Abstract
Multiple sclerosis is a chronic demyelinating inflammatory disease of the central nervous system (CNS). As the tissue macrophage of the CNS, microglia have the potential to regulate and be regulated by cells of the CNS and by CNS-infiltrating immune cells. The exquisite sensitivity of microglia to these signals, coupled with their ability to develop a broad range of effector functions, allows the CNS to tailor microglial function for specific physiological needs. However, the great plasticity of microglial responses can also predispose these cells to amplify disproportionately the irrelevant or dysfunctional signals provided by either the CNS or immune systems. The consequences of such an event could be the conversion of self-limiting inflammatory responses into chronic neurodegeneration and may explain in part the heterogeneous nature of multiple sclerosis.
Keywords: multiple sclerosis, pathogenesis, microglia, autoimmunity
INTRODUCTION
The onset, progression, and termination of tissue-specific immune responses are determined in large part by the interactions between the tissue-infiltrating immune cells, the tissue stromal cells, and the tissue macrophage/dendritic cells (Knight and Stagg, 1993; Medzhitov and Janeway, 1998; Lo et al., 1999; Carson and Lo, 2001). Whether the outcome of inflammation is deleterious or beneficial for tissue function and integrity is a function of the complex summation of these interactions. Within the central nervous system (CNS), microglia are the tissue macrophage with demonstrated potential to regulate and be regulated by CNS-infiltrating immune cells (lymphocytes, macrophages, B cells) and by the stromal cells of the CNS (neurons and glia) (Becher et al., 2000; Aloisi, 2001; Carson and Sutcliffe, 2001; Neumann, 2001). Found scattered throughout the CNS, microglia constitute 5–15% of the total brain cell population and thus are strategically located to participate in the onset and progression of CNS inflammatory responses (Kreutzberg, 1996; Streit et al., 1999). Consequently, microglia are thought to play key regulatory and effector roles in the pathogenesis of multiple sclerosis, one of the first chronic CNS disorders recognized to have an inflammatory component.
To what extent, and by what mechanisms, microglia contribute to the onset and progression of MS is a subject of substantial debate. Outlined simplistically, three opposing views have been presented in the literature: (1) activated microglia are highly detrimental for CNS function; in this view, activated microglia cause nonspecific CNS-damage through their production of neurotoxic molecules and immune cell chemoattractants and specific damage as antigen-presenting cells; (2) mcroglial responses may help shape CNS inflammation, but it is the inappropriate presence of CNS-infiltrating macrophages/dendritic cells and anti-myelin T-cell responses that are the prime villains driving MS pathology; and (3) activated microglia limit CNS inflammation by the production of neurotrophic and immunosuppressive factors and may even drive antigen-specific neuroprotective T-cell responses.
The number of variables in MS that remain to be defined hinders the resolution of this debate. Namely, if MS is a single disease, what accounts for the extreme heterogeneity of clinical symptoms and histopathology? If MS is a collection of symptomatically related disorders, can the same pathogenic mechanisms be assumed to operate in the different forms? Are the myeloid cells in and around MS lesions microglia or CNS-infiltrating macrophages? To what extent are microglia responding to pathogenic signals from CNS cells versus immune cells in each stage of MS? Which of the broad range of effector functions described for microglia in both in vitro and in animal models are induced during the initiation versus the progression of MS? In this review, we explore these issues by examining in detail what is currently known about the pathogenesis of MS and the ability of microglia to interact with both the immune system and the CNS. Finally, we speculate as to how these microglial interactions may contribute to the relapsing, remitting, and progressive courses of MS.
WHAT IS MULTIPLE SCLEROSIS?
MS is the most common inflammatory demyelinating disease of the CNS, afflicting 250,000–350,000 individuals in the United States alone (Wingerchuk and Weinshenker, 2000; Keegan and Noseworthy, 2002). Characterized as a disease of young adults, it develops most commonly between the ages of 20–40. While MS is twice as frequent in women than in men, men are at greater risk of developing more severe forms of MS (Weinshenker et al., 1991). Most individuals experience a relapsing-remitting form of the disease, with clinical episodes followed by periods of full or partial remission of symptoms. The frequency, severity, and types of clinical episodes vary widely between individuals. Initial symptoms can include transient bouts of extreme fatigue, vertigo, optic neuritis, and weakness and/or numbness in extremities. With time, symptoms become much more severe and disabling and can include debilitating ataxia, paraparesis, painful limb spasms, and cognitive impairment. After several years, the progression of disease also tends to convert from an episodic to a progressive neurological deterioration, although 10–15% of patients experience a progressive deterioration from the initial onset of symptoms.
ETIOLOGY OF MS
To understand how microglial activation begins in MS, ideally the nature and timing of the event(s) or agent(s) initiating the disease would be defined. Unfortunately, the causes of MS have eluded conclusive identification, despite more than a century of research. However, both genetic and environmental components contribute to disease susceptibility, as most clearly demonstrated by twin studies (Noseworthy, 1999; Wingerchuk and Weinshenker, 2000). While the risk of contracting MS is 0.1–0.4% in the general population, concordance between dizygotic twins is 3–5% and between monozygotic twins is 25–30%. Although analysis is complicated by the strong environmental influence on susceptibility (70–75%), linkage studies indicate susceptibility is polygenic, with the HLA DR2 locus as the most consistently implicated locus. Based on this linkage to a human MHC class II locus, the genetic predisposition for MS has often been suggested to be due to autoimmune responses generated against an HLA DR2 presented antigen(s).
