Abstract
The olfactory bulbectomized (OBX) rat is considered to be a good model of the pathology of human depression and also of the functional actions of antidepressant drug therapy. It has been proposed that antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) can be accelerated by blocking 5-HT1A/B autoreceptors with pindolol. The underlying mechanism is thought to involve acute unrestricting of 5-HT release and, consequently, relatively enhanced 5-HT turnover throughout the forebrain serotonergic networks. The effect of this combination on 5-HT turnover in sham-operated or OBX rats can be assessed at the level of 5-HT synthesis, very important presynaptic step in serotonergic neurotransmission, using the α-[14C]methyl-L-tryptophan autoradiography method. In sham rats, acute citalopram (20 mg/kg) treatment increased synthesis at almost all serotonergic terminal regions but slightly decreased synthesis at serotonergic cell body regions (i.e. dorsal and median (not significant) raphe; ∼16%). Combining pindolol (10 mg/kg) with citalopram further increased synthesis at many regions in sham rats (relative to treatment with only citalopram). In OBX rats, citalopram decreased synthesis at a few terminal regions and greatly decreased synthesis at the dorsal and median raphe (∼45%; relative to OBX rats treated with saline). Combining pindolol with citalopram greatly increased synthesis at almost all regions in OBX rats (relative to treatment with only citalopram). These results suggest that acute citalopram effects result in elevated terminal 5-HT synthesis, but these effects are restrained by 5-HT1A/B autoreceptor feedback to different degrees in sham and OBX rats. Moreover, 5-HT1A/B autoreceptor feedback is stronger in OBX rats and may underlie the delay of SSRI effects in OBX rats and, correspondingly, in human depression. Pindolol acceleration and augmentation of SSRI antidepressant therapy for human depression may be mediated by attenuation of 5-HT1A/B autoreceptor feedback, permitting unhindered SSRI effects on serotonergic terminals.
Keywords: 5-HT autoreceptor, citalopram, olfactory bulbectomy, pindolol, serotonin, SSRI
Introduction
Testing antidepressant drugs in normal rats has provided information about their effector sites and the sequela of neurophysiological alterations that they induce. However, this information may not represent the precise therapeutic effector sites and alterations that produce remission in the pathological neural systems of depressed humans. To elucidate the neuropathological substrates of depression and, subsequently, the mechanisms of antidepressant therapy, it is useful to assess antidepressant drugs in an animal model that manifests behavioural and neurophysiological pathology that parallels human depression.
The present study uses the well validated olfactory bulbectomy (OBX) rat model of depression (Kelly 1997). OBX induces a syndrome of limbic dysfunction (e.g. behavioral, neurochemical and endocrine abnormalities) that can be normalized only upon chronic treatment with proven antidepressant drugs (van Riezen and Leonard 1990). It is thought that the OBX syndrome arises from abnormal neuronal function and transmission between various brain regions. Particularly, bulbectomy directly damages serotonergic collaterals in the bulbs, which are part of the broadly branched projections from neurons in dorsal and medial raphe nuclei. This leads to reactive sprouting (Bjorklund et al., 1981) and other serotonergic abnormalities in the remaining collateral branches: abnormal 5-HT content and turnover (Janscar and Leonard 1984, Lumia et al., 1992, Redmond et al., 1997, Song and Leonard 1997); abnormal expression or function of 5-HT receptors (Earley and Glennon 1994), reuptake transporters and synthetic enzymes (Huether et al., 1997, Zhou et al., 1998); serotonergic hyperinnervation (Grecksch et al., 1997); heterosynaptic network alterations (Norrholm and Ouimet 2001). Using the α-[14C]methyl-l-tryptophan (α-MTrp) tracer and autoradiography method, we observed that bulbectomy results in abnormally high 5-HT synthesis at serotonergic terminal regions (Watanabe et al., 2003 and 2006, Hasegawa et al., 2005a), which is in line with elevated turnover of 5-HT (Lumia et al., 1992) and reduction in the density of 5-HT1A receptors in OBX rats (Sato et al., 2008). Although elevation in 5-HT synthesis in the OBX rat model seemingly differs from the conventional hypothesis that human depression is a deficit in serotonergic transmission in neural networks regulating affective behaviour, is probably related to an altered regulation of 5-HT turnover related to pathological serotonergic transmission in various neural networks and creation of nonphysiological neuronal circuitry (Spoont, 1992). Elevated tissue 5-HT and the synthesis probably create large number of nonphysiological circuitry. Nonetheless, this pathological transmission likely underlies OBX behavioural dysfunction that may be similar to human depression (Spoont, 1992). More practically, the OBX serotonergic pathology can be normalized (brought to the levels found in sham rats) after chronic treatment with drugs known to possess antidepressant activity (Zhou et al., 1998)Hasegawa et al., 2005, Watanabe et al., 2006; Sato et al., 2008).
Among the first generation selective serotonin reuptake inhibitors (SSRI) used for antidepressant therapy, citalopram (via its active S-enantiomer) has the highest selectivity for 5-HT reuptake inhibition (Hyttel, 1994). This probably accounts for its relatively higher clinical effectiveness (Stahl, 2000), and further implicates the serotonergic system in depression and antidepressant therapy. Its therapeutic effects become evident only after 2 or more weeks of citalopram treatment (Stahl, 2000). Thus citalopram's mechanism of action probably derives not only from its acute direct augmentation of extracellular 5-HT in serotonergic networks (Artigas, 1993) but also from gradual indirect changes in synaptic function, including network transmitter balance, firing activity and molecular adaptations (i.e. desensitization of autoreceptor feedback, changes in 5-HT synthesis enzyme and or postsynaptic receptor function). The gradualness of these changes likely underlies the delay in therapeutic onset that is characteristic of SSRI therapy (Blier et al., 1990a). Treatment strategies now focus on evoking SSRI-mediated 5-HT overflow concurrent with blockade of autoreceptor feedback, which may be central in delaying the synaptic changes essential for therapeutic effects (Blier and Bergeron 1995).
SSRIs acutely block 5-HT reuptake, resulting in accumulation of extracellular 5-HT at release sites along the soma, axons and terminals of serotonergic neurons (Invernizzi et al., 1992). Subsequently, enhanced activation of both pre- and postsynaptic 5-HT receptors may occur. Activation of presynaptic 5-HT1A/B autoreceptors can restrain the SSRI-mediated 5-HT overflow at axonal and other release sites (Rutter and Auerbach 1993). Moreover, 5-HT1A/B autoreceptors (5-HT1A in the cell bodies and 5-HT1B in the terminal regions) directly modulate serotonergic neuronal firing (Chaput et al., 1986, Sinton and Fallon 1988), 5-HT release (Blier et al., 1990b, Hjorth and Sharp 1991) and 5-HT synthesis (Okazawa et al., 1999, Invernizzi et al., 1991, Barton and Hutson 1999; Hasegawa et al., 2005b, Tohyama et al., 2007, Skeline et al., 2008). It has been suggested that blockade of 5-HT1A/B autoreceptors, using the partial agonist pindolol, could accelerate the antidepressant effects of SSRIs (Artigas, 1993) and improve clinical treatment of depression (Blier and Bergeron, 1995, Blier, 2003). Animal studies have demonstrated that co-administration of citalopram and pindolol enhances 5-HT overflow over that which results from treatment with only citalopram (Romero et al., 1996). Pindolol is a better autoreceptor antagonist than other drugs, such as WAY100635, because its partial agonist properties attenuate only presynaptic 5-HT autoreceptor activity during states of SSRI-mediated 5HT overflow. Because pindolol does not substantially affect postsynaptic 5-HT1A receptor function (Romero et al., 1996) and is a partial 5-HT1A autoreceptor agonist, whereby it has predominant antagonist effects during SSRI-mediated 5-HT overflow, it is a better adjuvant for SSRI therapy than other 5-HT1A receptor antagonists such as WAY100635 (Martinez et al., 2001). In addition pindolol is also a 5-HT1B antagonist (Tsuchihashi et al., 1990) the sites proposed to be important in treatment of depression (Sari, 2004). Selection of pindolol was also supported by a substantial number of the clinical studies in which pindolol supplementation was shown to be beneficial in treatment of depression (Ballesteros and Callado, 2004).
There are no previous animal studies assessing the acute effects of combining pindolol and citalopram on 5-HT synthesis. Because a drug action on the neurochemistry of a system starts acutely after administration, it is important to assess any alterations (i.e. 5-HT synthesis) after acute treatment with antidepressants. The clinical importance of assessing 5-HT synthesis is emphasized by the finding that 5-HT synthesis is abnormal in depressed humans (Rosa-Neto et al., 2004). Similarly in a recent study using positron emission tomography and 11C-labelled α-MTrp, it has been shown that pindolol augmentation, in a region-specific manner, effect 5-HT synthesis in depressed subjects treated with citalopram (Berney et al., 2008). Using the α-MTrp method, the present study has assessed regional 5-HT synthesis after administration of citalopram alone or in combination with pindolol in sham operated and OBX rats.
The main objective was to assess the effect of adding pindolol to citalopram treatments in sham rats or OBX rats, because it seems work in humans and effected 5-HT synthesis in limbic structures in humans (Berney et al., 2008). A relationship between reduction of 5-HT synthesis and normalization of the open field activity has been established in treatments with buspirone (Watanabe et al., 2006, Sato et al., 2008). It has been shown in the OBX rats that only treatment with 20 mg/kg/day and not 10 mg/kg/day normalizes both open field (Sato et al., 2008) and the regional 5-HT synthesis (Watanabe et al., 2006) linking directly 5-HT synthesis and open field activity. From animal model study we should be able to get better understanding of action responsible for improvement observed in humans (Ballesteros and Callado, 2004). A separate emphasis was noted for brain regions known to be preferentially innervated by serotonergic projections originating from either the dorsal raphe or the median raphe. The main working hypothesis was that an addition of pindolol, acting as a partial agonist, to citalopram will produce an elevation of the synthesis, the most important presynaptic process in 5-HT neurotransmission.
Materials and Methods
Materials
Male Sprague Dawley rats (200-220 g, Charles River Canada) were habituated for at least 72 hrs before sham or OBX surgery. Rats were housed with a 12 hour light-dark cycle and had free access to food and water. All animal procedures were in strict accordance with the Canadian Council on Animal Care guidelines, and were approved by the Animal Care Committee of McGill University. Citalopram (a generous gift from H. Lundbeck A/S, Copenhagen, Denmark) and pindolol (HBr-salt; Tocris Bioscience, USA) were dissolved in saline (0.9%) vehicle to yield appropriate dose injections of <0.5 ml/kg per treatment. α-[14C]methyl-l-tryptophan (55 mCi/mmol specific activity) at a concentration of 30 μCi/ml in 1 ml was injected over 2 min in all experiments.
