Abstract
Stimuli-responsive polymers are arguably the most widely considered systems for a variety of applications in biomedical arena. We report here a novel triple stimuli sensitive block copolymer assembly that responds to changes in temperature, pH and redox potential. Our block copolymer design constitutes an acid-sensitive THP-protected HEMA as the hydrophobic part and a temperature-sensitive PNIPAM as the hydrophilic part with an intervening disulfide bond. The micellar properties and the release kinetics of the encapsulated guest molecule in response to one stimulus as well as combinations of stimuli have been evaluated. Responsiveness to combination of stimuli not only allows for fine-tuning the guest molecule release kinetics, but also provides the possibility of achieving location-specific delivery.
Introduction
Driven by the need to develop technologically smart materials for use in various applications such as catalysis,1 nanotechnology,2 and drug delivery,3 a number of stimuli-responsive polymers have been developed and extensively investigated. Of various polymeric systems, amphiphilic block copolymers with stimuli-responsive elements are of particular interest for two reasons: (i) they can self-assemble into various supramolecular structures and thus provide interiors that can non-covalently encapsulate guest molecules4 and (ii) the release of guest molecules can be triggered by external stimuli. Self-assembly of amphiphilic molecules in aqueous media is of fundamental interest for applications in biotechnology and medicine, since most drug molecules are hydrophobic and therefore can be useful in drug delivery. A number of micellar systems have been successfully developed so far. However, precisely switching on and off the release of the encapsulated guest molecules in response to environmental changes is still a challenging task for chemists. Towards this end, systems that respond to various stimuli such as light5, temperature6, pH,7,8 and redox potential9 are becoming more prevalent for applications in biology,10 drug delivery,3 recyclable catalysis,11 and separations.12
During the past two decades, there have been numerous reports on stimuli-sensitive polymeric micellar systems. But a majority of them deal with response to single stimulus.5-9 In nature however, the change in behavior of a macromolecule (proteins and nucleic acids) is often a result of its response not to a single factor, but to a combination of environmental changes. To mimic this feature, formulation of materials which can sense specific changes and respond to multiple stimuli in a predictable manner would be of great interest.3e,10f Dual responsive systems have been relatively underexplored, especially systems that exhibit redox-sensitive behavior.13,14 Engineering new materials endowed with responsive properties for multiple stimuli can be highly beneficial to obtain more systematic release kinetics. These systems would provide a unique opportunity to fine tune their response to each stimulus independently, as well as precisely regulate release profile during the combined effect of multiple stimuli. Here, we describe the design and synthesis of a polymeric micellar system which can respond to multiple stimuli, viz., temperature, pH and redox potential.
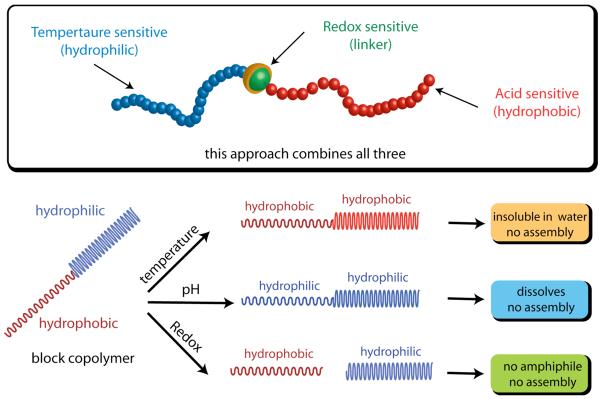
Recently, we have introduced a facile method for the synthesis of block copolymers linked via disulfide functionality.15 We envisaged the possibility of designing a triple stimuli sensitive polymeric system by incorporating a temperature sensitive functionality on one block of an amphiphilic block copolymer, acid sensitive functionalities on the other block, and connecting the two with a redox sensitive disulfide linker. If the design also renders the polymer amphiphilic and thus micelle-forming, then this design would result in a supramolecular assembly that can respond to changes in temperature, pH, and redox potential (Scheme 1). In this report, we disclose the findings on the micellar properties of this amphiphilic block copolymer (BCP), study the responsiveness of the assembly to the stimuli, and the release kinetics of the encapsulated guest molecules in response to a stimulus by itself or to combinations of stimuli.
Scheme 1.
Design of amphiphilic diblock copolymer: Schematic representation of amphiphilic block copolymer which can respond to three stimuli; pH, temperature and redox.
Results and Discussion
Design, synthesis and characterization of amphiphilic block copolymer and assembly
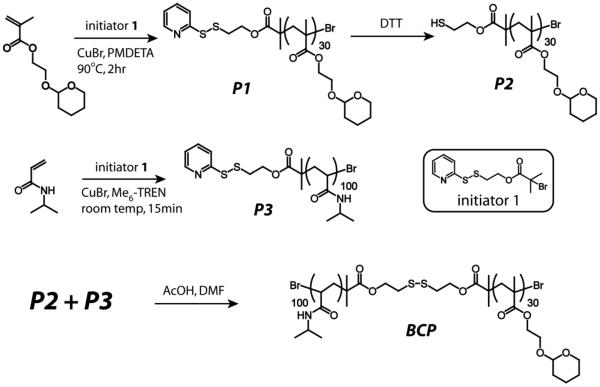
Three different components of the amphiphilic block copolymer were targeted to engineer the responsive characteristics for each of the three stimuli. Thus, the polymer is designed in such a way that: (i) the hydrophilic part is temperature sensitive; (ii) hydrophobic part is acid sensitive; and (iii) the linker connecting the hydrophilic and the hydrophobic blocks is redox sensitive. The specific functionalities for the stimuli sensitive characteristics have been chosen as follows. For the temperature sensitive block, poly(N-isopropylacrylamide) (PNIPAM) is used, which is well-known for exhibiting a reversible thermosensitive phase transition in aqueous solution.16 The character of this block changes from hydrophilic to hydrophobic above its lower critical solution temperature (LCST). For the acid-sensitive functionality, we were interested in utilizing a functionality that converts the hydrophobic block to a hydrophilic one upon encountering the stimulus. Acetals are commonly used to protect alcohols in organic synthesis.17 The simple deprotection of acetals under mild acidic conditions is well established in literature.7f,8a,8b Hence, 2-hydroxyethyl methacrylate (HEMA) was chosen, where the alcohol group of HEMA was protected as a tetrahydropyran (THP) derivative. Deprotection of the THP moiety from the polymer under acidic conditions will convert this hydrophobic block to a hydrophilic PHEMA. The hydrophilic and hydrophobic blocks were linked by redox sensitive disulfide functionality. This linkage can cleave the block copolymer into its constituent homopolymers in the presence of a mild reducing agent, such as dithiothreitol (DTT). The structure of the polymer that contains all these structural features is shown as BCP in Scheme 2.