Epidemiological studies following individuals moving between areas with different levels of risk of MS suggest that there may be a “window of opportunity” for environmental factors to influence MS susceptibility (Weinshenker et al., 1991; Wingerchuk and Weinshenker, 2000). If individuals move before puberty, their adult risk level is the same as that of the general population in the area they move into, but if they move after puberty, individuals retain the risk level of their area of origin. These and other studies have been used to suggest that exposure to a viral, bacterial, or environmental antigen during childhood may be the priming event for MS, if this antigen is structurally similar to an antigen expressed in CNS myelin (a molecular mimic) (Fujinami and Oldstone, 1985; Aw 1986; Gran et al., 1999; Schmidt, 1999; Liblau and Gautam, 2000; Stohlman and Hinton, 2001). Immune responses generated against a molecular mimic would thus prime the immune system to generate an anti-myelin autoimmune response. Onset in young adulthood has then been suggested to be due to a reactivation of this immune response by antigen-specific (i.e., viral/bacterial infection) or by nonspecific mechanisms (local chemokine/cytokine reactivation of bystander T cells).
CLINICAL DEFINITION OF MS
Without a clear understanding of the events initiating MS, identification of the pathogenic mechanisms operating in MS depends on three factors: accurate diagnosis of the disease, careful documentation of the pathological lesions at all stages of the disease, and the development of accurate animal models to test which pathological mechanisms are capable of inducing MS-like pathologies. Unfortunately, a definitive laboratory diagnostic test for MS does not exist. The diagnosis of MS is made only if all three of the following criteria are met: (1) the occurrence of two or more attacks of clinical disability separated in time, (2) confirmation of two or more lesions in separate locations within the white matter tracts of the CNS, and (3) all other causes of these clinical signs must be excluded. Cerebrospinal fluid (CSF) abnormalities are often used to support a diagnosis of MS but are not by themselves sufficient for diagnosis. For example, higher levels of immunoglobulin G (IgG) in CSF than serum and/or the presence of oligoclonal immunoglobulin bands in the CSF are often found in the mid and late stages of MS. However, these same signs can be found in such distinct diseases as acute disseminated encephalomyelitis, sarcoidosis and systemic lupus erythematosus (Scolding, 2001). Although a variety of acute, focal demyelinating disorders can also temporarily masquerade as MS, with disease progression these diseases can usually be appropriately diagnosed (Scolding, 2001).
MS PATHOGENESIS
Using these consensus diagnostic criteria to identify patients with confirmed acute MS, Lucchinetti et al. 2000 performed a large-scale analysis of MS lesions from biopsy and autopsy material. Two striking observations were made in this study. First, all the analyzed MS demyelinating lesions could be grouped into four distinct patterns, and second, all lesions from a single individual belonged to only one pattern. By implication, these observations suggest that symptomatic MS results from four distinct types of pathogenic mechanisms.
Interestingly, the authors note that lesion patterns I and II were reminiscent of the autoimmune destruction observed in T-cell-mediated and antibody-augmented forms of experimentally induced autoimmune encephalomyelitis (EAE). In both types of lesions, myelin loss was seen predominantly in perivenous locations and the edges of the lesions were sharply demarcated. Active demyelination was associated with lymphocytic and myeloid inflammation but was coupled with apparent remyelination and preservation of oligodendrocytes. In damaged myelin sheaths, all myelin proteins were lost at the same rate. The primary difference between patterns I and II was the deposition of IgG and activated complement in pattern II lesions and the absence of such deposition in pattern I lesions.
By contrast, the pathologies of pattern III and IV lesions were noted to be reminiscent of viral or toxin-induced oligodendrocyte dystrophy. Most notably, unlike lesions of patterns I and II, oligodendrocyte death was quite prominent in both pattern III and IV lesions. The apparent method of death differed between the two lesions. In pattern III lesions, oligodendrocytes displayed nuclear condensation and fragmentation characteristic of apoptotic death, while those in pattern IV lesions displayed features suggesting necrotic death. The pattern of demyelination also differed between lesion patterns III and IV. In pattern III lesions, there was preferential loss of MAG from the damaged myelin sheaths, while in pattern IV lesions all myelin proteins were lost in similar proportions. Significantly, pattern III lesions were not centered around veins and lesions borders were diffuse and irregular. Deposition of IgG and activated complement was not detected in either pattern III or IV lesions. However, both lesion types contained lymphocytes and activated myeloid cells.
It must be cautioned that the number of cases examined were too few to make conclusive associations between pathology and clinical disease; however, some trends did emerge. Among the 83 cases analyzed (51 from autopsy, 32 from biopsy), pattern II lesions were most common and tended to be found in patients with classical relapsing, remitting disease and pattern IV lesions were found only in patients with primary progressive disease. Intriguingly, pattern III lesions were only observed in patients with clinical courses of less than 8 weeks before biopsy or death. It is unknown whether pattern III lesions may represent a distinct stage of “starter” lesions capable of converting into another pattern. However, because pattern III lesions types were not found in individuals with other lesion patterns, the evolution from pattern III to another lesion form would need to be synchronous and rapid. Although impractical and complicated by ethical concerns, continued biopsies from individuals with pattern III lesions would be needed to resolve this issue.