Sham and Olfactory Bulbectomy Surgery
Anaesthesia was induced with 3% halothane and maintained at 1%. The scalp was incised and a cranial window was drilled 5 mm anterior from Bregma. The olfactory bulb tissue was aspirated by means of a syringe attached to a vacuum pump as previously detailed (Watanabe et al., 2003). Sham operated rats were treated similarly except that the olfactory bulbs were not cut or removed, but the dural membrane was penetrated. The aspirated cavity was filled with gelfoam to reduce haemorrhaging and the scalp wound was sutured and disinfected with iodine. Animals were monitored for at least 2 hours for recovery from anaesthesia. A period of 2 weeks was allowed for recovery from the surgical procedure and development of the OBX syndrome (Kelly, 1997), subsequently animals where subjected to the α-MTrp tracer experiments. At the end of the tracer experiments, dissected brains were visually inspected to ensure that complete removal of the olfactory bulbs was achieved and that no excess damage to other brain areas occurred.
Surgical and Drug Treatment Groups
The following drug treatment groups were successfully tested and analyzed: 9 sham (SVV) and 10 OBX rats (XVV) treated with saline (0.9%) vehicle injections; 10 sham (SCV) and 7 OBX rats (XCV) treated with citalopram (20 mg/kg, s.c.); 9 sham (SCP) and 8 OBX rats (XCP) treated with a combination of citalopram (20 mg/kg, s.c.) and (±)pindolol (10 mg/kg, s.c.). Doses of both citaloprama and pindolol were based on previous works and have been considered to be clinically relevant (Kugelberg et al., 2001; Hadrava et al., 1996). Furthermore the brain concentration achieved with this dose of citalopram effectively inhibits 5-HT reuptake transporters while avoiding non-specific interactions with other transmitter systems (Owens et al., 2001). The dose of pindolol is at the level at which pindolol interacts with presynaptic and not postsynaptic 5-HT1A receptors (Hadrava et al., 1996). The groups used are summarised in Table 1 with identification of comparisons in the statistical post hoc analysis.
Table 1. Study groups and identification of comparisons of interest.
| Group | Comparisons |
|---|---|
| Sham; saline twice (SVV) | SCV, XVV |
| Sham; saline + 20 mg/kg citalopram (SCV) | SCP, SVV |
| Sham; 20 mg/kg citalopram + 10 mg/kg pindolol (SCP) | SCV |
| OBX; saline twice (XVV) | SVV, XCV |
| OBX; saline + 20 mg/kg citalopram (XCV) | XVV, XCP |
| OBX; 20 mg/kg citalopram + 10 mg/kg pindolol (XCP) | XCV |
α-MTrp Experimental Procedure
Regional 5-HT synthesis rates were determined using the autoradiographic α-MTrp method as detailed in (Nagahiro et al., 1990, Diksic and Young 2000). Two weeks after the OBX or sham surgery, animals were fasted overnight to stabilize the plasma concentration of amino acids, especially tryptophan. Water was provided ad libitum. Certain physiological parameters were monitored in all rats during tracer exposure: arterial pH, PCO2, PO2 and hematocrit. Full details of procedures are given in our previous publications (Nagahiro et al., 1990, Tohyama et al., 2001 and 2002).
Autoradiograms of the brain slices (Fig. 1, 2) were digitized with a computer based image analysis system (MCID, Image Research Co., Canada) and the regional optical densities were calibrated to six co-exposed [14C]-polymer activity standards (calibrated to brain tissue density; Amersham Co). The regional synthesis rates (R; pmol/g/min) were calculated from the trapping constant (K*; mL/g/min) as detailed in our previous publications (R=Cp K*/LC) (Nagahiro et al., 1990, Diksic and Young 2000). In this equation LC (=0.42) is equal the conversion factor measured in the rat brain, which has been shown to be uniform throughout the brain (Vanier et al., 1995). In pathological conditions (e.g. epilepsy) this tracer could be partially metabolized through kynurine pathway (Chugani and Muzik, 2000; Fedi et al., 2001) but in normal brain this pathway does not exist (Diksic and Young 2000). The brain areas were identified based on a rat brain atlas (Paxinos and Watson 1986). Each brain region was quantified in at least three consecutive slices and on left and right sides of the brain. An average of these values was used in further calculations.
Figure 1.
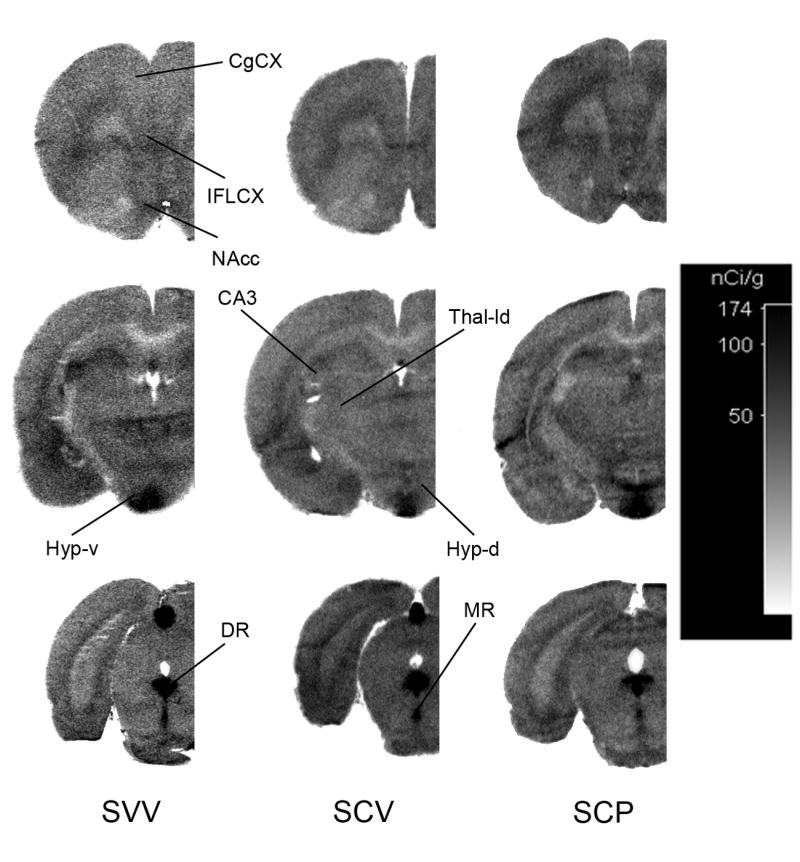
Representative autoradiograms from sham rats treated with either vehicle (SVV), citalopram (SCV) or citalopram and pindolol (SCP). Autoradiographic images of tissue radioactivity at rostral, central and caudal coronal sections through a brain after 150 min of exposure to systemic α-[14C]-methyl-L-tryptophan. CgCX, cingulate cortex; IFLCX, infralimbic cortex; NAcc, nucleus accumbens; CA3, hippocampal area CA3; Thal-ld, lateral-dorsal thalmus; Hyp-d, dorsal hypothalamus; Hyp-v, ventral hypothalamus; DR, dorsal raphe; MR, median raphe.
Figure 2.
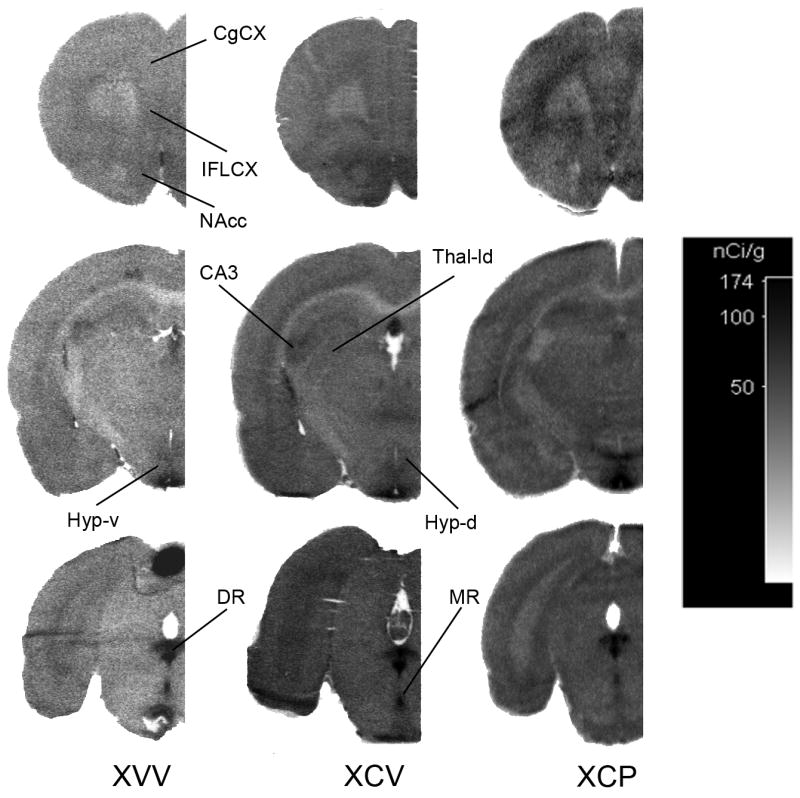
Representative autoradiograms from OBX rats treated with either vehicle (XVV), citalopram (XCV) or citalopram and pindolol (XCP). Autoradiographic images of tissue radioactivity at rostral, central and caudal coronal sections through a brain after 150 min of exposure to systemic α-[14C]-methyl-L-tryptophan. CgCX, cingulate cortex; IFLCX, infralimbic cortex; NAcc, nucleus accumbens; CA3, hippocampal area CA3; Thal-ld, lateral-dorsal thalmus; Hyp-d, dorsal hypothalamus; Hyp-v, ventral hypothalamus; DR, dorsal raphe; MR, median raphe.