Scheme 2.
Synthesis of BCP from its homopolymers
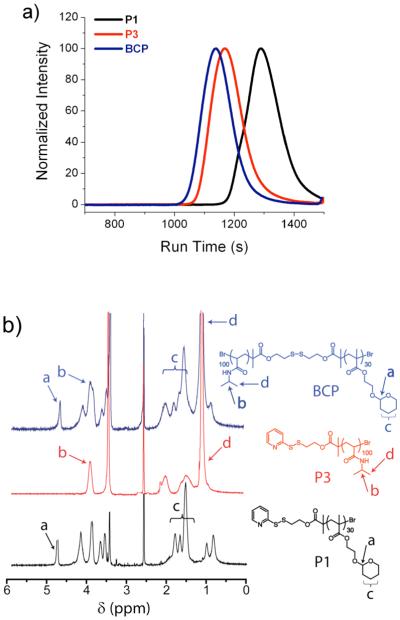
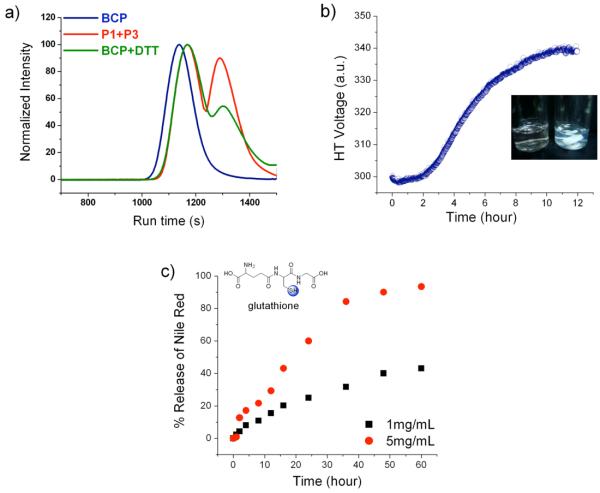
THP-protected HEMA monomer and NIPAM were polymerized using initiator 1 by ATRP (Scheme 2). Both homopolymers were obtained with low polydispersity and with molecular weights (Mn) of 6400 g/mol and 11300 g/mol, respectively (Table 1). In order to obtain polymer P2 with a free thiol, polymer P1 was reduced using dithiothreitol (DTT). Finally, polymer P3 was coupled with polymer P2 to achieve the block copolymer, BCP (Scheme 2). The molecular weights of P1, P3 and BCP were determined by gel permeation chromatography (GPC) using DMF as the solvent and PMMA as the standard (Figure 1a). The molecular weight of the block copolymer BCP is nearly equal to the sum of the constituent homopolymers P2 and P3 (Table 1). Moreover in GPC, the peak corresponding to BCP is shifted towards a higher molecular weight compared to both P2 and P3, indicating that the targeted block copolymer is indeed achieved (Figure 1a). The above observations are further supported by 1H NMR (Figure 1b).
Table 1.
Properties of polymers
estimated by GPC (DMF) using PMMA standard.
Figure 1.
Characterization of block copolymer (BCP) (a) the GPC profile showing the formation of block copolymer (BCP) (b) 1H NMR of BCP and its homopolymers.
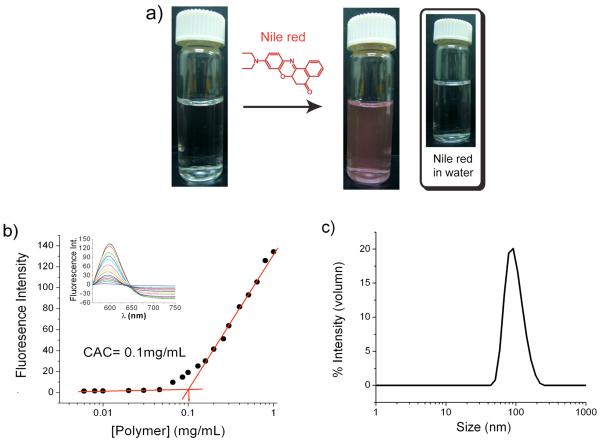
Critical aggregation concentration (CAC) of the block copolymer was determined at pH 7.4 using a fluorescence probe. Nile red is a hydrophobic dye which by itself is not soluble in water, as can be discerned from the lack of absorption or emission spectral intensity from its aqueous solution. However, this dye can be sequestered inside the hydrophobic pocket generated by micelles. To obtain the CAC of BCP, the aqueous solution of BCP was prepared by dialysis method and Nile red was encapsulated into the hydrophobic micellar interior (Figure 2a). The concentration of BCP was varied and the change in the relative emission intensity of Nile red was plotted. A sudden decrease in emission intensity was observed at a concentration of about 0.1 mg/mL of BCP, indicating the onset of micelle formation (Figure 2b). Dynamic light scattering (DLS) experiments were carried out to further verify the formation of micelle from BCP and measure the size of the assembly. The BCP solution (0.2 mg/mL, above the CAC) was prepared and the hydrodynamic radius of the micelle was determined. An average size of about 90 nm was obtained with excellent correlation function (Figure 2c), suggesting that BCP indeed aggregated to form micelle-type assemblies in water.