MS IS NOT JUST A DISEASE OF MYELIN
Axonal transection was not quantified in the studies by Lucchinetti et al. (2000). However, several studies have found that axonal damage and transection are major features of many MS lesions that occur in brain regions apparently unaffected by inflammation and in neurons with apparently intact myelin sheaths (De Stefano et al., 1998, De Stefano et al., 2001; Trapp et al., 1999a,Trapp et al., 1999b; Ciccarelli et al., 2001; Brex et al., 2002). Examining lesions from patients with different durations of clinical disease (2-week to 26-year duration), either histologically upon autopsy or by magnetic resonance imaging and spectroscopy during the course of the disease, it is now clear that axonal transection begins early in MS disease process. Furthermore, in actively inflamed lesions, the abundance of transected axons per lesion does not change with the duration of clinical disease. Within lesions, axonal damage is likely to be caused by two mechanisms: the presence of inflammatory products, and sustained demyelination. The causes of axonal damage occurring in noninflamed normal appearing brain regions are less clear, but it is likely that Wallerian degeneration of neurons damaged within the inflamed lesions are large contributors to this pathology. As yet, it is also unknown whether microglial clustering and the global expression of activation markers by microglia displaying an “unactivated” ramified morphology in these noninflamed “normal” regions precedes or is a consequence of axonal damage (Banati et al., 2000; Gobin et al., 2001). Altogether, these and other studies suggest that the initial episodic clinical symptoms are the consequences of reversible inflammatory events, while progressive disability is due to ever increasing levels of axonal damage and neuronal death (De Stefano et al., 1998, De Stefano et al., 2001; Trapp et al., 1999a, b; Ciccarelli et al., 2001; Brex et al., 2002).
IS MS A SINGLE DISEASE?
From the analysis of Lucchinetti and colleagues, it would appear that MS is a collection of related disorders with similar clinical outcomes. However, MS may also be a single disorder with different pathogenic mechanisms being induced in different individuals. For instance, the propensity of an individual to develop myelin-specific antibody responses may influence the propensity to develop pattern II lesions. Alternatively, as an individual’s immune response “matures” and antibody responses develop, rapid deposition of IgG and activated complement may occur at most lesion sites within a short period. For this reason, it would be informative (but ethically questionable) to examine sequential lesion biopsies from individuals as they convert from oligoclonal immunoglobulin band negative CSF to oligoclonal immunoglobulin band positive CSF. Even the variations in oligodendrocyte survival (preservation of oligodendrocytes in patterns I and II), and manner of death (apoptotic in pattern III and necrotic in pattern IV) may reflect individual differences in the intensity of similar pro-inflammatory/neuroprotective immune responses. Finally, there are numerous examples of the same agent causing vastly different effects, depending on concentration or timing of exposure. For example, factors such as NGF and BDNF can promote such opposite consequences for cells such as survival or death, depending on the differentiation state of the responding cell (Lee et al., 2001). Similarly, toxic agents such as lysolecithin can cause demyelination without oligodendrocyte death at low concentrations but can induce destruction of oligodendrocytes and axons at higher concentration (Fressinaud et al., 1996).
INITIATION OF CNS INFLAMMATION
In this heterogeneous disease, one feature was common to all lesion patterns and all forms of MS: lymphocytes and activated myeloid cells were always found within areas of active demyelination. Activated T cells will readily infiltrate the CNS in search of their target antigen, even in the presence of an intact BBB, but if these T cells are specific for non-CNS antigens, they will not remain within the CNS (Wekerle, 1997; Matyszak, 1998; Lo et al., 1999). Thus, the universal presence of lymphocytes within active MS lesions, indicates that these T cells are being actively and specifically retained within myelinated regions of the CNS. Two types of mechanisms may retain T cells within a tissue: T cells can be nonspecifically retained by locally produced chemokines or specifically retained by local macrophage/dendritic cells presenting the target antigen of the T cell.
INDIRECT AND BYSTANDER RECRUITMENT OF LYMPHOCYTES
The production of numerous macrophage and T-cell chemoattractants (e.g., IP-10, MIP-1, MIP-2, CCL19, MCP-1, MCP-2) is among the earliest microglial (and astrocytic) responses to injury, pathogens (bacterial and viral), alterations in the blood-brain barrier, or even abrupt changes in neuronal activity (Sun et al., 1997; Aschner et al., 1999; Ransohoff, 1999; Aloisi et al., 2000a; Streit, 2000). These and other chemokines have also been detected in MS and in inflammatory infiltrates of autoimmune and viral models for MS (Ransohoff, 1999). Rodent studies have shown that these chemoattractants are highly effective in recruiting macrophages into the CNS but by themselves are insufficient to retain active T-cell responses within the CNS. Transgenic expression of potent T-cell chemoattractants such as CCL19 and CCL21 within the CNS failed to induce lymphocyte infiltration (Chen et al., 2002). The ability of transgenic CCL21 expression in the CNS to induce a strong innate immune response (neutrophils, eosinophils, and macrophages) and disrupt the blood-brain barrier demonstrates that the circulating immune system has the potential to sense the chemokine within the CNS. This inability of T-cell chemoattractants to cause CNS lymphocyte accumulation appears to be specific to the CNS. Transgenic expression of this same chemokine in the pancreas was by itself sufficient to recruit T cells, initiate de novo formation of lymph node-like structures and promote progression of autoimmunity (Fan et al., 2000; Ploix et al., 2001).
By contrast, T cells specific for non-CNS antigens can be nonspecifically recruited to the CNS, as bystanders to a myelin-specific response (Krakowski and Owens, 2000; Bauer et al., 2001). While the bystander T cells were presumably recruited by the chemoattractant factors produced within the CNS, bystander recruitment apparently requires additional factors and events induced by myelin-specific T cells. Altogether, these results suggest that while some of the lymphocytes found in MS lesions may be recruited and retained by bystander mechanisms, the presence of bystander T cells is dependent on the presence of antigen-specific interactions between myelin-specific T cells and antigen-presenting cells present within the lesions.
