Table 3.
Ba ENR inhibition and antibacterial activity for modification of ring B, where ring A is a 2-hydroxy-4-propylphenyl group.
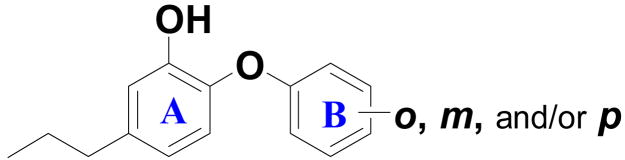
| |||||
|---|---|---|---|---|---|
| Compound | ortho | meta | para | IC50 (μM) or % inhibition at 1μM | MIC[c] (μg/mL) |
| Triclosan | 0.6 ± 0.0 | 3.1 | |||
| 27 | --- | --- | --- | >0.8[a] | 22.8 |
| 8 | --- | --- | NO2 | 1.1 ± 0.1 | 3.4 |
| 9 | --- | NO2 | --- | >0.8[a] | 1.7 |
| 10 | NO2 | --- | NO2 | 3.6 ± 0.8 | 4 |
| 11 | Cl | --- | NO2 | 0.5 ± 0.0 | 1.9–3.1 |
| 12 | Cl | --- | CN | >0.8[a] | 1.8 |
| 15 | Cl | --- | –C(=O)NH2 | 1.1 ± 0.1 | 30.6 |
| 40 | --- | --- | –C(=O)Me | 0.8 ± 0.1 | 13.5 |
| 43 | --- | --- | –S(=O)Me | 3.6 ± 0.3 | 116 |
| 44 | --- | --- | –SO2Me | 2.2 ± 0.3 | 61.3 |
| 42 | --- | --- | CH(OH)Me | 17.1% | 54.5 |
| 21 | --- | --- | NH2 | 8.8 ± 1.0 | >97 |
| 41 | --- | --- | SMe | 0.6 ± 0.0 | 13.5 |
| 45 | --- | N(Me)2 | --- | 12.6% | 13.6 |
| 46 | --- | CF3 | --- | 1.24 | 7.4 |
| 37 | --- | CO2Me | --- | 2.0 ± 0.3 | 3.6 |
| 39 | --- | CO2H | --- | 9.2% | 6.8 |
| 38 | --- | CH2OH | --- | 20.3 ± 1.3 | 12.9 |
| 36 | --- | Ph | --- | 0.5 ± 0.1[b] | 1.9 |
| 34 | --- |
|
--- | 20.6% | 1.2 |
| 35 | --- | --- |
|
10.0% | >151 |
| 48 | --- |
|
--- | >6.25 | 2 |
| 49 | --- | CH2CH2CO2Me | --- | 7.5 ± 1.9 | 7.9 |
| 50 | --- | CH=CHCO2H | --- | 17.9% | 14.9 |
| 51 | --- | CH2CH2CO2H | --- | 17.6% | 60.1 |
| 13 | --- | CH2CH2Br | --- | 6.0 ± 1.5 | 1.7 |
Saturation with inhibitor was not obtained over the concentration range tested. The percent inhibition of BaENR showed a linear response to increasing inhibitor concentrations.
100% inhibition was not observed. The response of the enzyme to inhibitor showed maximum saturation at ~50% inhibition.
MIC values are against ΔANR B. anthracis.