Figure 2.
Micellar assembly (a) Photograph shows an aqueous solution of BCP; (left) before adding Nile red, (right) after adding Nile red. (b) Plot of fluorescence intensity of Nile red vs. concentration of BCP. (c) Size of the micelle at 0.2 mg/mL determined by DLS experiment.
pH-Responsive disassembly of block copolymer micelle
The hydrophobic part of the BCP consists of an acid-sensitive cyclic acetal functionality, which can be cleaved under mildly acidic conditions. Upon cleavage of the acetal group, the hydrophobic part of the block would transform into hydrophilic PHEMA, thus creating an imbalance in the hydrophilic / lipophilic ratio to cause disruption of the micelle. In order to examine if the BCP micelle is indeed sensitive to variations in pH, we monitored the assembly using DLS. The BCP solution (0.2 mg/mL) was treated with pH 4.0 sodium acetate buffer (50 mM) and the size of the micellar assembly was monitored for 2 days. As indicated in Figure 3b, the size of the micelle slowly decreases with time. After the 48 hours period, significant formation of a smaller assembly at 30 nm was observed.
Figure 3.
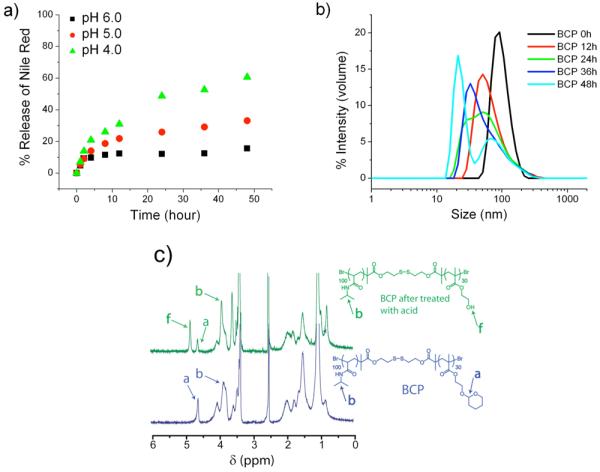
Acid sensitivity of BCP a) pH dependent release of Nile red from micellar assembly, b) Time dependent DLS profile of BCP solution in sodium acetate buffer of pH 4.0 (50mM), c) 1HNMR of BCP before and after treatment with sodium acetate buffer of pH 4.0 (50mM).
It is interesting that the remnant of a supposedly disassembled double hydrophilic structure is still 30 nm large. But there have been reports wherein the double hydrophilic block copolymers are known to assemble into core-shell nanostructures and vesicles.18 Ultimately however, as mentioned earlier, we are interested in releasing the non-covalently sequestered guest molecules. Therefore, we were interested in assessing whether such a change in the assembly size in response to pH will result in the concomitant release of the guest molecules. For this purpose, the BCP solution (0.2 mg/mL, above the CAC) was used to encapsulate Nile red and this solution was treated with 50 mM sodium acetate buffer (pH varying from 4.0, 5.0, and 6.0). The release of Nile red from the micellar interior was monitored as a function of time and is depicted in Figure 3a. Slow release of Nile red, up to 20% was observed at pH 6.0, while the release rate increased with decrease in pH from 5.0 to 4.0. Up to 60% of Nile red was released at pH 4.0 in 2 days in contrast to that of 20% at pH 6.0, suggesting faster cleavage kinetics of acetal at low pH with concomitant disassembly of micelle.
Although the disassembly of the micelle and release study of the Nile red provides good evidences to establish the acid sensitivity of BCP, we wanted to further investigate if the disassembly is indeed the result of the hydrolysis of acetal moiety of the BCP. To test this, the BCP solution was treated with sodium acetate buffer of pH 4 (50 mM) and incubated at room temperature for 2 days. The resulting solution was then dialyzed and lyophilized to remove the byproduct, 2-hydroxytetrahydropyran, which is generated due to the cleavage of acetal. The 1H NMR spectra of the BCP before and after treatment with acid were recorded (Figure 3c). The acetal proton of BCP appears at 4.6 ppm in the 1H NMR. After treatment with acid, the intensity of the acetal peak has significantly reduced and a new peak was observed at 4.8 ppm, which corresponds to the –OH group of poly (2-hydroxyethyl methacrylate) (PHEMA), suggesting that the acetal was indeed accessible and cleaved by the reduced pH of the solution.
Thermosensitive behavior of the block copolymer
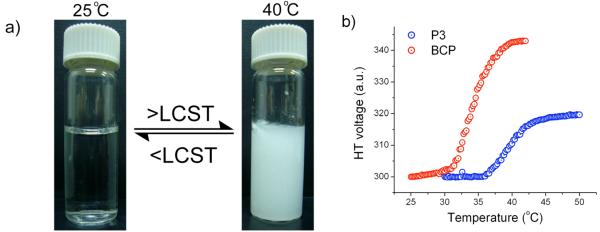
Poly-NIPAM is considered hydrophilic, because the amide side chain of the polymer is hydrated and thus is soluble in water at ambient temperature. It is suggested that this degree of hydration of PNIPAM reduces with increasing temperature resulting in the polymer being more hydrophobic and thus precipitation of the polymer. Our BCP assembly is comprised of the acetal-protected HEMA as the hydrophobic part and PNIPAM as the hydrophilic part. In the assembly, the poly-NIPAM part is presented as the outer hydrophilic shell to optimize the surface contact with water. If this hydrophilic component is converted to a hydrophobic one in response to temperature, then the BCP should precipitate out. To test this possibility of the LCST behavior, a solution of BCP was taken and heated up to 40 °C. Formation of a precipitate was observed with increase in temperature, and this process is thermally reversible (Figure 4a), indicating that the BCP does exhibit the LCST behavior.
Figure 4.
Temperature sensitivity of BCP a) photograph showing an aqueous solution of BCP; left-at room temperature, right-after heating to 40 °C, b) Turbidity experiment showing the change in HT voltage with temperature of BCP and PNIPAM.