ANTIGEN-SPECIFIC RECRUITMENT OF LYMPHOCYTES
In most tissues, resident macrophage/dendritic cells are capable of transforming from tissue phagocytes and cytokine producers to antigen-presenting cells capable of driving T-cell proliferation and differentiation (Knight and Stagg, 1993; Medzhitov and Janeway, 1998; Lo et al., 1999). Here we will concentrate on antigen-presentation events leading to CD4+ T-cell activation for four reasons: linkage studies have implicated an MHC class II locus in MS susceptibility, lesions resembling those in MS are induced by CD4+ T-cell responses in EAE, CD4+ T cell-mediated responses are required for antibody mediated responses and CD4+ T cells play pivotal roles in CD8+ T cell-induced demyelination (Knight and Stagg, 1993; Mos-mann and Sad, 1996; Lo et al., 1999; Lane et al., 2000; Keegan and Noseworthy, 2002).
In brief, tissue macrophages/dendritic cells become activated by nonspecific “danger” or inflammatory signals to capture antigen from pathogens or cellular debris (Knight and Stagg, 1993;Medzhitov and Janeway, 1998; Lo et al., 1999). Whole proteins are not recognized by T cells. Therefore, an antigen-presenting cell must proteolytically process the captured antigen by reducing the protein into antigenic peptides. After antigen capture, tissue macrophages/dendritic cells migrate to the draining lymph nodes where they present antigen to T cells and initiate primary immune responses. CD4+ T cells are activated by antigen presented in the binding cleft of MHC class II and CD8+ T cells by antigen bound to MHC class I. Upon activation, these T cells leave the lymph node and circulate throughout the body in search of their target antigen. Within the target tissue, local antigen-presenting cells, tissue-infiltrating macrophages, and dendritic cells present the target antigen and thus retain the activated T cells at the site of damage or pathology. The functional outcome of this series of events can be diverse. Antigen presentation can lead to T-cell proliferation, differentiation of effector function, T-cell anergy, or even T-cell apoptosis. Many factors, including the affinity of the TCR for its antigen, the expression levels of MHC class II, co-stimulatory molecules, and chemokines have been shown to determine the outcome of this series of interactions between a T cell and its antigen-presenting cell. In the inflamed CNS, three populations have the potential to act as antigen-presenting cells: parenchymal microglia, perivascular macrophages, and CNS-infiltrating inflammatory macrophages/dendritic cells. In MS, expression of molecules necessary to present antigen (MHC and co-stimulatory molecules) is detected on ramified myeloid cells surrounding plaques, on non-myelin- and myelin-containing myeloid cells within plaques. Expression of these molecules is even detected in microglia in normal-appearing MS brain tissue (Sriram and Rodriguez, 1997; Aloisi, 2001; Gobin et al., 2001). Thus, the question in MS is: which populations present antigen during MS and is the outcome dependent on the identity of the antigen-presenting cell?
ARE MICROGLIA OR INFILTRATING IMMUNE CELLS ACTING AS ANTIGEN-PRESENTING CELLS IN MS LESIONS?
The identities of the myeloid cells within MS lesions are uncertain. Depending on the study, these myeloid cells have been variously identified as resident microglia or CNS-infiltrating macrophages based primarily on their morphology (Trapp et al., 1999b; Lucchinetti et al., 2000; Keegan and Noseworthy, 2002). Cells with ramified morphologies have been generally labeled resident microglia and those with amoeboid morphologies, macrophages. The unreliability of morphological based identification is demonstrated by a simple series of experiments. When fluorescently labeled rodent microglia were placed on cultured rodent brain slices, they developed both ramified and amoeboid morphologies that appeared to be dependent on brain region and type of tissue damage (Hailer et al., 1997). Conversely, ramified morphologies do not necessarily represent micro-glia. Fluorescently labeled myeloid dendritic cells when injected into the CNS parenchyma retain a ramified morphology even after migration into the CNS (Carson et al., 1999b). Similarly, in autoimmune responses outside of the CNS, both macrophages and dendritic cells can display ramified rather than amoeboid morphologies (Scott et al., 1994; Ploix et al., 2001). At present, there are no definitive markers that distinguish activated microglia from macrophages in histological sections. Activated microglia express all of the common macrophage markers, including Fc receptor, CD11b, F4/80, and CD45 (Kreutzberg, 1996). While CD14 expression has often been used to differentiate microglia from macrophages, microglia can be induced to express CD14 (Becher et al., 1996). Therefore, from histological sections alone, neither the identity of the myeloid cells in the MS lesions nor their relative functions can be determined. However, the relative functions of these cells are being dissected in rodent models of autoimmune and virus-induced demyelination.
In rodent models, irradiation bone marrow chimeras have been used to show that parenchymal microglia are phenotypically distinct from the macrophage populations located in nonparenchymal CNS sites (the meninges, subarachnoid spaces, choroid plexus, and perivascular regions) or macrophages that acutely infiltrate the CNS in response to tissue damage or inflammatory signals. In these studies, the host bone marrow is killed by lethal irradiation and replaced with bone marrow from a genetically distinct donor. These studies find that, in contrast to macrophage populations located in nonparenchymal sites that are relatively short-lived and replenished by bone marrow-derived cells every few days, microglia in the healthy CNS are long-lived and rarely replenished (Matsumoto and Fujiwara, 1987; Hickey and Kimura, 1988). In comparison with the macrophage populations, parenchymal microglia also express very low levels of CD45, a protein tyrosine phosphatase expressed by all nucleated cells of hematopoietic lineage (Ford et al., 1995; Carson et al., 1998). In response to chronic pathology or robust in vivo inflammatory signals, microglial levels of CD45 increase to levels intermediate between those of unactivated microglia or of mature macrophages (Ford et al., 1995; Carson et al., 1998). While this gradation in levels of CD45 expression can be easily quantitated by flow cytometric analysis of cell suspensions, distinguishing activated microglia from macrophages in histological sections on the basis of differential CD45 immunoreactivity is less certain.