Brain Regions Selected for Study and assignment of the origin
Generally brain regions receive innervations from both DR and MR, but many brain regions have an absolute majority of projections from one of these regions (see details below). Based on studies by Vertes et al., (1999) and (Azmitia and Segal 1978) the present study assessed 5-HT synthesis at two midbrain regions- dorsal and median raphe (DR and MR, respectively), which contain the serotonergic neurons that innervate the entire forebrain, and twenty-six other regions known to be preferentially innervated by either DR- or MR-terminals (Fig. 1, 2). Justification for assignment of certain cortical areas to the DR-terminal group was based reports that cortical injections (frontal, parietal or visual cortices) of retrograde tracers yields two to four times more labelling of DR neurons than MR neurons (O'Hearn and Molliver 1984). Furthermore, microdialysis studies demonstrate that stimulation of DR neurons causes 80-100% rise in extracellular 5-HT at the frontal cortex, caudate putamen or globus pallidus, whereas stimulation of MR neurons has no significant effect. Conversely, only stimulation of MR neurons can evoke a significant rise in 5-HT at the dorsal hippocampus (McQuade and Sharp 1997). Other forebrain regions were assigned to either the DR- or MR-terminal groups based on reports of preferential labelling after injections of anterograde tracers to either the DR or MR (Azmitia and Segal 1978, Vertes and Kocsis 1994). The core of nucleus accumbens was assigned to the DR-terminal group based studies that indicate that terminals in this region have morphological and toxin-sensitivities that are characteristic of DR-terminals (Brown and Molliver 2000).
Eighteen regions were considered to be preferentially innervated by DR-terminals: cortical areas (McQuade and Sharp 1997, Kosofsky and Molliver 1987, O'Hearn and Molliver 1984)- pyriform (anterior and posterior), frontal motor, somatosensory, auditory, and visual cortex; subcortical areas (Brown and Molliver 2000, Vertes and Kocsis 1994)- bed nucleus of the stria terminalis, nucleus accumbens, caudate-putamen (ventral, medial and lateral) and globus pallidus; amygdala areas-cortical, central and basolateral; thalamic paraventricular nucleus, substantia nigra compacta and locus coeruleus.
Eight regions were considered to be preferentially innervated by MR-terminals: medial anterior olfactory area (Azmitia and Segal 1978); cortical areas (Vertes et al., 1999, Kosofsky and Molliver 1987)- medial orbital, infralimbic and cingulate cortex; subcortical areas (Azmitia and Segal 1978, Vertes et al., 1999)- suprachiasmic nucleus and dorsal hippocampus (CA3, CA1 and dentate gyrus).
Data Analysis
The physiological parameters were compared using tow-factor (factor 1=sham, OBX; factor 2=six treatment groups) ANOVA followed by Newman-Keuls post hoc comparisons (Table 1). There were four main comparisons of interest in this study: SVV compared to SCV; SCV compared to SCP; XVV compared to XCV; and XCV compared to XCP. The rational for these comparisons stem from the fact that the vehicle rats in sham and control groups (SVV and XVV) were used as controls for citalopram treated groups (SCV and XCV) while the citalopram + vehicle groups (SCV and XCV) were used as the control groups for citalopram+pindolol groups (SCP and XCP). In addition a comparison was made between SVV and XVV groups jut to asses previously reported effect of bulbectomy, elevation of the regional synthesis (Watanabe et al., 2003 and 2006a) even this was not objective of the present investigation.
The global effect of each drug treatment group was evaluated by two-factor ANOVA (1st factor sham-OBX, and the 2nd factor six treatment groups) followed by Newman-Keuls post hoc test. In addition to asses if a treatment had a greater effect in regions innervated by MR or DR (see above regions assigned to those projections) the ratios between treatment groups and respective controls (SCV/SVV; SCP/SCV; XCV/XVV; XCP/XCV) were evaluated by one-sample two-tail t-test. The mean of the ratios of regional synthesis was compared to 1±0 (an ideal ratio with N=1 and indicating no effect of treatment). Further to see if there is a differential effect in the DR and MR mainly innervated regions the mean ratios were compared by ANOVA. All statistical comparisons were done by SIGMASTAT 7.0 (StatSoft Inc., USA) software. A p<0.05 was considered significant.
Results
In general six physiological parameters measured are similar among these groups (Table 2). However, the weight gain was significantly different among groups (F(5,52)=4.4; p=0.002), and there was significantly lower between sham operated and corresponding OBX groups. There were also some significant differences in pH (F(5,52)=9.93; p<0.001) and total plasma tryptophan (F(5,52)=17.2; p<0.001) between experimental groups. Post hoc analysis revealed differences between SVV and SCP, SCV and SCP, and XVV and XCP groups for pH. In addition there were differences in total plasma tryptophan between SCV and SCP, XVV and XCP, and XVV and XCV groups. There are no significant differences among groups in PaO2, hematocrit or plasma free tryptophan.
Table 2. Average physiological parameters of the rats tested in various treatment groups. The values are given as mean±SD.
| Sham operated groups1 | OBX groups2 | |||||
|---|---|---|---|---|---|---|
| SVV (n=9) |
SCV (n=10) |
SCP (n=9) |
XVV (n=10) |
XCV (n=7) |
XCP (n=8) |
|
| Weight gain (g) | 81 ±13§ | 82 ±14§ | 81 ±11§ | 62.2 ±5.4 | 62 ±17 | 66 ±22 |
| PaO2 | 88.1 ±5.4 | 92.3 ±2.8 | 91.1 ±4.6 | 91 ±10 | 88.6 ±8.3 | 88 ±11 |
| PaCO2 | 36.8 ±1.8 | 37.1 ±5.1 | 41.0 ±3.3 | 37.3 ±1.9 | 41.5 ±2.9 | 39.8 ±3.1 |
| pH | 7.45 ±0.02¶ | 7.43 ±0.02† | 7.40 ±0.03 | 7.43 ±0.03‡ | 7.40 ±0.02 | 7.38 ±0.02 |
| Hematocrit (%) | 44 ±3 | 46 ±5 | 47 ±2 | 45 ±5 | 48 ±6 | 47 ±3 |
| Total Trp (nmol/mL) | 43 ±11.0¶¶ | 66.4 ±5.2 | 67.9 ±8.5 | 45.0 ±7.2 | 73 ±11# | 67 ±12 |
| Free Trp (nmol/mL) | 7.4 ±2.12 | 9.2 ±2.4 | 8.9 ±2.5 | 7.9 ±2.8 | 8.9 ±2.3 | 10.3 ±0.9 |
The sham groups are: SVV, sham rats treated with vehicle for both citalopram and pindolol; SCV, sham rats treated with citalopram and vehicle for pindolol; SCP, sham rats treated with both citalopram and pindolol. n=number of rats per group.
The OBX groups are: XVV, OBX rats treated with vehicle for both citalopram and pindolol; XCV, OBX rats treated with citalopram and vehicle for pindolol; XCP, OBX rats treated with both citalopram and pindolol. n = number of rats per group.
Significantly different from respective OBX group.
Significantly different from the value in the SCP group.
Significantly different from both SCV and SCP groups
Significantly different from the value in the SCP group.
Significantly different from the value in the XCP group.
Significantly different from both XVV and XCP groups.
Sham rats treatments
A set of representative autoradiograms illustrating the regional variations in 5-HT synthesis, as determined by the α-MTrp method, is shown in Fig. 1. One can note a substantial variability between different brain regions, but actual values is very difficult to appreciate from photographic image because of logarithmic dependence of density and concentration (reflection of the regional synthesis). Because of this the white matter regions with the synthesis of zero still show a small density in the autoradiogram. Groups' comparisons of interest are identified in Table 1. A comparison of regional 5-HT synthesis rates for sham rats treated with either vehicle (SVV) or citalopram (SCV) is provided in Table 3 (cf. Fig. 1- SVV and SCV). Two-factor ANOVA analysis showed highly significant differences between groups [F(5,47)=15.5; p<0.001] and group-region interaction [group*region F(135,1269)=64.5; p<0.001] suggesting significant differences between regions between different groups. The regions found to be significant by post hoc between groups of interest (see Methods) are identified in Table 3. Of the two regions containing serotonergic cell bodies (i.e. DR and MR), 5-HT synthesis at the DR was significantly lower in the SCV group compared to the SVV group. At almost all regions containing serotonergic terminals, 5-HT synthesis was significantly higher in the SCV group. However, three regions (medial olfactory area, suprachiasmic nucleus and CA3) mainly innervated by MR did not show any significant difference between SCV and SVV groups (Table 3). This observation prompted us to segregate forebrain regions into groups based on anatomical and functional reports indicating preferential innervation by either DR or MR terminals (see Table 3- column 2 for assignments and Methods for justifications).
Table 3.
Regional 5-HT synthesis ratesa (mean± SD [pmol/g/min]) in sham rats treated with either vehicle (SVV), citalopram alone (SCV) or in combination with pindolol (SCP).