To determine the actual LCST of the BCP, temperature dependent turbidity measurements were carried out using a circular dichroism (CD) spectrometer. BCP (1.4 mg/mL) solution was prepared and the change in the high tension (HT) voltage was monitored at 600 nm by varying the temperature by 1 °C/min.6b,19 The LCST of BCP was found to be around 35 °C (Figure 4b). It is known that the copolymerization of NIPAM with hydrophobic comonomer would decrease the LCST, while the hydrophilic comonomers have the opposite effect. Since the BCP is comprised of a hydrophilic PNIPAM (P3) and hydrophobic THP protected HEMA (P2), the LCST of the BCP is indeed expected to be lower than that of PNIPAM (P3). To verify this, the LCST of the PNIPAM homopolymer of same weight percentage (1.4 mg/mL) was determined and compared with that of BCP. The LCST of PNIPAM was found to be about 40°C, which corroborates the fact that hydrophobic comonomer decreases the LCST of PNIPAM. Note that the LCST of PNIPAM here is slightly higher than the typically reported LCST of 32 °C. Note, however, that it is also known in the literature that the LCST of PNIPAM does change with molecular weight, concentration and even the presence of various end group functionalities.20 Considering the molecular weight of the PNIPAM block and the concentrations used here, the observed LCST is indeed consistent with literature.
Redox-responsive disassembly of block copolymer micelle
The hydrophobic and the hydrophilic blocks of BCP are linked by disulfide bond and the BCP is shown to form micelles in water. We have examined the disassembly of the micelle under redox conditions such as using dithiothreitol (DTT) and glutathione. Treatment of BCP solution with reducing agents should cleave the BCP into two separate homopolymers, the water-soluble PNIPAM and the water-insoluble THP-protected HEMA. The insoluble part would precipitate out and since the homopolymers are no more intact the micelle would be disrupted, thus resulting in the co-precipitation of the guest molecules from solution. To demonstrate that BCP is sensitive to changes in the redox environment, BCP (0.2mg/mL) was dissolved in DMF (a good solvent for both parts of the copolymer) and treated with DTT (5mg/mL) (a mild reducing agent for 30 min). The GPC of the reaction mixture before and after addition of DTT were recorded (Figure 5a). The GPC of the physical mixture of polymers P1 and P3 is also shown in Figure 5a. The GPC profile of the reaction mixture after treatment with DTT matches with that of the physical mixture of the two constituent polymers, indicating the complete cleavage of BCP. However, note that while this experiment demonstrates that the BCP can be redox sensitive in solution, this does not guarantee the availability of disulfide bond to be cleaved by hydrophilic reducing agent such as DTT in aqueous solution, because it might be more deeply embedded within the hydrophobic / hydrophilic interface of the supramolecular assembly.
Figure 5.
Redox sensitivity of BCP a) GPC profile of BCP compared with the BCP solution treated with DTT and physical mixture of P1 and P3, b) turbidity of micellar solution upon treatment with DTT, c) % release of Nile red from micellar interior.
In the BCP assembly in water, disruption of the disulfide linkage by DTT would result in the formation of the insoluble hydrophobic part, which should increase the turbidity of the solution. To test this, an aqueous solution of BCP (0.2 mg/mL) was taken and the turbidity of the solution in presence of DTT (5mg/mL) was measured using CD spectrophotometer. The change in HT voltage was measured over time and is plotted in Figure 5b. The turbidity was found to increase with time, suggesting that the disulfide bond in polymeric micelle is indeed accessible to the hydrophilic reducing agents.
By the same method, when the hydrophobic part of the assembly precipitates out of the solution, it is likely that the non-covalently sequestered dye molecule within the hydrophobic interior of the assembly also precipitates out of solution. We tested this possibility with glutathione as the reducing agent. Glutathione is a tripeptide that is found to be present in higher concentrations in certain cell types and the redox behavior is similar to that of DTT.21 We chose glutathione as the reducing agent for the study here mainly to test whether our system will indeed be sensitive to both DTT and glutathione. To elucidate the fate of the guest molecule in the redox environment, we once again used the emission spectrum of Nile red as the probe. Nile red was encapsulated into the BCP micelle and treated with glutathione. The percent Nile red retained in micellar interior with time was monitored (Figure 5c). The precipitation of Nile red increased with time, suggesting the disassembly of the polymeric micelles. At a concentration of 1 mg/mL (3.2 mM) glutathione, 40% of Nile red was precipitated in 3 days, while 100% precipitation was observed at higher glutathione concentration (5 mg/mL, 16 mM) over the same period of time. Note that the intracellular glutathione concentrations have been estimated to be as high as 10 mM, while the concentration is even higher in tumor cells.22
Multiple stimuli responsive polymer micelles
The primary purpose of our molecular design is to bring about the multi-stimuli sensitive property to a single macromolecular assembly. Even though the responsiveness of the micelle to pH, temperature and redox potential has been demonstrated independently, such a behavior would be more enticing if there were a combination of two or more stimuli in one system as it would provide a unique opportunity to fine tune the release kinetics of guest molecules at specific circumstances where more than one stimulus is present. The responsiveness of our BCP micelle for the following combination of stimuli was investigated: (i) temperature and pH; (ii) temperature and redox potential; (iii) pH and redox potential; and (iv) pH, temperature and redox potential.
