Table 2.
Ba ENR inhibition and antibacterial activity for modification of ring B.
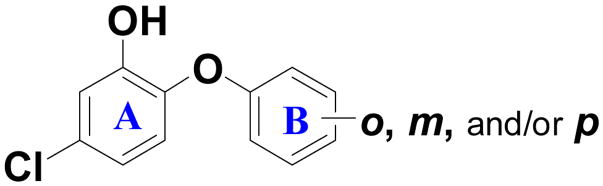
| |||||
|---|---|---|---|---|---|
| Compound | ortho | meta | para | IC50 (μM) or %inhibition at 1μM | MIC[b] (μg/mL) |
| Triclosan | Cl | --- | Cl | 0.6 ± 0.0 | 3.1 |
| 55 | --- | --- | --- | 0.5 ± 0.1 | 32 |
| 3 | --- | --- | OH | 2.6% | >64 |
| 20 | --- | --- | NH2 | 7.1 ± 1.2 | 12 |
| 18 | --- | --- |
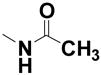
|
1.6% | 111 |
| 19 |
|
--- | --- | 0% | 111 |
| 22 | --- | --- |
|
>12[a] | 4.9 |
| 4 | --- | --- | NO2 | 7.2% | 3.3 |
| 5 | NO2 | --- | --- | 7.7 ± 0.7 | 13.3 |
| 6 | Cl | --- | NO2 | 0.3 ± 0.0 | <0.1 – 3.1 |
| 7 | --- | --- | Ph | >6.25 | 1.9 |
| 32 | --- | --- |
|
15.5% | >145 |
| 33 | --- |
|
--- | 9.9% | 2.3 |
Saturation with inhibitor was not obtained over the concentration range tested. The percent inhibition of BaENR showed a linear response to increasing inhibitor concentrations.
MIC values are against ΔANR B. anthracis.