| Region | Predominant Nucleus of Origin | SVVa (n=9) |
SCVa (n=9) |
% change in SCV relative to SVV | SCPa (n=9) |
% change in SCP relative to SCV |
|---|---|---|---|---|---|---|
| aPCX | DR | 21 ± 6 | 34 ± 8 | 61* | 45 ± 10 | 32# |
| pPCX | DR | 27 ± 6 | 42 ± 7 | 55* | 52 ± 10 | 22# |
| FCX | DR | 18 ± 6 | 38 ± 9 | 112* | 56 ± 13 | 46# |
| SCX | DR | 12 ± 5 | 32 ± 8 | 164* | 52 ± 10 | 61# |
| AudCX | DR | 13 ± 6 | 43 ± 7 | 238* | 60 ± 11 | 39# |
| VCX | DR | 19 ± 5 | 43 ± 7 | 129* | 60 ± 10 | 40# |
| BNST | DR | 31 ± 6 | 41 ± 9 | 34* | 53 ± 11 | 30# |
| Nacc | DR | 40 ± 7 | 52 ± 9 | 31* | 56 ± 11 | ns |
| CPL | DR | 22 ± 7 | 35 ± 8 | 59* | 49 ± 9 | 39# |
| CPM | DR | 34 ± 8 | 46 ± 9 | 37* | 58 ± 11 | 25# |
| CPV | DR | 34 ± 8 | 50 ± 8 | 47* | 54 ± 11 | ns |
| GP | DR | 21 ± 7 | 37 ± 6 | 78* | 47 ± 9 | 30# |
| AC-Amyg | DR | 27 ± 6 | 43 ± 8 | 61* | 55 ± 10 | 29# |
| CN-Amyg | DR | 28 ± 6 | 47 ± 6 | 68* | 59 ± 13 | 26# |
| BLN-Am | DR | 30 ± 6 | 52 ± 7 | 71* | 59 ± 9 | ns |
| PV-Thal | DR | 25 ± 8 | 42 ± 9 | 69* | 71 ± 13 | 69# |
| SNc | DR | 20 ± 6 | 38 ± 9 | 87* | 58 ± 12 | 52# |
| LC | DR | 28 ± 8 | 47 ± 8 | 69* | 61 ± 8 | 30# |
| MOA | MR | 36 ± 5 | 46 ± 8 | ns | 55 ± 10 | 20# |
| MOCX | MR | 26 ± 6 | 42 ± 8 | 59* | 49 ± 10 | ns |
| IFLCX | MR | 32 ± 5 | 41 ± 8 | 29* | 54 ± 10 | 30# |
| CgCX | MR | 21 ± 6 | 35 ± 8 | 67* | 48 ± 11 | 39# |
| SCH | MR | 36 ± 7 | 46 ± 12 | ns | 60 ± 14 | 30# |
| Hipp-d | MR | 30 ± 6 | 50 ± 7 | 66* | 56 ± 9 | ns |
| DG | MR | 29 ± 6 | 50 ± 7 | 75* | 56 ± 9 | ns |
| CA3 | MR | 46 ± 16 | 55 ± 7 | ns | 51 ± 10 | ns |
| DR | 99 ± 11 | 83 ± 11 | -16* | 90 ± 15 | ns | |
| MR | 69 ± 10 | 59 ± 10 | ns | 81 ± 12 | 38* | |
Region names: medal anterior olfactory area (MOA); cortical areas- medial orbital (MOCX), infralimbic (IFLCX), anterior cingulate (CgCX), anterior and posterior pyriform (aPCX and pPCX), primary motor (FCX), somatosensory (SCX), auditory (AudCX), and visual (VCX); amygdala areas- anterior cortical (AC-Amyg), central nucleus (CN-Amyg), basolateral nucleus (BLN-Amyg); subcortical areas- bed nucleus of the stria terminalis (BNST), nucleus accumbens (Nacc), caudate-putamen (lateral-CPL; medial-CPM; ventral-CPV), globus pallidus (GP), paraventricular thalamus (PV-Thal), suprachiasmic nucleus (SCH), dorsal hippocampus (Hipp-d), dentate gyrus (dorsal; DG), CA3 (dorsal; CA3), substantia nigra compacta (SNc), locus coeruleus (LC), median raphe (MR), dorsal raphe (DR).
Significant difference between SVV and SCV groups.
Significant difference between SCV and SCP groups.
ns, not significant.
An evaluation of the overall effect of citaloprama using ratios (see Methods), with respect to groupings of DR- or MR-terminal regions, shows that the mean synthesis rate change (SCV relative to SVV rates in Table 3) was 1.81±0.12 (N=18; t=6.79; p<0.0001) for DR-terminal regions and 1.46±0.07 (N=8; t=5.88; p<0.001) for MR-terminal regions. A formal comparison of these ratios by ANOVA indicated that there is only a trend (F(1,25)=3.4; p=0.08; power=0.304) for a greater effect in the regions mainly innervated by MR despite all regions innervated by DR showed significant difference (p<0.05) between SVV and SCV. The main reason for difference between these ratios being nonsignificant is relatively large regional differences between ratios in regions innervated by DR producing low power of the comparison with the present number of animals (Table 3; column 5). Indeed the largest increases were observed at DR-terminal regions (i.e. cortical regions: auditory (238%), somatosensory (164%), frontal motor area (112%) and globus pallidus (78%)). Only moderate or insignificant increases were observed at MR-terminal regions (i.e. dorsal hippocampal regions (66-75%), cingulate (67%) and medial orbital cortex (59%)).
A comparison of regional 5-HT synthesis rates for sham rats treated with either citalopram (SCV) or citalopram combined with pindolol (SCP) is provided in Table 3 (cf. Fig. 1- SCV and SCP). As mentioned above two-factor ANOVA indicated highly significant differences among groups and interaction between groups and regions. At the MR, but not the DR, 5-HT synthesis was higher in the SCP group. At 15 of 18 DR-terminal regions and only 4 of 8 MR-terminal regions, 5-HT synthesis was higher in the SCP group. An evaluation of the overall effect of pindolol shows that the mean synthesis rate change (SCP relative to SCV rates in Table 3) was 1.33±0.04 (N=18; t=8.43; p<0.0001) for DR-terminal regions and 1.19±0.05 (N=8; t=3.79; p<0.01) for MR-terminal regions. This evaluation indicates that pindolol induced an increase in 5-HT synthesis that was significantly (F(1,25)=4.42; p=0.046) more pronounced at DR-terminal regions of sham rats treated with citalopram. Furthermore, evaluation of the overall combined effect of citalopram and pindolol shows that the mean synthesis rate change (SCP relative to SVV rates in Table 3) was 2.45±0.21 (N=18; t=6.25; p<0.0001) for DR-terminal regions and 1.74±0.12 (N=8; t=6.14; p<0.001) for MR-terminal regions. This evaluation indicates that the effect of the pindolol and citalopram combination was significantly (F(1,25)=4.7; p=0.041) more pronounced at DR-terminal regions of sham rats.
OBX rats treatments
A comparison of regional 5-HT synthesis rates for OBX rats treated with either vehicle (XVV) or citalopram (XCV) is provided in Table 4 (cf. Fig. 2- XVV and XCV). As mentioned above two-way ANOVA analysis showed highly significant differences between groups and group-regions interaction. The regions found to be significant by post hoc between groups of interest (see Methods) are identified in Table 4. 5-HT synthesis at the DR and MR was significantly lower in the XCV than in the XVV group. At two out of eighteen DR-terminal regions and three out of eight MR-terminal regions 5-HT synthesis was significantly lower in the XCV group. Interestingly, these affected regions (i.e. medial anterior olfactory area, infralimbic cortex, nucleus accumbens, and suprachiasmatic nucleus) correspond to regions in sham rats that expressed the smallest responses to citalopram (cf. Table 3). An evaluation of the overall effect of citalopram shows that the mean synthesis rate change (XCV relative to XVV rates in Table 4) was 0.95±0.03 (N=18; p=0.13) for DR-terminal regions and 0.72±0.05 (N=9; t=-6.12; p<0.001) for MR-terminal regions. This evaluation indicates that citalopram induced a decrease in 5-HT synthesis that was significantly (F(1,25)=15.8; p=0.001) more pronounced at MR-terminal regions of OBX rats.
Table 4.
Regional 5-HT synthesis ratesa (mean± SD [pmol/g/min]) in OBX rats treated with either vehicle (XVV), citalopram alone (XCV) or in combination with pindolol (XCP).
| Region† | Predominant Nucleus of Origin | XVVa (n=10) |
XCVa (n=7) |
% change in XCV relative to XVV | XCPa (n=8) |
% change in XCP relative to XCV |
|---|---|---|---|---|---|---|
| aPCX | DR | 39 ± 5 | 33 ± 5 | ns | 45 ± 16 | ns |
| pPCX | DR | 37 ± 7 | 46 ± 4 | 25* | 53 ± 8 | ns |
| FCX | DR | 27 ± 7 | 24 ± 6 | ns | 57 ± 6 | 138# |
| SCX | DR | 23 ± 7 | 24 ± 9 | ns | 50 ± 6 | 107# |
| AudCX | DR | 25 ± 7 | 30 ± 10 | ns | 54 ± 7 | 83# |
| VCX | DR | 28 ± 7 | 28 ± 9 | ns | 60 ± 6 | 112# |
| BNST | DR | 37 ± 8 | 31 ± 6 | ns | 56 ± 9 | 79# |
| Nacc | DR | 54 ± 8 | 41 ± 7 | -24* | 56 ± 14 | 37# |
| CPL | DR | 31 ± 8 | 28 ± 7 | ns | 51 ± 6 | 81# |
| CPM | DR | 43 ± 8 | 34 ± 6 | ns | 60 ± 9 | 80# |
| CPV | DR | 42 ± 8 | 36 ± 6 | ns | 65 ± 10 | 82# |
| GP | DR | 30 ± 7 | 31 ± 4 | ns | 45 ± 7 | 45# |
| AC-Amyg | DR | 38 ± 6 | 39 ± 4 | ns | 55 ± 10 | 42# |
| CN-Amyg | DR | 38 ± 7 | 36 ± 6 | ns | 59 ± 9 | 64# |
| BLN-Amyg | DR | 44 ± 8 | 44 ± 7 | ns | 62 ± 11 | 41# |
| PV-Thal | DR | 36 ± 8 | 37 ± 7 | ns | 51 ± 7 | 40# |
| SNc | DR | 34 ± 6 | 27 ± 6 | -20* | 50 ± 5 | 83# |
| LC | DR | 33 ± 8 | 29 ± 7 | ns | 52 ± 7 | 78# |
| MOA | MR | 50 ± 9 | 30 ± 5 | -40* | 45 ± 22 | ns |
| MOCX | MR | 39 ± 7 | 29 ± 11 | ns | 49 ± 13 | 67# |
| IFLCX | MR | 44 ± 8 | 25 ± 9 | -42* | 53 ± 9 | 108# |
| CgCX | MR | 31 ± 7 | 24 ± 7 | ns | 54 ± 7 | 131# |
| SCH | MR | 44 ± 9 | 25 ± 12 | -42* | 76 ± 6 | 198# |
| Hipp-d | MR | 37 ± 7 | 32 ± 10 | ns | 57 ± 11 | 78# |
| DG | MR | 38 ± 7 | 31 ± 8 | ns | 54 ± 11 | 75# |
| CA3 | MR | 43 ± 7 | 37 ± 8 | ns | 65 ± 9 | 77# |
| DR | 111 ± 5 | 61 ± 13 | -45* | 107 ± 25 | 76# | |
| MR | 78 ± 4 | 43 ± 6 | -45* | 70 ± 13 | 63# | |
Region names are abbreviated under Table 2.
Significant difference between XVV and XCV groups.
Significant difference between XCV and XCP groups.
ns, not significant.
A comparison of regional 5-HT synthesis rates for OBX rats treated with either citalopram (XCV) or citalopram combined with pindolol (XCP) is provided in Table 4 (cf. Fig. 2- XCV and XCP). At the DR, MR and almost all DR- and MR-terminal regions 5-HT synthesis was higher in the XCP group. An evaluation of the overall effect of pindolol shows that the mean synthesis rate change (XCP relative to XCV rates in Table 4) was 1.69±0.08 (N=18; t=9.14; p<0.0001) for DR-terminal regions and 1.98±0.17 (N=8; t=5.69; p<0.001) for MR-terminal regions. This evaluation indicates that pindolol induced a similar (p=0.08) increase in 5-HT synthesis at DR- and MR-terminal regions of OBX rats treated with citalopram. Furthermore, evaluation of the overall combined effect of citalopram and pindolol shows that the mean synthesis rate change (XCP relative to XVV rates in Table 4) is 1.59±0.08 (N=18; t=7.50; p<0.0001) for DR-terminal regions and 1.41±0.10 (N=9; t=4.11; p<0.005) for MR-terminal regions. A formal comparison of these ratios by ANOVA indicates that the effect of the pindolol and citalopram combination was similar (p=0.173) at DR- and MR-terminal regions of OBX rats.