These phenotypic differences between parenchymal microglia and nonparenchymal macrophages have largely been confirmed in humans, most notably, in bone marrow transplant studies (Unger et al., 1993; Bauer et al., 2001). Bone marrow donor cells that are well matched for histocompatibility antigens can be mismatched by gender and still used in transplants. In these situations when male donor cells are transplanted into female recipients, donor cells can be identified by in situ hybridization analysis using Y-chromo-some-specific probes. When CSF and brain tissue were examined by these methods after donor cell reconstitution of the peripheral immune system, parenchymal microglia were found to be of the recipient genotype, while cells in nonparencyhmal sites and in the CSF were found to be of the donor genotype.
ACTIVATED MICROGLIA EXPRESS THE MOLECULAR MACHINERY REQUIRED TO PRESENT ANTIGEN
Under nonpathological conditions, microglia in the CNS of healthy rodents and humans are largely MHC class I and II negative and thus incapable of acting as antigen-presenting cells (Kreutzberg, 1996; Aloisi, 2001). However, microglia appear poised to acquire the ability to present antigen. Even in the absence of MHC expression, microglia often express very low levels of co-stimulatory molecules B7.2 and CD40 (Becher and Antel, 1996; Carson et al., 1998; Tan et al., 1999). However, it is important to note that the expression levels of B7.1, B7.2, and CD40 varied from low to undetectable among different mouse strains (M.J. Carson, unpublished observations). It is likely that similar variations exist in the diverse human population. Microglia do become MHC class II positive in response to a wide variety of signals including increasing age and blunt trauma. In the presence of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), the expression of ICAM-1, CD40, B7.2, and B7.1 expression is dramatically upregulated (Aloisi, 2001; Nguyen and Benveniste, 2002). Thus, as one of their most universal responses to pathology, microglia acquire the potential to interact with T cells in an antigen-specific manner.
CAN MICROGLIA INITIATE ANTIGEN-DRIVEN IMMUNE RESPONSES?
Direct measures of the microglial potential to initiate and sustain antigen-specific T-cell responses suggest that their potential is dependent on their prior history. Microglia isolated from healthy adult rodent CNS were found to be ineffective as antigen-presenting cells, if tested immediately ex vivo, consistent with their MHC class II negative status (Ford et al., 1995; Carson et al., 1998, Carson et al., 1999a; Matyszak et al., 1999). If these microglia were acutely treated with lipopolysaccharide (LPS) and IFN-γ, these cells remained CD45low but became MHC class II positive. They also remained much less effective than splenic macrophages or CNS-infiltrating macrophages at stimulating naive or primed T-cell proliferation or interleukin-2 (IL-2) production. However, if microglia were activated by in vivo pathology (during EAE or transgenic CNS overexpression of IL-3) to become not only MHC class II positive, but also CD45intermediate, a slightly different picture was observed (Carson et al., 1999a; Juedes and Ruddle, 2001). These microglia were still relatively poor at stimulating T-cell proliferation but were found to be very potent at promoting T-cell production of pro-inflammatory Th1 cytokines. Indeed, microglia were found to be much more potent at driving Th1 T-cell effector function than the other macrophage populations examined in these studies because in the absence of antigen-induced T-cell proliferation fewer T cells were producing greater levels of IFN-γ.
The mechanisms by which T-cell proliferation was inhibited apparently differed in the two situations. Activated CNS APCs isolated from a MOG model of EAE inhibited T-cell proliferation by the production of nitric oxide (NO), while microglia isolated from IL-3 transgenic mice inhibited proliferation by the production of prostaglandins (Carson et al., 1999a; Juedes and Ruddle, 2001). The production of NO and prostaglandins not only directly inhibit T-cell proliferation, but these substances also repress the ability of microglia, macrophages and dendritic cells to present antigen by suppressing their expression of MHC class II and costimulatory molecules (Lo et al., 1999; Minghetti et al., 1999; Willenborg et al., 1999). Therefore, in vivo, the net effect of microglia antigen-presentation would be twofold: a strong brief burst of Th1 cytokine production coupled with the inhibition of antigen-presenting function of surrounding microglia, CNS-infiltrating macrophages, and/or dendritic cells.
By contrast, rat microglia activated in vivo by graft vs host disease were also activated to become MHC class II positive and CD45intermediate. When assayed in vitro, these activated microglia not only failed to induce T-cell proliferation and IL-2 production, they induced T-cell apoptosis (Ford et al., 1996). Because these activated microglia did not express co-stimulatory factors B7.2 and B7.1, this result was not unexpected. However, it does reveal that not all microglial activation states are equal.