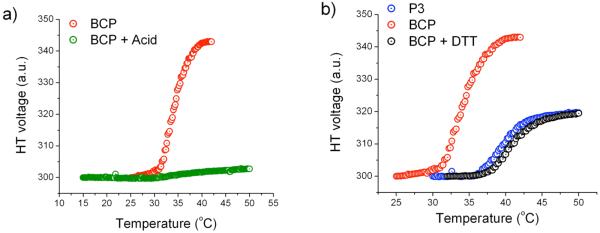
To study the effect of the combination of pH and temperature, the BCP (0.2 mg/mL) solution was treated with sodium acetate buffer (50 mM, pH 4.0) and incubated at room temperature for 2 days. The solution was filtered and the LCST was measured by monitoring the turbidity of the solution using the HT voltage measurements. The BCP solution after treatment with acid didn't exhibit any phase transition (Figure 6a). A control experiment was also performed by measuring the LCST of PNIPAM with acid (10 % v/v) to test if the acidic environment has any inherent effects on the LCST of PNIPAM. But the acid was found to have no effect on the LCST of PNIPAM (see Supporting Information). It is understandable that the thermal behavior of the polymer changed upon subjecting it to an acidic environment, because the hydrophilicity of the polymer increases upon deprotection of PHEMA, which causes the overall hydrophilicity of the BCP to increase. Apparently, this change in property is sufficient for significantly changing the thermal behavior of the BCP.
Figure 6.
Dual stimuli responsive micelle a) Temperature-acid sensitive behavior, b) Temperature-redox sensitive behavior.
To study the effect of temperature and redox potential, BCP (0.2 mg/mL) solution was treated with DTT (5mg/mL) and incubated at room temperature for 2 days. After incubation, the BCP solution turned turbid, suggesting that the disulfide bond was cleaved by DTT. The solution was then filtered to remove the insoluble particles and LCST of the filtrate was determined by monitoring the HT voltage using CD spectrophotometer (Figure 6b). Treatment of BCP solution with DTT should result in a mixture of PNIPAM (P3) and THP-protected HEMA (P1). Since P1 is hydrophobic, this polymer is not soluble in water and thus the filtrate should contain only PNIPAM. Therefore, the observed LCST should resemble that of PNIPAM. As can be seen from Figure 6b, the LCST of the filtrate matches exactly with that of PNIPAM (same weight percentage), suggesting the complete disruption of disulfide bonds.
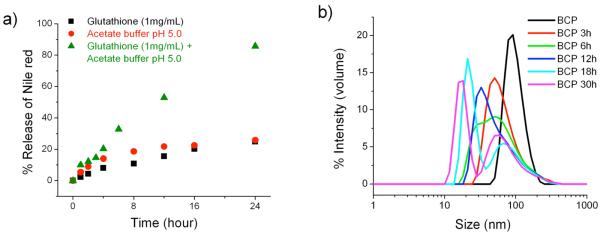
As mentioned earlier, the release kinetics of Nile red from the polymer micelle was found to be rather slow in response to either pH or redox potential. Such materials could find use in certain controlled release applications. However, as mentioned in the introduction, it is also desirable to tune the release of the guest molecules in response to the simultaneous presence of two different stimuli. We hypothesized that if we can combine both pH and redox potential, then simultaneous cleavage of the disulfide linkages as well as the acetal group should result in a rapid collapse of the micelle, thus providing an enhancement in the release kinetics of the encapsulated guest molecule. To explore this possibility, we used 1 mg/mL (3.2 mM) glutathione and sodium acetate buffer of pH 5.0, because these conditions had earlier resulted in very slow release kinetics of Nile red and thus would be the optimal parameters to be investigated. To test our hypothesis, the Nile red encapsulated BCP solution was subjected to glutathione (1 mg/mL, 3.2 mM) and acetate buffer of pH 5.0. The release profile of Nile red was monitored and plotted against time (Figure 7a). Interestingly, a dramatic increase in the release rate of Nile red was observed and complete release was obtained within 24 h. DLS experiments were carried out to further investigate the rapid disassembly of micelle. The solution of BCP was treated with glutathione (1 mg/mL, 3.2 mM) and acetate buffer of pH 5.0 and the size of the micelle were monitored with the progress of time (Figure 7b). The result indicates that the micelles were indeed cleaved faster. Thus, the BCP decorated with multiple stimuli sensitive elements would provide an opportunity to fine tune various parameters to obtain the desired release profile. It should also be noted that we did not observe any precipitation of the polymer, as was the case with redox-sensitive cleavage of the BCP.
Figure 7.
pH-redox responsive micelle (a) % release of Nile red from BCP solution treated with sodium acetate buffer of pH 5.0 (50mM) and Glutathione (1mg/mL, 3.2 mM) (b) Time dependent DLS profile of BCP solution in sodium acetate buffer of pH 5.0 (50mM) and Glutathione (1mg/mL, 3.2 mM)
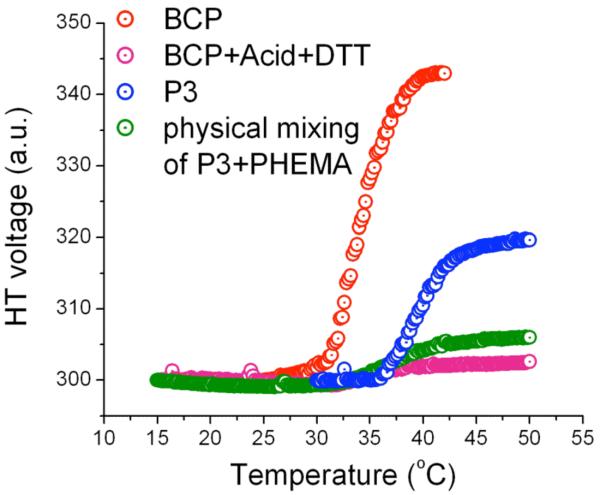
So far, we have demonstrated that the BCP can respond to dual stimuli viz., pH-temperature, redox-temperature, and redox-pH. Since the BCP is comprised of three key stimuli-sensitive functionalities, we also were interested in exploring if the system can simultaneously respond to all these stimuli. The BCP solution (0.2 mg/mL) was subjected to 5 mg/mL of DTT in presence of 5% (v/v) of acetic acid, and was incubated at room temperature for overnight. The solution was filtered and the LCST was measured by monitoring the HT voltage. Treatment of BCP solution with acid and DTT would result in PNIPAM and PHEMA, both of which are water soluble. We expected that the LCST of resulting solution would be similar to that of PNIPAM. Surprisingly, no phase transition was observed even up to the temperature of 50 °C (Figure 8). We wanted to examine if the presence of PHEMA in solution has any effect on the LCST behavior of PNIPAM. A physical mixture of PNIPAM and PHEMA of same weight percentage was prepared and the LCST was determined. Interestingly, the solution exhibited the similar behavior as that of the BCP solution treated with acid and DTT. This demonstrates that the expected change did occur between the BCP and the acid-catalyzed deprotection of THP to afford PHEMA and PNIPAM. The suppression of the LCST behavior of PNIPAM in presence of PHEMA can be attributed to the fact that PHEMA can participate in H-bonding with PNIPAM and thus can disrupt the interaction of PNIPAM with water. The participation of polymers such as poly (2-hydroxyethyl methacrylate), poly (2-hydroxyethyl acrylate) and poly (acrylic acid) in H-bonding with PNIPAM is well established in case of hydrogels.23 Thus there is literature precedence for our assertions regarding the mixture of these polymers.