The regional 5-HT synthesis rates in sham (SVV) and OBX (XVV) rats were also compared (actual comparisons not shown because the main objective of this work was to evaluate the effects of citalopram, and citalopram combined with pindolol; cf. Table 3 and 4). At the DR and MR, 5-HT synthesis was only slightly higher in the XVV group. As we have previously reported (Hasegawa et al., 2005b; Watanabe et al., 2006), 5-HT synthesis rates at terminal regions were typically greater in the XVV group than those in the SVV group. There was no significant difference observed at the bed nucleus of the stria terminalis, CA3 and locus coeruleus. The current results are consistent with our previous observations in OBX rats (Watanabe et al., 2003, Hasegawa et al., 2005a) where greater increases in 5-HT synthesis were observed at the cingulate, somatosensory and frontal cortex, lateral caudate putamen, globus pallidus, and substantia nigra compacta; in contrast to regions that were less affected by bulbectomy, such as the medial caudate putamen, dorsal hippocampus, CA3, locus coeruleus, DR and MR.
An evaluation of the overall effect of bulbectomy shows that the mean synthesis rate change (XVV relative to SVV rates in Tables 3 and 4) was 1.47±0.05 (N=18; t=9.55; p<0.001) for DR-terminal regions and 1.30±0.04 (N=8; t=7.10; p<0.001) for MR-terminal regions (Fig. 3). This evaluation indicates that bulbectomy induced similar (p=0.053) increases in 5-HT synthesis at DR- and MR-terminal regions.
Discussion
Influence on Physiological Parameters
There were some significant differences in the plasma total tryptophan values between groups (Table 2). Since only plasma free tryptophan values are used in calculating regional 5-HT synthesis (Diksic et al., 1990, Nagahiro et al., 1990), and many studies have correlated plasma free tryptophan with brain tryptophan concentrations and brain 5-HT synthesis (Bloxam and Curzon 1978, Salter et al., 1989, discussed in detail in Diksic et al., 1990, Diksic and Young 2000, Nagahiro et al., 1990), the presently observed differences in plasma total tryptophan are not expected to influence our assessment of regional 5-HT synthesis. The small differences found in blood pH between some groups (Table 2) are not expected to have any influence on the comparisons made. More importantly, the variables like blood oxygen and plasma free tryptophan, which are known to influence brain 5-HT synthesis both in laboratory animals (Diksic 2001) and humans (Nishizawa et al., 1997, Nishikawa et al., 2005) are not different among the current experimental groups (Table 2).
Effect in sham rats
Although many studies in rats have assessed SSRIs effects on 5-HT synthesis at terminal regions, only a few have assessed their effects at the cell body regions, MR and DR. A 5-HTP accumulation study by Long et al., (1983) did not observe any fluoxetine effects at the DR (MR was not assessed). Tsuiki et al., (1995) observed that a high dose of fluoxetine increased 5-HT synthesis at the DR and MR, but attributed this effect to other mechanisms (pyrrolase inhibition and/or norfluoxetine effects). More similar to the present findings (Table 3: DR and MR), Mück-Šeler et al., (1996) observed that a moderate dose of fluoxetine decreased 5-HT synthesis significantly at the DR but not the MR. It is thought that SSRI-mediated extracellular 5-HT accumulation at the DR and MR causes inhibition of 5-HT synthesis via somatodendritic 5-HT1A autoreceptors (Hjorth et al., 1995, Invernizzi et al., 1991). Indeed there is a high density of 5-HT1A autoreceptors on the somatodendrites of serotonergic neurons, particularly in the DR (Pazos and Palacios 1985, Sato et al., 2008), and they are coupled to inhibition of neuronal firing and TPH activity (Boadle-Biber 1993). Correspondingly, 5-HT synthesis is decreased by 5-HT1 autoreceptor agonists (Tohyama et al., 2002 and 2007, Okazawa et al., 1999, Skelin et al. 2008) and increased by 5-HT1A antagonists (unpublished pindolol experiments; Tohyama et al., 2001). In the present study somatodendritic 5-HT1A autoreceptor feedback may underlie, at least in part, the citalopram-induced decrease in 5-HT synthesis measured at the DR.
In sham rats citalopram had relatively small or no effects on various MR-terminal regions (Table 3). One possible explanation is that a stronger terminal 5-HT1B autoreceptor inhibition of 5-HT release exists at MR-terminals (Hjorth et al., 2000), which presumably mitigates citalopram-induced reduction of 5-HT stores and moderates the demand for 5-HT synthesis at MR-terminals. 5-HTP accumulation studies have suggested that other SSRIs (e.g. fluoxetine or paroxetine), may decrease terminal 5-HT synthesis via 5-HT autoreceptor feedback (Long et al., 1983, Baldessarini et al., 1992, Barton and Hutson 1999). Although autoreceptor feedback may generally occur after acute and subchronic SSRI treatments, at the time points used in this study such feedback does not completely attenuate citalopram-mediated extracellular 5-HT accumulation (Invernizzi et al., 1997) and, subsequently, increases in 5-HT synthesis at terminal regions apparently occur in sham rats (Table 3).
The effect of combining pindolol with citalopram treatment in sham rats was also evaluated. Previous microdialysis and electrophysiological studies on citalopram-treated rats indicate that co-treatment with pindolol (10-15 mg/kg, but not 5 mg/kg) attenuates somatodendritic 5-HT1A autoreceptor regulation of serotonergic neuronal firing, permitting more citalopram-mediated extracellular 5-HT accumulation at terminal regions (Romero et al., 1996). In the mouse forced swimming test addition of pindolol to SSRI potentiated SSRI's effect (Bourin et al. 1998) suggesting that co-administration of pindolol with a SSRI could have an effect on the antidepressant-like activity of SSRI. Correspondingly, the present study observed that addition of pindolol enhanced 5-HT synthesis at almost all terminal regions (Table 3). This enhanced synthesis could, in part, be a response to replenish 5-HT stores that are presumably depleted subsequent to pindolol blockade of 5-HT1A autoreceptor regulation of somatic firing, ongoing terminal 5-HT release and citalopram blockade 5-HT reuptake (as discussed for citalopram treatment). Additionally, enhanced terminal synthesis might also be attributed to pindolol inhibition of 5-HT1B autoreceptor feedback (Artigas et al., 2001) because it has been reported that 5-HT1B receptors facilitate paroxetine's, a SSRI, antidepressant-like effect in the forced swim test in rats (Tatarczyńska et al., 2002). 5-HT1B autoreceptors are expressed exclusively on serotonergic terminals (Riad et al., 2000) and inhibit terminal 5-HT release and TPH function (Hasegawa et al., 2005b, Boadle-Biber 1993). Microdialysis studies on frontal cortex observe that pindolol alone has no effect but can augment SSRI-induced extracellular 5-HT accumulation when combined with a 5-HT1A but not a 5-HT1B antagonist (Dawson and Nguyen 2000). This additive effect was attributed to pindolol's blockade of 5-HT1B rather than 5-HT1A autoreceptor feedback on terminal release. Additionally, we have observed that 5-HT synthesis can be increased by treatment with only pindolol (M. Diksic et al., unpublished results). Since in these conditions, where 5-HT1A autoreceptors do not have a tonic activity, pindolol did not exert any partial agonist inhibition of 5-HT synthesis, this suggests that pindolol may be acting as an antagonist of 5-HT1B autoreceptors, which are tonically inhibiting terminal 5-HT synthesis (Hasegawa et al., 2005b). The presently observed effects are probably not mediated by postsynaptic 5-HT1A receptors because pindolol does not alter postsynaptic 5-HT1A receptor signalling (Haddjeri and Blier 2000, Romero and Artigas 1997). Furthermore, pindolol's effects on 5-HT synthesis are probably not via its antagonistic actions on β-adrenergic receptors since other β-adrenergic receptor antagonists do not augment basal or citalopram-induced extracellular 5-HT accumulation at terminal regions (Hjorth et al., 1996). The fact that pindolol seems to be beneficial in combination with SSRIs in treatment of human depression (Ballesteros and Callado, 2004) suggests that the effect through 5-HT1B receptors might not be predominant mode of action in humans (Gobert and Millan, 1999), but it can not be excluded as a contributor in these rat experiments.
In sham rats pindolol had more of an effect on DR-terminal regions: fifteen of eighteen DR-terminal regions and only four of eight MR-terminal regions were affected by the addition of pindolol (Table 3). 5-HT synthesis at MR-terminal regions may not be as tightly regulated by 5-HT1A autoreceptors but, rather, by 5-HT1B autoreceptors (see above). Previous studies indicate that MR-somatic firing and MR-terminal 5-HT release and synthesis are less sensitive 5-HT1A autoreceptor regulation (Sinton and Fallon 1988, Blier et al., 1990b, Invernizzi et al., 1991, Casanovas et al., 1997). Indeed the density of 5-HT1A autoreceptors at the MR region is only a fraction of that at the DR region (Pazos and Palacios 1985). Correspondingly, differential effects of 5-HT1A autoreceptor antagonists on MR- vs. DR-terminal regions have been demonstrated. For example, paroxetine- or citalopram-induced extracellular 5-HT accumulation at DR-terminal regions was greatly enhanced by co-treatment with various selective 5-HT1A receptor antagonists, whereas this effect was smaller at MR-terminal regions (Romero and Artigas 1997, Invernizzi et al., 1997). Together these observations suggest that pindolol may block somatodendritic 5-HT1A and terminal 5-HT1B (see above) autoreceptor feedback, resulting in a preferential enhancement of citalopram-induced extracellular 5-HT accumulation and 5-HT synthesis at DR-terminal regions.
