 30.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144(1):151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83(2):279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 32.Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J Cell Biol. 2000;150(5):1215–1221. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7(6):862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 34.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D, Yanuka O, Kotkow KJ, Rubin LL, Benvenisty N, Eiges R. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum Reprod. 2004;19(12):2875–2883. doi: 10.1093/humrep/deh529. [DOI] [PubMed] [Google Scholar]
- 36.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 37.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 39.Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75(2):233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 40.Hopfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mol Biol. 2004;254:79–98. doi: 10.1385/1-59259-741-6:079. [DOI] [PubMed] [Google Scholar]
- 41.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 42.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98(1):216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiles MV. Embryonic stem cell differentiation in vitro. Methods Enzymol. 1993;225:900–918. doi: 10.1016/0076-6879(93)25057-9. [DOI] [PubMed] [Google Scholar]
- 44.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88(9):3424–3431. [PubMed] [Google Scholar]
- 45.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27(36):6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Gerecht S, Brudick JA, Ferreira L, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cell. Proc Natl Acad Sci U S A. 2007;104(27):11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 48.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29(6):752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Torisawa YS, Chueh BH, Huh D, Ramamurthy P, Roth TM, Barald KF, Takayama S. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip. 2007;7(6):770–776. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- 52.Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94(5):938–948. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 53.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield and homogeneity of embryoid body differentiation. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 54.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23(9):1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 55.Gerecht-Nir S, Cohen S, Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnology and Bioengineering. 2004;86(5):493–502. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 56.Zweigerdt R, Burg M, Willbold E, Abts H, Ruediger M. Generation of confluent cardiomyocyte monolayers derived from embryonic stem cells in suspension: a cell source for new therapies and screening strategies. Cytotherapy. 2003;5(5):399–413. doi: 10.1080/14653240310003062. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, Field LJ, Lehmann J, Zweigerdt R. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92(7):920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 58.Sargent CY, Berguig GY, McDevitt TC. Cardiomyogenic Differentiation of Embryoid Bodies is Promoted by Rotary Orbital Suspension Culture. Tissue Engineering. 2008 doi: 10.1089/ten.tea.2008.0145. In Press. [DOI] [PubMed] [Google Scholar]
- 59.Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92(2):193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 60.Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 61.Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microencapsulation: effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnology and Bioengineering. 2006;93(3):581–591. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 62.Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Annals of the New York Academy of Sciences. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 63.Randle WL, Cha JM, Hwang YS, Chan KL, Kazarian SG, Polak JM, Mantalaris A. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng. 2007;13(12):2957–2970. doi: 10.1089/ten.2007.0072. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28(17):2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60(2):199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68(4–5):167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107(14):1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 69.Gajovic S, St-Onge L, Yokota Y, Gruss P. Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation. 1997;62(4):187–192. doi: 10.1046/j.1432-0436.1998.6240187.x. [DOI] [PubMed] [Google Scholar]
- 70.Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Engineering. 2001;7(1):89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 71.Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007;25(9):2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nature Methods. 2005;2(2):119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 73.Hwang YS, Randle WL, Bielby RC, Polak JM, Mantalaris A. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng. 2006;12(6):1381–1392. doi: 10.1089/ten.2006.12.1381. [DOI] [PubMed] [Google Scholar]
- 74.Rathjen J, Lake JA, Bettess MD, Washington JM, Chapman G, Rathjen PD. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112(Pt 5):601–612. doi: 10.1242/jcs.112.5.601. [DOI] [PubMed] [Google Scholar]
- 75.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30(1):65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 76.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23(9):1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 77.Sachlos E, Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: A strategy for stem cell differentiation. Biomaterials. 2008;29(34):4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Hay ED. Extracellular matrix. J Cell Biol. 1981;91(3 Pt 2):205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26(31):6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 82.Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23(2):288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 83.Fujiwara H, Hayashi Y, Sanzen N, Kobayashi R, Weber CN, Emoto T, Futaki S, Niwa H, Murray P, Edgar D, Sekiguchi K. Regulation of mesodermal differentiation of mouse embryonic stem cells by basement membranes. J Biol Chem. 2007;282(40):29701–29711. doi: 10.1074/jbc.M611452200. [DOI] [PubMed] [Google Scholar]
- 84.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12(8):2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 85.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 86.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 87.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 88.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 89.Lowell S, Benchoua A, Heavey B, Smith AG. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol. 2006;4(5):e121. doi: 10.1371/journal.pbio.0040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh J, Andrews PW. Expression of Wnt and Notch pathway genes in a pluripotent human embryonal carcinoma cell line and embryonic stem cell. APMIS. 2003;111(1):197–210. doi: 10.1034/j.1600-0463.2003.1110124.x. discussion 210–1. [DOI] [PubMed] [Google Scholar]
- 91.Fox V, Gokhale PJ, Walsh JR, Matin M, Jones M, Andrews PW. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem Cells. 2008;26(3):715–723. doi: 10.1634/stemcells.2007-0368. [DOI] [PubMed] [Google Scholar]
- 92.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113(Pt 23):4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 93.Beckstead BL, Santosa DM, Giachelli CM. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. Journal of Biomedical Materials Research A. 2006;79(1):94–103. doi: 10.1002/jbm.a.30760. [DOI] [PubMed] [Google Scholar]
- 94.Mahoney MJ, Saltzman WM. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat Biotechnol. 2001;19(10):934–939. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- 95.Ferreira L, Squier T, Park H, Choe H, Kohane DS, Langer R. Human embryoid bodies containing nano- and microparticulate delivery vehicles. Advanced Materials. 2008;20(12) 2285−+ [Google Scholar]