Figure 8.
Triple stimuli sensitive micelle.
Summary
We have designed and synthesized an amphiphilic block copolymer (BCP) which comprises of an acid-sensitive hydrophobic core, temperature-sensitive hydrophilic shell and a redox-sensitive interface. Our amphiphilic BCP was shown to self-assemble into micellar structure in aqueous solution and is capable of encapsulating hydrophobic guest molecules, such as Nile red. We have shown that the stimuli-sensitive degradation of the assembly can be achieved under the following conditions: (i) above the LCST, the hydrophilic thermo-responsive block is converted to a hydrophobic one, rendering the polymer insoluble in water and hence no assembly; (ii) lowering the pH has transformed the acid sensitive hydrophobic block to a hydrophilic one, resulting in the dissolution of the assembly; and (iii) a reducing environment affords the scission of the BCP into individual homopolymers and hence disruption of the assembly. Thus, it has been clearly demonstrated that the BCP is sensitive to multiple stimuli, viz., pH, temperature, and redox stimuli. We have also shown that our amphiphilic BCP is sensitive not only to a single stimulus, but to the simultaneous presence of multiple stimuli. Thus the proposed amphiphilic block copolymer has the potential to be used as a redox, acid, and temperature responsive system, which would provide us with a unique opportunity to fine tune the release kinetics of the encapsulated hydrophobic guest molecules. For example, we have shown that while the pH and redox stimulus by itself exerts slow or incomplete release of the guest molecules over a long period of time, combination of these two stimuli results in significantly accelerated and more complete release of the encapsulated hydrophobic guests. It is possible that these types of systems have implications in areas such as the targeted drug delivery to cancer cells, as it is known that pH or redox triggers could be used for releasing drugs in tumor cells.3 Our work here demonstrates the feasibility of combining these triggers. Note, however, that our current polymers were not designed with biocompatibility in mind and this is indeed part of the ongoing investigations in our laboratories. These multiple stimuli responsive systems also have the prospect of more broadly impacting fields such as controlled release, catalysis, and separation.
Supplementary Material
Acknowledgment
We thank NSF-Center for Hierarchical Manufacturing and the NIMGS of the NIH for partial funding. The polymer assembly designed here was made possible due to the synthetic methods developed under support from the NSF-Center for Fueling the Future at the University of Massachusetts Amherst.
Footnotes
Supporting Information Available: Synthetic and other experimental details. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Astruc D, Chardac F. Chem. Rev. 2001;101:2991–3024. doi: 10.1021/cr010323t. [DOI] [PubMed] [Google Scholar]; (b) Persigehl P, Jordan R, Nuyken O. Macromolecules. 2000;33:6977–6981. [Google Scholar]; (c) Vriezema DM, Comellas Aragones M, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem. Rev. 2005;105:1445–1490. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]; (d) Jung HM, Price KE, McQuade DT. J. Am. Chem. Soc. 2003;125:5351–5355. doi: 10.1021/ja0271983. [DOI] [PubMed] [Google Scholar]
- 2.(a) Tew GN, Pralle MU, Stupp SI. Angew. Chem., Int. Ed. 2000;39:517–521. doi: 10.1002/(sici)1521-3773(20000204)39:3<517::aid-anie517>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; (b) Lee M, Cho B-K, Zin W-C. Chem. Rev. 2001;101:3869–3892. doi: 10.1021/cr0001131. [DOI] [PubMed] [Google Scholar]; (c) Fuhrhop J-H, Wang T. Chem. Rev. 2004;104:2901–2937. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]; (d) Brunsveld L, Folmer BJB, Meijer EW, Sijbesma RP. Chem. Rev. 2001;101:4071–4098. doi: 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]; (e) Estroff LA, Hamilton AD. Chem. Rev. 2004;104:1201–1218. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- 3.(a) Okano T, Bae YH, Jacobs H, Kim SW. J. Controlled Release. 1990;11:255–265. [Google Scholar]; (b) Hoffman AS. J. Controlled Release. 1987;6:297–305. [Google Scholar]; (c) Brønsdsted H, Kopecek J. Biomaterials. 1991;12:584–592. doi: 10.1016/0142-9612(91)90056-g. [DOI] [PubMed] [Google Scholar]; (d) Yeh P, Kopečková P, Kopeček J. J. Polym. Sci., Polym. Chem. 1994;32:1627–1637. [Google Scholar]; (e) Ganta S, Devalapally H, Shahiwala A, Amiji M. J. Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]; (f) Kataoka K, Harada A, Nagasaki Y. Adv. Drug Delivery Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]; (g) Rapoport N. Prog. Polym. Sci. 2007;32:962–990. [Google Scholar]