Although serotonergic function (e.g. 5-HT synthesis and release) at DR-terminals may be more strongly regulated by somatodendritic 5-HT1A autoreceptors, regulation at the MR and DR cell body regions seem to be different (Hervás et al., 1998, Romero and Artigas 1997, Casanovas et al., 1997, Tohyama et al., 2001). First, 5-HT release at the MR can be more sensitive to 5-HT1A receptor drugs. Microdialysis studies indicate that, relative to the DR, 5-HT release at the MR is preferentially reduced by 5-HT1A receptor agonists (Casanovas et al., 1997) or enhanced by antagonists (Hervás et al., 1998). Second, 5-HT synthesis at the MR can be more sensitive to 5-HT1A autoreceptor drugs. Agonists reduced 5-HT synthesis equally at the MR and DR (Tohyama et al., 2002, Okazawa et al., 1999), whereas the antagonist WAY100635 enhanced synthesis significantly at the MR but not DR (Tohyama et al., 2001). In the present study, pindolol significantly enhanced synthesis at the MR but not DR (Table 3). Together these observations suggest that 5-HT1A autoreceptor control of 5-HT synthesis is stronger at the MR than DR. However, the MR receives a significant afferent input from DR neurons and vice versa (Vertes et al., 1999). Thus 5-HT synthesis measured at the MR may also be determined by synthesis in afferent terminals from the DR, which is regulated directly by terminal 5-HT1B and indirectly by somatodendritic 5-HT1A autoreeptors (Hjorth et al., 2000). Since 5-HT1B autoreceptors are expressed exclusively on serotonergic terminals (Riad et al., 2000), microdialysis studies demonstrating that 5-HT1B receptor agonists decrease extracellular 5-HT levels to a greater extent at the MR than DR (Adell et al., 2001) are suggestive of a stronger 5-HT1B autoreceptor regulation of synthesis is stronger at the MR than DR. We have previously demonstrated that 5-HT1B autoreceptor agonists can decrease 5-HT synthesis at the MR (Watanabe et al., 2006). Also, it has been suggested that 5-HT1B autoreceptor feedback on 5-HT release and synthesis is weaker at the DR than MR due to differences in efficacy or density of 5-HT1B autoreceptors (Sari, 2004). Thus compared to treatment with citalopram alone, combining pindolol with citalopram enhances 5-HT synthesis at the MR possibly due to blockade of terminal 5-HT1B and somatodendritic 5-HT1A autoreceptors on DR neurons.
Effect of treatments in OBX rats
The present experiments in sham rats observed effects that correspond to the putative actions of SSRIs and 5-HT1A/B receptor antagonists on non-pathological brain models. Citalopram and pindolol were also tested in OBX rats to observe their effects on a pathological brain model of depression. A predominant hypothesis (reviewed in Jesberger and Richardson 1988) of pathological serotonergic network function in OBX rats has been established on observations of serotonergic neuronal sprouting (Lumia et al., 1992, Zhou et al., 1998) and enhanced TPH expression (Zhou et al., 1998) and function (Watanabe et al., 2003, Hasegawa et al., 2005a) in OBX rats. Accordingly, the present study observed that 5-HT synthesis was enhanced at many brain regions of OBX compared to sham rats (cf. SVV vs. XVV in Tables 3 and 4): large differences were observed at cortical regions while only small or no differences were observed at the dorsal hippocampus, CA3, locus coeruleus, DR and MR of OBX rats. This is corresponds to our previous studies in OBX rats where large differences occurred at cortical regions but only small differences at the dorsal hippocampus, CA3, locus coeruleus, DR and MR (Watanabe et al., 2003, Hasegawa et al., 2005a). The present observations are consistent with a serotonergic pathology in OBX rats and warrant further assessment of this abnormal serotonergic function and its response to serotonergic drugs.
In OBX rats, citalopram either had no effect or decreased 5-HT synthesis at terminal regions and at the DR and MR (Table 4). The induced decrease in synthesis the DR and MR of OBX rats was similar to that observed for sham rats and thus may be similarly attributed to citalopram-induced activation of somatodendritic 5-HT1A and terminal 5-HT1B autoreceptors in the DR and MR (as discussed for sham rats). Additionally, the decrease was larger in OBX rats, suggesting a stronger autoreceptor control of somatic functions at the MR and DR in OBX rats. Because 5-HT1A autoreceptor expression in the MR and DR is decreased in OBX rats compared to sham rats (Sato et al., 2008), it could be hypothesized that the presently observed effects on the MR and DR are probably attributable to enhanced 5-HT1B autoreceptor function in OBX rats. This assumes that decreases in the receptor expression correlates to decreases in functionality, which remains to be proven in future experiments.
The lack of citalopram effects at many terminal regions in OBX rats may also be attributed to a strong terminal 5-HT1B autoreceptor feedback on 5-HT synthesis at MR- and DR-terminals. Moreover, citalopram-induced extracellular 5-HT accumulation at terminals is known to activate presynaptic 5-HT1B autoreceptors, which can directly deactivate TPH activity (Sari, 2004) and attenuate 5-HT release (Dawson and Nguyen, 2000), thus mitigating citalopram-induced depletion of terminal 5-HT stores and reducing the demand for 5-HT synthesis (Table 4). Furthermore, the preferential citalopram-induced decrease of 5-HT synthesis at MR-terminal regions (ratios of XVV vs. XCV) suggests an enhanced 5-HT1B autoreceptor regulation of MR-terminals in OBX rats. This is consistent with observations of preferential 5-HT1B autoreceptor regulation of MR-terminals (Hjorth et al., 2000). Together these results support the hypothesis that an altered autoreceptor regulation underlies the characteristic delay of antidepressant treatments in normalizing the behavioural deficits of OBX rats (Cryan et al., 1998, Cairncross et al., 1975).
Serotonergic autoreceptor function in OBX rats was presently assessed by observing the effect of combining pindolol with citalopram treatment. In OBX rats this combination treatment enhanced 5-HT synthesis at all regions relative to treatment with only citalopram (Table 4: XCV vs. XCP). Similar to sham rats, the pindolol-induced enhancement of 5-HT synthesis may be, in part, attributed to pindolol blockade of somatodendritic 5-HT1A and terminal 5-HT1B autoreceptor feedback and, in combination with ongoing citalopram inhibition of 5-HT reuptake, results in an increased demand for 5-HT synthesis (as discussed for sham rats). Furthermore, the larger pindolol-induced enhancement of 5-HT synthesis in OBX relative to sham rats (Tables 3 and 4: compare % changes for SCP vs. XCP groups) suggests a stronger autoreceptor regulation in OBX rats. Few other studies have assessed the effects of 5-HT autoreceptor antagonists in OBX rats. Cyran et al., (1998) observed that pindolol combined with an SSRI more effectively attenuated 5-HT1A receptor agonist-mediated hypothermia in OBX compared to sham rats. They interpreted this to be indicative of an elevated functional state of 5-HT1A receptors or other autoreceptors in OBX rats. Presently we observed that pindolol induced a larger enhancement of 5-HT synthesis at MR-terminals in OBX compared to sham rats (1.98±0.17 vs. 1.19±0.05, respectively; see results section) and is suggestive of an enhanced terminal 5-HT1B autoreceptor function in OBX rats. These observations are in line with the theory of an upregulated autoreceptor function in OBX rats (Cyran et al., 1998) and the theory of a strong 5-HT1B autoreceptor regulation at MR-terminals (Hjorth et al., 2000). Thus pindolol blockade of both somatodendritic 5-HT1A and terminal 5-HT1B autoreceptors could underlie the enhancement of SSRI-induced effects at DR- and MR-terminals in OBX rats. Further studies in OBX rats using localized administration of selective 5-HT1A or 5-HT1B receptor drugs could clarify the currently proposed abnormal autoreceptor regulation and provide insight into the pathology and the mechanisms of antidepressant drugs in this animal model of depression.
Antidepressant effects are not apparent after acute citalopram treatment because inhibition of 5-HT reuptake causes 5-HT overflow that acutely activates autoreceptors, resulting in a net restriction of terminal 5HT release. Indeed studies on extracellular 5HT levels at various forebrain regions observe that acute citalopram administration (∼10 mg/kg, s.c.) does not augment 5-HT levels as much as it does after chronic administration (Bosker et al., 2001) or in the presence of 5-HT autoreceptor antagonists (Invernizzi et al., 1992). Acute citalopram administration nonetheless yields gradual extracellular 5-HT accumulation at terminal regions (Invernizzi et al., 1997, David et al., 2003). This presumably reduces intracellular 5-HT stores (Kim et al., 2002) and consequently upregulates denovo 5-HT synthesis by activation of tryptophan hydroxylase (TPH) to replenish these stores (Boadle-Biber 1993). Such a mechanism may underlie the observed citalopram-induced increases in 5-HT synthesis at terminal regions of sham rats. Other SSRIs have been observed to induce an upregulation of TPH expression and function: in cultured RBL-2H3 cells, sertraline induced an increase in mRNA levels and enzymatic activity of TPH that was correlated with ex vivo observed decrease in intracellular 5-HT stores (Kim et al., 2002); fluoxetine induced an increase in 5-HT synthesis at several brain regions in conscious rats (Mück-Šeler et al., 1996, Tsuiki et al., 1995). Indeed drugs that extrude and reduce intracellular 5-HT stores, such as reserpine, 3,4-methylenedioxymethamphetamine and fenfluramine, consequently upregulate 5-HT synthesis at terminal regions (Hjorth et al., 1995, Nishisawa et al., 1999, Yamane et al., 1999). These studies corroborate the results reported here.
The potential contribution of the β-adrenergic receptor antagonist actions of pindolol on the acute neurochemical and behavioural effects of citalopram may be less relevant than with other SSRIs. A recent study where noradrenergic afferents to the raphe nuclei were specifically lesioned demonstrated that citalopram-induced behavioural effects remain intact whereas fluoxetine-induced effects were attenuated (O'Leary et al., 2007). Furthermore, pindolol's effects on 5-HT synthesis are probably not via its antagonistic actions on β-adrenergic receptors since other β-adrenergic receptor antagonists do not augment basal or citalopram-induced extracellular 5-HT accumulation at terminal regions (Hjorth et al., 1996).
Studies on OBX rats treated with SSRI-5-HT1A receptor antagonist combinations have thus far failed to correlate their acute and chronic effects on 5-HT1A receptor function with behavioural improvements in the open field test for stress response (Cryan et al., 1998 and 1999). However, this standard test may not be sufficiently sensitive and thus may require some modification to detect the expected behavioural effects of this drug combination- as was necessary to improve the sensitivity of the forced swim test for SSRI effects (Cryan et al., 2002). Indeed other limbic-related behavioural tests, such as open field-induced hyperthermia and tachycardia, have demonstrated good correlation to regional neurochemical changes induced by OBX induction of depressive symptoms and are congruent with chronic antidepressant drug treatments (Roche et al., 2007). Future experiments will focus on these latter behavioural tests to establish functional correlations with the neurochemical effects of combination SSRI-pindolol treatments.