Analysis of cultured microglia further illustrates how strongly environmental factors can influence microglial effector function. Even if maintained in the presence of other CNS cells and in the absence of known stimulatory factors, cultured microglia already display a quasi-activated phenotype (Becher and Antel, 1996; Carson et al., 1998, Carson et al., 1999a; Aloisi, 2001). They express intermediate levels of CD45 and, dependent on culture conditions, may be weakly positive for co-stimulatory factors and MHC class I and II. Using these cells, one set of studies has suggested that weakly activated microglia induce T-cell anergy or unresponsiveness, despite low level expression of co-stimulatory molecules, CD40 and B7.2 (Matyszak et al., 1999). If cultured microglia were treated with granulocyte-macrophage-colony-stimulating factor (GM-CSF) and IFN-γ, molecules found in abundance in inflammatory infiltrates, these cells rapidly transformed into potent antigen-presenting cells (Matyszak et al., 1999). Similar culture conditions also cause microglia to express high levels of the dendritic cell markers, CD11c and Dec-205 (Santambrogio et al., 2001). Even in the absence of stimulatory factors, a small percentage of cultured microglia spontaneously express the dendritic cell marker, Dec-205 (Carson et al., 1998). This developmental plasticity is not just a feature of microglia derived from neonatal glial cultures. Microglia isolated from adult murine CNS cultured with astrocytes and GM-CSF also begin to express dendritic cell antigens (Santambrogio et al., 2001).
CAN MICROGLIA PROCESS ANTIGEN?
Most of these studies examined the ability of microglia to present pre-processed peptide antigens. However, in vivo, it is more likely that antigen would be processed after phagocytosis of cellular debris. While unactivated cultured microglia could not efficiently process antigen, upon activation by IFN-γ or by viral infection, microglia could be stimulated to efficiently process and present endogenous (viral antigens) and exogenous myelin antigens (Olson et al., 2001a). Several studies also suggest that myelin phagocytosis itself can prime microglia to become antigen-presenting cells (Cash et al., 1993; Cash and Rott, 1994). Having the ability to process antigen, microglia have the potential to participate in the epitope switching that has been observed in viral models of CNS demyelination. Here, an initial immune response is generated against viral antigens. However, as myelin damaged occurs (as a consequence of oligodendrocyte infection and/or death or inflammatory products), macrophages and microglia phagocytose the debris. Presentation of the myelin epitopes causes the immune response to shift from a viral-specific response to a myelin-specific response.
While microglia in vitro and in vivo have been demonstrated to phagocytose damaged or complement-coated myelin readily, the studies of Prineas et al. (2001) suggest that this process may be subject to additional levels of regulation. In these studies, myelin lesions were characterized in autopsy material from two patients in which remitting disease had converted to a severe progressive from of MS. In chronic lesions lacking signs of active inflammation, myeloid cells identified as microglia failed to phagocytose myelin debris completely despite the presence of disrupted myelin and C3d (an opsonin formed during complement activation). Rather, fragments of myelin were detected on the cell surface. Such “frustrated” phagocytosis was not observed in acute MS lesions. In acute, actively inflamed lesions, myelin debris was internalized. Thus, the propensity to process antigen may be actively regulated by the process of inflammation (Cash et al., 1993; Cash and Rott, 1994; Smith, 2001).
It should also be stressed that antigen-presenting cell phenotypes are not the only or default differentiation pathways for microglia. Exposure to bacteria components, antibody and/or activated complement coated cellular structures/debris induces microglial activation through pattern recognition, Fc and complement receptors, respectively (Becher et al., 2000; Aloisi, 2001). In response to these stimuli, microglia (both in vitro and in vivo) produce reactive oxidative products, metal-lomatrix proteases, pro-inflammatory cytokines and phagocytose antibody/complement-coated targets.
WHEN DO MICROGLIA HAVE THE OPPORTUNITY TO PRESENT ANTIGEN?
As immature dendritic cells develop into mature dendritic cells, their ability to process antigen is lost, while their ability to present antigen increases (Lo et al., 1999). Upon maturation, dendritic cells home to the draining lymph node. Although in vitro studies suggest that microglia can express dendritic cell markers and present antigen, there is currently no evidence that microglia are capable of homing to the cervical lymph nodes. Thus, microglia must wait for T cells to infiltrate the CNS.
Although the CNS is isolated from the immune system by the blood-brain barrier, activated T cells easily infiltrate the CNS (Hickey and Kimura, 1988; Bauer et al., 2001). Thus, microglia have the opportunity to retain and restimulate T cells already primed to differentiate into Th1 cell producing pro-inflammatory cytokines (IL-2, IFN-γ, TNF-α) or into Th2 cells producing cytokines that support antibody-mediated responses (IL-4, Il-5, IL-10, 1L-13) (Mosmann and Sad, 1996).
However, it is has only recently been realized that antigen-inexperienced, and thus unprimed T cells (naive T cells) can enter the CNS. Unlike primed T cells that are found infiltrating all tissues, naive T cells are found preferentially in lymph nodes (Lo et al., 1999). They express only very low levels of CD44, a molecule that increases efficiency of extravasation and high levels of CD62L, an integrin that binds them to the high endothelial venules within the lymph node. The ratios of these two markers are reversed on antigen-activated cells. Using these and other markers, Krakowski and Owens (2000) demonstrated that naive T cells expressing a transgenic TCR specific for an antigen not found in rodents (ovalbumin) could accumulate in the CNS along with myelin-specific T cells during EAE, presumably by bystander recruitment.
Antigen-inexperienced T cells can also enter the CNS even in the absence of an ongoing CNS inflammatory response or a disrupted BBB (Brabb et al., 2000). It has been previously shown that in response to lymphopenia (lymphocyte deficiency) caused by either genetic or environmental factors, T cells proliferate in an antigen-independent manner until the circulating T-cell population has been restored to its normal “setpoint” (Ploix et al., 2001). This antigen-independent proliferation is called homeostatic proliferation. Recently, Ploix and colleagues demonstrated that CD4+ T cells become transiently semi-activated by homeostatic proliferation (CD62Llow, CD44intermediate/high) and acquire the ability to enter the CNS (Ploix et al., 2001; and unpublished observations). However, these T cells do not express other T-cell activation markers, such as CD40 ligand (also called CD154) and thus cannot interact with microglia through CD40, the receptor for CD40 ligand.