- 4.(a) Zhang L, Yu K, Eisenberg A. Science. 1996;272:1777–1779. doi: 10.1126/science.272.5269.1777. [DOI] [PubMed] [Google Scholar]; (b) Zhang L, Eisenberg A. J. Am. Chem. Soc. 1996;118:3168–3181. [Google Scholar]; (c) Discher DE, Eisenberg A. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]; (d) Zhou Z, Li Z, Ren Y, Hillmyer MA, Lodge TP. J. Am. Chem. Soc. 2003;125:10182–10183. doi: 10.1021/ja036551h. [DOI] [PubMed] [Google Scholar]; (e) Sanji T, Kitayama F, Sakurai H. Macromolecules. 1999;32:5718–5720. [Google Scholar]; (f) Neiser MW, Muth S, Kolb U, Harris JR, Okuda J, Schmidt M. Angew. Chem., Int. Ed. 2004;43:3192–3195. doi: 10.1002/anie.200353259. [DOI] [PubMed] [Google Scholar]
- 5.(a) Goodwin AP, Mynar JL, Ma Y, Fleming GR, Frechet JMJ. J. Am. Chem. Soc. 2005;127:9952–9953. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]; (b) Jiang J, Tong X, Zhao Y. J. Am. Chem. Soc. 2005;127:8290–8291. doi: 10.1021/ja0521019. [DOI] [PubMed] [Google Scholar]; (c) Anderson VC, Thompson DH. Biochim. Biophys. Acta. 1992;1109:33–42. doi: 10.1016/0005-2736(92)90183-m. [DOI] [PubMed] [Google Scholar]; (d) Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, Takahashi H, Yanagi Y, Tamaki Y, Koyama H, Kataoka K. Nat. Mater. 2005;4:934–941. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zhang Y, Zhu W, Wang BB, Yu L, Ding JD. J. Pharm. Sci. 2005;94:1676–1684. doi: 10.1002/jps.20310. [DOI] [PubMed] [Google Scholar]; (b) Aathimanikandan SV, Savariar EN, Thayumanavan S. J. Am. Chem. Soc. 2005;127:14922–14929. doi: 10.1021/ja054542y. [DOI] [PubMed] [Google Scholar]; (c) Maeda Y, Taniguchi N, Ikeda I. Macromol. Rapid. Commun. 2001;22:1390–1393. [Google Scholar]; (d) Zhang Q, Clark CG, Jr., Wang M, Remsen EE, Wooley KL. Nano Lett. 2002;2:1051–1054. [Google Scholar]; (e) Sundararaman A, Stephan T, Grubbs RB. J. Am. Chem. Soc. 2008;130:12264–12265. doi: 10.1021/ja8052688. [DOI] [PubMed] [Google Scholar]; (f) You Y, Oupicky D. Biomacromolecules. 2007;8:98–105. doi: 10.1021/bm060635b. [DOI] [PubMed] [Google Scholar]; (g) Morishima Y. Angew. Chem., Int. Ed. 2007;46:1370–1372. doi: 10.1002/anie.200603405. [DOI] [PubMed] [Google Scholar]
- 7.(a) Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. J. Controlled Release. 2003;93:105–120. doi: 10.1016/j.jconrel.2003.06.001. [DOI] [PubMed] [Google Scholar]; (b) El-Sayed MEH, Hoffman AS, Stayton PS. J. Controlled Release. 2005;101:47–58. doi: 10.1016/j.jconrel.2004.08.032. [DOI] [PubMed] [Google Scholar]; (c) Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE. Mol. Pharmacol. 2006;3:340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]; (d) Bae Y, Fukushima S, Harada A, Kataoka K. Angew. Chem., Int. Ed. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]; (e) Li Y, Du W, Sun G, Wooley KL. Macromolecules. 2008;41:6605–6607. [Google Scholar]; (f) Jung J, Lee I-H, Lee E, Park J, Jon S. Biomacromolecules. 2007;8:3401–3407. doi: 10.1021/bm700517z. [DOI] [PubMed] [Google Scholar]
- 8.(a) Gillies ER, Fréchet JMJ. Chem. Commun. 2003;14:1640–1641. doi: 10.1039/b304251k. [DOI] [PubMed] [Google Scholar]; (b) Gillies ER, Jonsson TB, Frechet JMJ. J. Am. Chem. Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]; (c) Gillies ER, Frechet JMJ. Bioconjugate Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]; (d) Apostolovic B, Klok HA. Biomacromolecules. 2008;9:3173–3180. doi: 10.1021/bm800746e. [DOI] [PubMed] [Google Scholar]; (e) Auguste D, Furman K, Wong A, Fuller J, Armes SP, Deming T, Langer R. J. Controlled Release. 2008;130:266–274. doi: 10.1016/j.jconrel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Du J, Tang Y, Lewis AL, Armes SP. J. Am. Chem. Soc. 2005;127:17982–17983. doi: 10.1021/ja056514l. [DOI] [PubMed] [Google Scholar]; (g) Chiu HC, Lin YW, Huang YF, Chuang CK, Chern CS. Angew. Chem., Int. Ed. 2008;47:1875–1878. doi: 10.1002/anie.200704078. [DOI] [PubMed] [Google Scholar]; (h) Du J, Armes SP. J. Am. Chem. Soc. 2005;127:12800–12801. doi: 10.1021/ja054755n. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ghosh S, Irvin K, Thayumanavan S. Langmuir. 2007;23:7916. doi: 10.1021/la700981z. [DOI] [PubMed] [Google Scholar]; (b) Napoli A, Boerakker MJ, Tirelli N, Nolte RJM, Sommerdijk N, Hubbell JA. Langmuir. 2004;20:3487–3491. doi: 10.1021/la0357054. [DOI] [PubMed] [Google Scholar]; (c) Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Nat. Mater. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]; (d) Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K. J. Am. Chem. Soc. 2008;130:6001–6009. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]; (e) Cerritelli S, Velluto D, Hubbell JA. Biomacromolecules. 2007;8:1966–1972. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]; (f) Li Y, Lokitz BS, Armes SP, McCormick CL. Macromolecules. 