Conclusions
The present study has demonstrated that the effects of acute citalopram treatment on regional 5-HT synthesis can be enhanced by co-treatment with pindolol. Interestingly in the OBX rat model of depression, the effects of acute SSRI treatment were greatly blunted but, however, could be greatly enhanced by co-treatment with pindolol. These observations support the notion of a pathological serotonergic system in this model of depression and in the mechanism of antidepressant therapy, particularly with respect to the function of serotonergic autoreceptors.
Acknowledgments
The research reported here was supported in part by the Croatian Ministry of Science, Education and Sports (219-1081970-2032) and Canadian Institute for Health Research (MOP-42438). We would also like to thank Dr. Pierre Blier for discussions of design of this study.
Abbreviations
- 5-HT
5-hydroxytryptamine
- α-MTrp
α-methyl-l-tryptophan
- NA
norepinephrine
- OBX
olfactory bulbectomy
- SSRI
selective serotonin reuptake inhibitor
Footnotes
In part submitted to the McGill University in fulfilment of requirement for M.Sc. degree. Data were also in part presented at the Society for Neuroscience Annual Meeting, November 8-13, 2003, New Orleans, LA. (Abstract#460.16)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Khanh Q. Nguyen, Cone Neurological Research Laboratory, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
Yoshihiro Tohyama, Cone Neurological Research Laboratory, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada.
Arata Watanabe, The permanent address of Arata Watanabe is Department of Neurosurgery, Yamanashi Medical University, 1110 Shimokato Tamaho-cho, Nakakoma-gun, Yamanashi 409–3898, Japan.
Shu Hasegawa, The permanent address of Shu Hasegawa is Department of Neurosurgery, Kumamoto University School of Medicine, 1-1-1 Honjo, Kumamoto, Kumamoto, 860–0816 Japan.
Ivan Skelin, Cone Neurological Research Laboratory, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada.
Mirko Diksic, Faculty of Medicine, the J.J. Strossmayer University, Osijek, Croatia; Address correspondence to: Mirko Diksic, Ph. D., McGill University, 3801 University Street, Montreal, Quebec, H3A-2B4, Canada. EM: mirko.diksic@mcgill.ca.
References
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. Journal of Neurochemistry. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Artigas F. 5-HT and antidepressants: new views from microdialysis studies. Trends in Pharmacological Sciences. 1993;14:262–262. doi: 10.1016/0165-6147(93)90125-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends in Pharmacological Sciences. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Callado LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled trials. Journal of Affective Disorders. 2004;79:137–47. doi: 10.1016/S0165-0327(02)00404-4. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Marsh ER, Kula NS. Interactions of fluoxetine with metabolism of dopamine and serotonin in rat brain regions. Brain Research. 1992;579:152–156. doi: 10.1016/0006-8993(92)90754-w. [DOI] [PubMed] [Google Scholar]
- Barton CL, Hutson PH. Inhibition of hippocampal 5-HT synthesis by fluoxetine and paroxetine: evidence for the involvement of both 5-HT1A and 5-HT1B/D autoreceptors. Synapse. 1999;31:13–19. doi: 10.1002/(SICI)1098-2396(199901)31:1<13::AID-SYN3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Berney A, Nishikawa M, Benkelfat C, Debonnel G, Gobbi G, Diksic M. An index of 5-HT synthesis changes during early antidepressant treatment: a–[11C]methyl–L-Tryptophan PET study. Neurochemistry International. 2008;52:701–8. doi: 10.1016/j.neuint.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Björklund A, Wiklund L, Descarries L. Regeneration and plasticity of central serotoninergic neurons: a review. Journal of Physiology (Paris) 1981;77:247–55. [PubMed] [Google Scholar]
- Blier P. The pharmacology of putative early-onset antidepressant strategies. European Neuropsychopharmacology. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- Blier P, Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. Journal of Clinical Psychopharmacology. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Chaput Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. Journal of Clinical Psychiatry. 1990a;51:14–20. [PubMed] [Google Scholar]
- Blier P, Serrano A, Scatton B. Differential responsiveness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and ρ-chloroamphetamine. Synapse. 1990b;5:120–133. doi: 10.1002/syn.890050206. [DOI] [PubMed] [Google Scholar]
- Bloxam DL, Curzon G. A study of proposed determinants of brain tryptophan concentration in rats after portocaval anastomosis or sham operation. J Neurochem. 1978;31:1255–1263. doi: 10.1111/j.1471-4159.1978.tb06250.x. [DOI] [PubMed] [Google Scholar]
- Boadle-Biber MC. Regulation of serotonin synthesis. Progress in Biophysics and Molecular Biology. 1993;60:1–15. doi: 10.1016/0079-6107(93)90009-9. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TIFH, Jongsma ME, Westerink BHC, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine1A receptor-mediated feedback: a microdialysis study in the amygdala. Journal of Neurochemistry. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Bourin M, Redrobe JP, Baker GB. Pindolol does not act only on 5-HT1A receptors in augmenting antidepressant activity in the mouse forced swimming test. Psychopharmacology (Berl) 1998;136:226–34. doi: 10.1007/s002130050560. [DOI] [PubMed] [Google Scholar]
- Brown P, Molliver ME. Dual Serotonin (5-HT) Projections to the Nucleus Accumbens Core and Shell: Relation of the 5-HT Transporter to Amphetamine-Induced Neurotoxicity. Journal of Neuroscience. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross KD, King MG, Schofield SPM. Effect of amitriptyline on avoidance learning in rats following olfactory bulb ablation. Pharmacology Biochemistry and Behavior. 1975;3:1063–1067. doi: 10.1016/0091-3057(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Lesourd M, Artigas F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. British Journal of Pharmacology. 1997;122:733–741. doi: 10.1038/sj.bjp.0701420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn-Schmiedeberg's Archives of Pharmacology. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. Journal of Cerebral Blood Flow and Metabolism. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. Review. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, McGrath C, Leonard BE, Norman TR. Combining pindolol and paroxetine in an animal model of chronic antidepressant action--can early onset of action be detected? European Journal of Pharmacology. 1998;352:23–28. doi: 10.1016/s0014-2999(98)00402-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mcgrath C, Leonard BE, Norman TR. Onset of the Effects of the 5-HT1A Antagonist, WAY-100635, Alone, and in Combination With Paroxetine, on Olfactory Bulbectomy and 8-OH-DPAT-Induced Changes in the Rat. Pharmacology Biochemistry and Behavior. 1999;63:333–338. doi: 10.1016/s0091-3057(98)00245-7. [DOI] [PubMed] [Google Scholar]
- David DJP, Bourin M, Jego G, Przybylski C, Jolliet P, Gardier AM. Effects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: a microdialysis study in Swiss mice. British Journal of Pharmacology. 2003;140:1128–1136. doi: 10.1038/sj.bjp.0705538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ. The role of 5-HT1A and 5-HT1B/1D receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: the mechanism of action of (±)pindolol. Neuropharmacology. 2000;39:1044–1052. doi: 10.1016/s0028-3908(99)00192-6. [DOI] [PubMed] [Google Scholar]
- Diksic M. Labelled alpha-methyl-l-tryptophan as a tracer for the study of the brain serotonergic system. Journal of Psychiatry and Neuroscience. 2001;26:293–303. [PMC free article] [PubMed] [Google Scholar]
- Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. Journal of Cerebral Blood Flow and Metabolism. 1990;10:1–12. doi: 10.1038/jcbfm.1990.1. [DOI] [PubMed] [Google Scholar]
- Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. Journal of Neurochemistry. 2000;78:1185–1200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- Earley B, Glennon M. Autoradiographic distribution of cholinergic muscarinic receptors and serotonin2 receptors in olfactory bulbectomized rats after chronic treatment with mianserin and desipramine. Human Psychopharm. 1994;9:397–407. [Google Scholar]
- Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, White C, Nakai A, Gross DW, Andermann E, Diksic M. Localizing value of α-methyl-L-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629–36. doi: 10.1212/wnl.57.9.1629. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–7. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Zhou D, Franke C, Schroder U, Sabel B, Becker A, Huether G. Influence of olfactory bulbectomy and subsequent imipramine treatment on 5-hydroxytryptaminergic presynapses in the rat frontal cortex: behavioral correlates. British Journal of Pharmacology. 1997;122:1725–1731. doi: 10.1038/sj.bjp.0701530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Blier P. Effects of sustained (±)pindolol administration on serotonin neurotransmission in rats. Journal of Psychiatry and Neuroscience. 2000;25:378–388. [PMC free article] [PubMed] [Google Scholar]
- Hadrava V, Blier P, Dennis T, Ortemann C, de Montigny C. Characterization of 5-hydroxytryptamine1A properties of flesinoxan: in vivo electrophysiology and hypothermia study. Neuropharmacology. 1995;34:1311–26. doi: 10.1016/0028-3908(95)00098-q. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Watanabe A, Nguyen KQ, Debonnel G, Diksic M. Chronic administration of citalopram in olfactory bulbectomy rats restores brain 5-HT synthesis rates: an autoradiographic study. Psychopharmacology. 2005a;179:781–790. doi: 10.1007/s00213-004-2122-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Watanabe A, Nishi K, Nguyen KQ, Diksic M. Selective 5-HT1B receptor agonist reduces serotonin synthesis following acute, and not chronic, drug administration: results of an autoradiographic study. Neurochemistry International. 2005b;46:261–272. doi: 10.1016/j.neuint.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hervás I, Bel N, Fernández AG, Palacios JM, Artigas F. In vivo control of 5-hydroxytryptamine release by terminal autoreceptors in rat brain areas differentially innervated by the dorsal and median raphe nuclei. Naunyn-Schmiedeberg's Archives of Pharmacology. 1998;358:315–322. doi: 10.1007/pl00005259. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. Journal of Psychopharmacology. 2000;14:177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Milano S. Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or β-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. European Journal of Pharmacology. 1996;316:43–47. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sciences. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–176. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- Huether G, Zhou D, Ruther E. Long-term modulation of presynaptic 5-HT-output: experimentally induced changes in cortical 5-HT transporter density, tryptophan hydroxylase content and 5-HT innervation density. Journal of Neural Transmission. 1997;104:993–1004. doi: 10.1007/BF01273313. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) International Clinical Psychopharmacology. 1994;1:19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Belli S, Samanin R. Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Research. 1992;584:322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Carli M, Di Clemente A, Samanin R. Administration of 8-hydroxy-2-(Di-n-propylamino)tetralin in raphe nuclei dorsalis and medianus reduces serotonin synthesis in the rat brain: differences in potency and regional sensitivity. Journal of Neurochemistry. 1991;56:243–247. doi: 10.1111/j.1471-4159.1991.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Velasco C, Bramante M, Longo A, Samanin R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology. 1997;36:467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Janscar SM, Leonard BE. Changes in neurotransmitter metabolism following olfactory bulbectomy in the rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1984;8:263–2269. doi: 10.1016/0278-5846(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Jesberger A, Richardson J. Brain output dysregulation induced by olfactory bulbectomy: an approximation in the rat of major depressive disorder in humans. Intern J Neurosci. 1988;38:241–265. doi: 10.3109/00207458808990688. [DOI] [PubMed] [Google Scholar]