T CELLS CAN SHAPE MICROGLIAL FUNCTIONS
While most studies have focused on the ability of microglia to shape T-cell effector function, fewer studies have examined the reverse angle. These studies show that T cells not only shape microglial function, but that the resulting microglial phenotype is dependent on the differentiation state of the T cell. During graft versus host disease, direct in vivo contact between previously unactivated microglia and alloreactive T cells by itself was sufficient to induce microglial activation (increased phagocytosis and expression of CD45 and MHC class II) (Ford et al., 1996). In organotypic cultures, Th1 T cells were able to increase micro-glial expression of B7.1 and decrease expression of B7.2, while Th2 cells could only decrease B7.2 without increasing B7.1 expression (Wolf et al., 2001). By contrast, Th2 cells, but not Th1 cells, decreased microglial expression of ICAM-1, and thus decreased the efficiency of antigen-presentation by microglia. Analyzed after ex vivo isolation from adult murine CNS, antigen-specific interactions between microglia and Th1 T cells, but not Th2 T cells, strongly induced microglial expression of MHC class II, CD40 and CD54 (Aloisi et al., 2000b).
Even in the absence of antigen, T cells can alter microglial phenotype and function. As with other antigen-presenting cells, interaction between CD40 ligand expressed by activated T cells and CD40 expressed by microglia activates microglia to express higher levels of MHC class II, CD40, B7.2, and B7.1 and to produce IL-12 (Aloisi et al., 1999; Tan et al., 1999; Nguyen and Benveniste, 2002). The production of IL-12 in turn increases T-cell production of IFN-γ. Ligation of CD40 on the surface of microglia also increases microglial responses to other signals. For example, CD40 ligation dramatically increases the β-amyloid and IFN-γ-induced production of TNF-α by microglia (Tan et al., 1999; Nguyen and Benveniste, 2002).
DO MICROGLIA PRESENT ANTIGEN IN VIVO?
Because of the difficulty of separately measuring the relative abilities of microglia and macrophages to present antigen in vivo, most of the studies we have described examined these functions in vitro. To specifically examine the antigen-presenting function of microglia in vivo, some groups have selectively depleted the peripheral macrophage populations by treating animals with mannosylated liposome-encapsulated dichloromethyline diphopho-nate (Cl2MDP) (Bauer et al., 1995; Tran et al., 1998). Without a peripheral myeloid population, an effective immune response cannot be generated after peripheral immunization of myelin proteins in adjuvant (the active immunization form of EAE). By contrast, depletion of peripheral macrophages by this method did not prevent T-cell extravasation or Th1 cytokine production after adoptive transfer of myelin-specific T cells, but it did inhibit demyelination, TNF-α production, T-cell infiltration into the CNS parenchyma, and induction of clinical EAE. These studies suggest the ability of microglia to present antigen is neither identical nor as potent as other antigen-presenting cells. These studies also show that parenchymal infiltration of T cells and/or activated macrophages are required for full-blown EAE. However, these studies do not distinguish between the roles of activated macrophages as initiators versus effectors of disease (antigen-presenting cells versus phagocytes/producers of neurotoxic molecules). An additional complication is that the effects of Cl2MDP are not limited to peripheral macrophages and may alter microglial function.
Another method to examine the antigen-presenting functions of microglia versus other myeloid populations in vivo separately is to use irradiation bone marrow chimeras. In these studies, rodents are generated in which only the antigen-presenting cells derived from the host (the radiation resistant microglia) or only the antigen-presenting cells derived from the donor bone marrow (macrophages and dendritic cell populations) express the appropriate MHC to interact with MBP-specific T cells (Hickey and Kimura, 1988; Myers et al., 1993). Using this type of a model, one set of studies has clearly shown that induction of EAE by the adoptive transfer of MBP-specific T cells was not dependent on antigen-presentation by microglia in rats (Hickey and Kimura, 1988). Another study using mice confirmed that the radiation insensitive component of the CNS (presumably microglia) was not required for induction of EAE. However, this study also demonstrated that the radiation-insensitive component was by itself sufficient to support induction of EAE (Myers et al., 1993).
DOES THE CNS ENVIRONMENT LIMIT ANTIGEN-PRESENTATION?
Finally, when characterizing the antigen-presenting function of microglia in vivo, or after their isolation in vitro, it is important to distinguish between functions that are inherent microglial traits and those that are determined by the CNS microenvironment. For example, although perivascular macrophages are potent antigen-presenting cells in vitro, studies in both human and rodent have long shown that the CNS does not readily support primary immune responses, but does support secondary immune responses (Matyszak, 1998; Perry, 1998). For example, greater than 90% of circulating CD4 T cells in MBP-T-cell receptor (TCR) transgenic mice are specific for myelin-basic protein (MBP) (Brabb et al., 2000). If the MBP-TCR T cells are nonspecifically activated in the periphery, they will enter the CNS and induce clinical EAE. However, the mere presence of autoreactive T cells (even in high abundance) is insufficient to induce autoimmunity. If these mice are kept in specific pathogen-free conditions, the MBP-TCR T cells fail to accumulate in the CNS or cause EAE. This is not because MBP-TCR T cells fail to enter the CNS. Naive MBP-TCR T cells can be found within the CNS, but ex vivo analysis reveals that they have been tolerized and cannot be subsequently activated by antigen (Brabb et al., 2000). Currently, it is unknown whether this tolerization is due to naive T-cell interactions with local antigen-presenting cells, or due to the CNS microenvironment.