2006;39:2726–2728. [Google Scholar]; (g) Li C, Madsen J, Armes SP, Lewis AL. Angew. Chem., Int. Ed. 2006;45:3510–3513. doi: 10.1002/anie.200600324. [DOI] [PubMed] [Google Scholar]; (h) Oupicky D, Parker AL, Seymour LW. J. Am. Chem. Soc. 2002;124:8–9. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]; (i) Manickam DS, Oupicky D. Bioconjugate Chem. 2006;17:1395–1403. doi: 10.1021/bc060104k. [DOI] [PubMed] [Google Scholar]; (j) Stevenson M, Ramos-Perez V, Singh S, Soliman M, Preece JA, Briggs SS, Read ML, Seymour LW. J. Controlled Release. 2008;130:46–56. doi: 10.1016/j.jconrel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 10.(a) Torchilin VP. J. Control. Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]; (b) Kataoka K, Harada A, Nagasaki Y. Adv. Drug Deliv. Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]; (c) Adams ML, Lavasanifar A, Kwon GS. J. Pharm. Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]; (d) Jones MC, Leroux J-C. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]; (e) Kumar A, Srivastava A, Galaev IY, Mattiasson B. Prog. Polym. Sci. 2007;32:1205–1237. [Google Scholar]; (f) Mano JF. Adv. Eng. Mater. 2008;10:515–527. [Google Scholar]
- 11.(a) Mariagnanam VM, Zhang L, Bergbreiter DE. Adv. Mater. 1995;7:69–71. [Google Scholar]; (b) Komori K, Matsui H, Tatsuma T. Bioelectrochemistry. 2005;65:129–134. doi: 10.1016/j.bioelechem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lee C-K, Su W-D. Sep. Sci. Technol. 1999;34:3267–3277. [Google Scholar]; (b) Li J-L, Bai R, Chen B-H. Langmuir. 2004;20:6068–6070. doi: 10.1021/la035975m. [DOI] [PubMed] [Google Scholar]
- 13.(a) Heras Alarcon CDL, Pennadam S, Alexander C. Chem. Soc. Rev. 2005;34:276–285. doi: 10.1039/b406727d. references therein. [DOI] [PubMed] [Google Scholar]; (b) Jones RA, Cheung CY, Black FE, Zia JK, Stayton PS, Hoffman AS, Wilson MR. Biochem. J. 2003;372:65–75. doi: 10.1042/BJ20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yin X, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]; (d) Henry SM, El-Sayed MEH, Pirie CM, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:2407–2414. doi: 10.1021/bm060143z. [DOI] [PubMed] [Google Scholar]; (e) You YZ, Zhou QH, Manickam DS, Wan L, Mao GZ, Oupický D. Macromolecules. 2007;40:8617–8624. doi: 10.1021/ma071176p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Saily CMJ, Ryhanen SJ, Lankinen H, Luciani P, Manicini Giovanna., Parry MJ, Kinnunen PKJ. Langmuir. 2006;22:956–962. doi: 10.1021/la052398o. [DOI] [PubMed] [Google Scholar]; (b) Jiang X, Lavender CA, Woodcock JW, Zhao B. Macromolecules. 2008;44:2632–2643. [Google Scholar]; (c) Gohy JF, Antoun S, Jrme R. Macromolecules. 2001;34:7435–7440. [Google Scholar]; (d) Liu SY, Billingham NC, Armes SP. Angew. Chem., Int. Ed. 2001;40:2328–2331. doi: 10.1002/1521-3773(20010618)40:12<2328::AID-ANIE2328>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Klaikherd A, Ghosh S, Thayumanavan S. Macromolecules. 2007;40:8518–8520. [Google Scholar]
- 16.Schild HG. Prog. Polym. Sci. 1992;17:163–249. [Google Scholar]
- 17.Greene TW, Wuts PGM. Protective groups in organic synthesis. 3rd Ed. Wiley-Interscience; New York: 1999. [Google Scholar]
- 18.(a) An Z, Shi Q, Tang W, Tsung CK, Hawker CJ, Stucky GD. J. Am. Chem. Soc. 2007;129:14493–14499. doi: 10.1021/ja0756974. [DOI] [PubMed] [Google Scholar]; (b) Pasparakis G, Alexander C. Angew. Chem., Int. Ed. 2008;47:4847–4850. doi: 10.1002/anie.200801098. [DOI] [PubMed] [Google Scholar]
- 19.Arvinte T, Bui TTT, Dahab AA, Demeule B, Drake AF, Elhag D, King P. Anal. Biochem. 2004;332:46–57. doi: 10.1016/j.ab.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 20.(a) Xia Y, Yin X, Burke NAD, Stöve HDH. Macromolecules. 2005;38:5937–5943. [Google Scholar]; (b) Rzaev ZMO, Dincer S, Piskin E. Prog. Polym. Sci. 2007;32:534–595. [Google Scholar]; (c) Schild HG. Prog. Polym. Sci. 1992;17:163–249. [Google Scholar]; (d) Aoshima S, Kanaoka S. Adv. Polym. Sci. 2008;210:169–208. [Google Scholar]; (e) Tong Z, Zeng F, Zheng X. Macromolecules. 1999;32:4488–4490. [Google Scholar]
- 21.Ostergaard H, Tachibana C, Winther JR. J. Cell Biol. 2004;166:337–345. doi: 10.1083/jcb.200402120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Hou Y, Guo Z, Li J, Wang PG. Biochem. Biophy. Res. Commun. 1996;228:88–93. doi: 10.1006/bbrc.1996.1620. [DOI] [PubMed] [Google Scholar]; (b) Balendiran GK, Dabur R, Fraser D. Cell Biochem. Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]; (c) Boubakari, Bracht K, Neumann C, Grunert R, Bednarski PJ. Arch. Pharm. Pharm. Med. Chem. 2004;337:668–671. doi: 10.1002/ardp.200400620. [DOI] [PubMed] [Google Scholar]; (d) Sun KH, Sohn YS, Byeongmoon Jeong B. Biomacromolecules. 2006;7:2871–2877. doi: 10.1021/bm060512r. [DOI] [PubMed] [Google Scholar]
- 23.Liu YY, Shao YH. Macromol. Biosci. 2006;6:452–458. doi: 10.1002/mabi.200600007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.