- Kelly JP. The olfactory bulbectomized rat as a model of depression. Pharmacology and Therapeutics. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kim SW, Park SY, Hwang O. Up-Regulation of Tryptophan Hydroxylase Expression and Serotonin Synthesis by Sertraline. Mol Pharmacol. 2002;61:778–785. doi: 10.1124/mol.61.4.778. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kugelberg FC, Apelqvist G, Carlsson B, Ahlner J, Bengtsson F. In vivo steady-state pharmacokinetic outcome following clinical and toxic doses of racemic citalopram to rats. British Journal of Pharmacology. 2001;132:1683–90. doi: 10.1038/sj.bjp.0704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JB, Youngblood WY, Kizer JS. Regional differences in the response of serotonergic neurons in rat CNS to drugs. European Journal of Pharmacology. 1983;88:89–97. doi: 10.1016/0014-2999(83)90395-3. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Research. 1992;587:181–185. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- Martinez D, Hwang D, Mawlawi O, et al. Differential occupancy of somatodendritic and postsynaptic 5HT(1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24:209–229. doi: 10.1016/S0893-133X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional Mapping of Dorsal and Median Raphe 5-Hydroxytryptamine Pathways in Forebrain of the Rat Using Microdialysis. Journal of Neurochemistry. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Mück-Šeler D, Jevric-Causevic A, Diksic M. Influence of Fluoxetine on Regional Serotonin Synthesis in the Rat Brain. J Neurochem. 1996;67:2434–2442. doi: 10.1046/j.1471-4159.1996.67062434.x. [DOI] [PubMed] [Google Scholar]
- Nagahiro S, Takada A, Diksic M, Sourkes TL, Missala K, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo. II. A practical autoradiographic method tested in normal and lithium-treated rats. Journal of Cerebral Blood Flow and Metabolism. 1990;10:13–21. doi: 10.1038/jcbfm.1990.2. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Kumakura Y, Young SN, Fiset P, Vogelzangs N, Leyton M, Benkelfat C, Diksic M. Increasing blood oxygen increases an index of 5-HT synthesis in human brain as measured using alpha-[(11)C]methyl-L-tryptophan and positron emission tomography. Neurochem Int. 2005;47:556–564. doi: 10.1016/j.neuint.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Nishisawa S, Mzengeza S, Diksic M. Acute effects of 3,4-methylenedioxymethamphetamine on brain serotonin synthesis in the dog studied by positron emission tomography. Neurochemistry International. 1999;34:33–40. doi: 10.1016/s0197-0186(98)00067-9. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression in response to antidepressant treatment. Synapse. 2001;42:151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: A quantitative retrograde study. Brain Research Bulletin. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. European Neuropsychopharmacology. 2007;17:215–226. doi: 10.1016/j.euroneuro.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Yamane F, Blier P, Diksic M. Effects of acute and chronic administration of the serotonin1A agonist buspirone on serotonin synthesis in the rat brain. Journal of Neurochemistry. 1999;72:2022–2031. doi: 10.1046/j.1471-4159.1999.0722022.x. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Research. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Redmond AM, Kelly JP, Leonard BE. Behavioural and Neurochemical Effects of Dizocilpine in the Olfactory Bulbectomized Rat Model of Depression. Pharmacology Biochemistry and Behavior. 1997;58:355–359. doi: 10.1016/s0091-3057(97)00259-1. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet É, Langlois X, Mestikawy SE, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. Journal of Comparative Neurology. 2000;417:181–194. [PubMed] [Google Scholar]
- Roche M, Harkin A, Kelly JP. Chronic fluoxetine treatment attenuates stressor-induced changes in temperature, heart rate, and neuronal activation in the olfactory bulbectomized rat. Neuropsychopharmacology. 2007;32:1312–1320. doi: 10.1038/sj.npp.1301253. [DOI] [PubMed] [Google Scholar]
- Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. Journal of Neurochemistry. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- Romero L, Bel N, Artigas F, de Montigny C, Blier P. Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology. 1996;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Diksic M, Okazawa H, et al. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Archives of General Psychiatry. 2004;61:556–563. doi: 10.1001/archpsyc.61.6.556. [DOI] [PubMed] [Google Scholar]
- Rutter JJ, Auerbach SB. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1319–1324. [PubMed] [Google Scholar]
- Salter M, Knowles RG, Pogson CI. How does displacement of albumin-bound tryptophan cause sustained increases in the free tryptophan concentration in plasma and 5-hydroxytryptamine synthesis in brain? Biochem J. 1989;262:365–368. doi: 10.1042/bj2620365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neuroscience & Biobehavioral Reviews. 2004;28:565–82. doi: 10.1016/j.neubiorev.2004.08.008. Review. [DOI] [PubMed] [Google Scholar]
- Sato H, Skelin I, Debonnel G, Diksic M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: Assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Research Bulletin. 2008;75:545–555. doi: 10.1016/j.brainresbull.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Fallon SL. Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. European Journal of Pharmacology. 1988;157:173–181. doi: 10.1016/0014-2999(88)90380-9. [DOI] [PubMed] [Google Scholar]
- Skelin I, Yamane F, Diksic M. Flesinoxan challenge suggests that chronic treatment with paroxetine in rats does not desensitize receptors controlling 5-HT synthesis. Nerochemistry International. 2008 doi: 10.1016/j.neuint.2008.04.005. (In press, April 2008) [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. Changes in Behaviour, Neurotransmitters and Neutrophil Function in Olfactory Bulbectomized Rats: Effects of Chronic Desipramine Treatment. Human Psychopharmacology. 1997;12:99–103. [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing: implications for human psychopathology. Psychology Bulletin. 1992;112:330–50. doi: 10.1037/0033-2909.112.2.330. Review. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biological Psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- Tatarczyńska E, Kłodzińska A, Chojnacka-Wójcik E. Effects of combined administration of 5-HT1A and/or 5-HT1B receptor antagonists and paroxetine or fluoxetine in the forced swimming test in rats. Polish Journal of Pharmacology. 2002;54:615–23. [PubMed] [Google Scholar]
- Tohyama Y, Yamane F, Fikre Merid M, Blier P, Diksic M. Effects of serotonine receptors agonists, TFMPP and CGS12066B, on regional serotonin synthesis in the rat brain: an autoradiographic study. J Neurochem. 2002;80:788–798. doi: 10.1046/j.0022-3042.2002.00757.x. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, Yamane F, Merid MF, Diksic M. Effects of selective 5-HT1A receptor antagonists on regional serotonin synthesis in the rat brain: an autoradiographic study with α-[14C]methyl-l-tryptophan. European Neuropsychopharmacology. 2001;11:193–202. doi: 10.1016/s0924-977x(01)00076-1. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, Mück-Šeler D, Diksic M. Acute flesinoxan treatment produces a different effect on rat brain serotonin synthesis than chronic treatment: An α-methyl-L-tryptophan autoradiographic study. Neurochemistry International. 2007;51:486–95. doi: 10.1016/j.neuint.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi H, Nakashima Y, Kinami J, Nagatomo T. Characteristics of 125I-iodocyanopindolol binding to beta-adrenergic and serotonin-1B receptors of rat brain: selectivity of beta-adrenergic agents. The Japanese Journal of Pharmacology. 1990;52:195–200. doi: 10.1254/jjp.52.195. [DOI] [PubMed] [Google Scholar]
- Tsuiki K, Yamamoto YL, Diksic M. Effect of acute fluoxetine treatment on the brain serotonin synthesis as measured by the α–methyl-tryptophan autoradiographic method. Journal of Neuroscience. 1995;65:250–256. doi: 10.1046/j.1471-4159.1995.65010250.x. [DOI] [PubMed] [Google Scholar]
- van Riezen H, Leonard B. Effects of psychotropic drugs on the behavior and neurochemistry of olfactory bulbectomized rats. Pharmacology and Therapeutics. 1990;47:21–34. doi: 10.1016/0163-7258(90)90043-2. [DOI] [PubMed] [Google Scholar]
- Vanier M, Tsuiki K, Grdisa M, Worsley K, Diksic M. Determination of the lumped constant for the alpha-methyltryptophan method of estimating the rate of serotonin synthesis. Journal of Neurochemistry. 1995;64:624–35. doi: 10.1046/j.1471-4159.1995.64020624.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. Journal of Comparative Neurology. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. Journal of Comparative Neurology. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Hasegawa S, Nishi K, Nguyen KQ, Diksic M. Chronic buspirone treatment normalizes regional serotonin synthesis in the olfactory bulbectomized rat brain: an autoradiographic study. Brain Research Bulletin. 2006;69:101–108. doi: 10.1016/j.brainresbull.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Tohyama Y, Nguyen KQ, Hasegawa S, Debonnel G, Diksic M. Regional brain serotonin synthesis is increased in the olfactory bulbectomy rat model of depression: an autoradiographic study. Journal of Neurochemistry. 2003;85:469–475. doi: 10.1046/j.1471-4159.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- Yamane F, Tohyama Y, Diksic M. Acute and Chronic D-Fenfluramine Treatments Have Different Effects on Serotonin Synthesis Rates in the Rat Brain: An Autoradiographic Study. Neurochemical Research. 1999;24:1611–1620. doi: 10.1023/a:1021120603457. [DOI] [PubMed] [Google Scholar]
- Zhou D, Grecksch G, Becker A, Franke C, Pulz J. Serotonergic hyperinnervation of the frontal cortex in an animal model of depression, the bulbectomized rat. Journal of Neuroscience Research. 1998;54:109–116. doi: 10.1002/(SICI)1097-4547(19981001)54:1<109::AID-JNR11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]