This question has been more directly examined in two related mouse models. In the first, dendritic cells were injected intracranially into the CNS of adult mice (Carson et al., 1999b). The ability of these dendritic cells to retain and activate T cells was lower for those located within the CNS parenchyma than for those in nonparenchymal sites (perivascular spaces, the subarachnoid spaces and ventricles). Within the parenchyma, infiltration occurred primarily in the white matter tracts, even in the absence of myelin-directed responses. Unexpectedly, while T-cell recruitment was antigen dependent, and thus mediated by CD4+ T cells in the models used in the study, CD8+ T cells accumulated in greater numbers than CD4+ T cells. Despite white matter inflammation, demyelination did not occur and mice appeared clinically healthy. These results indicate that myelin-restricted inflammation need not be initiated by a myelin-specific antigen and can be benign.
To determine whether the CNS could limit a myelinspecific response, a model of molecular mimicry was used (Evans et al., 1996). In this model, the nucleoprotein (NP) of lymphocytic choriomeningitis virus (LVMV) was expressed in oligodendrocytes. Peripheral infection of these mice with LCMV led only to a mild lymphocytic infiltration of CNS white matter, even though the immune system was sufficiently activated to clear LCMV from peripheral tissues. To test whether autoimmunity was limited by deficiencies in local antigen presentation, dendritic cells loaded with a MHC class I restricted LCMV NP peptide were injected directly into the CNS (C.F. Evans, C. Ploix, L. Shriver, M.J. Carson, submitted). Injection of the dendritic cells before lymphocytes entered the CNS failed to accelerate lymphocytic infiltration, even though CNS-injected dendritic cells migrated to the draining lymph nodes. By contrast, injection of cells into LCMV infected mice at the time lymphocytes first appeared within the CNS induced massive inflammation throughout CNS white matter, but without causing demyelination or clinical signs of EAE. Interestingly, expression of lymphocyte activation markers and IFN-γ was suppressed in those T cells found within the parenchyma but not in those found in nonparenchymal sites. Altogether these results suggest that local deficiencies in antigen presentation within the CNS coupled with additional immunosuppressive factors may inhibit autoimmunity.
These studies do not imply that molecular mimicry mechanisms are unable to operate in the CNS and cause demyelinating disease. Olson et al. (2001b) have generated a virus-induced molecular mimicry model by engineering a Theiler’s murine encephalomyelitis (TMEV) to encode a myelin-protein epitope. These investigators find that the initial virus-specific immune response includes responses to the myelin protein that lead to early onset CNS demyelination. Two primary differences exist between the viruses used in the two studies. In the LCMV model, the virus did not infect cells within the CNS due to the administration route, while in the TMEV model, many cells in the CNS including the microglia were actively infected. This infection caused robust production of inflammatory products and changes in neuronal and glial physiology. Thus, TMEV acted not only on the immune system, but it acted within the CNS to change the local microenvironment.
HOW DOES THE CNS REGULATE ANTIGEN PRESENTATION?
Several studies suggest that the failure to retain and accumulate T cells is due in part to the lymphocytetoxic nature of the CNS parenchyma (Bonetti et al., 1997; Aloisi, 2001; Bauer et al., 2001; Pender and Rist, 2001). If alloreactive lymphocytes are injected directly into the CNS, they disappear from the CNS by 24 hours. In a more explicit example, lymphocytes infiltrating the CNS parenchyma during EAE (but not those in nonparenchymal sites) have been shown to die by apoptosis. The mechanism of this lymphocyte “toxicity” is unclear but has been shown to be antigen independent and at least partly dependent on Fas-FasL interactions (Bonetti et al., 1997; Aloisi, 2001; Bauer et al., 2001; Pender and Rist, 2001).
More recently, several groups have discovered multiple factors present in the healthy CNS capable of directly regulating microglial function (Neumann, 2001). For example, microglial expression of molecules required for antigen presentation is inhibited by electrically active neurons, but is induced when electrical activity is suppressed (Neumann, 2001). Neuronal production of neurotrophins also suppresses IFN-γ-induced MHC class II expression (Neumann et al., 1998). Many neuropeptides have been shown in vitro to shape the innate immune responses of microglia. Neuropep-tides such as α-MSH and VIP inhibit pro-inflammatory cytokine and NO production by LPS-activated microglia (Delgado et al., 1998; Kim et al., 2000). Strikingly, studies using knockout mice illustrate that CD200 expression by neurons actively prevents activation of myeloid cells expressing the CD200 receptor (i.e., micro-glia, macrophages, dendritic cells) (Hoek et al., 2000). Even in the CNS of healthy CD200-deficient mice, microglia display an activated phenotype (elevated expression of CD45, MHC class II, complement receptor 3). Furthermore, in CD200-deficient mice, microglial activation was accelerated in response to axonal degeneration, and EAE occurred with more rapid onset. Not all neuronal products inhibit microglia (Neumann, 2001). Fractalkine released from injured neurons prevents microglial apoptosis, while the neuropeptide substance P augments microglial production of pro-inflammatory factors.
ADDITIONAL COMPLICATIONS TO THE STORY
This review identifies multiple potential microglial activation states, as well as multiple levels of regulation, treating all microglia as equivalent to each other. However, regional differences in cell morphology, antigenic markers, response to IFN-γ, constitutive and inducible MHC expression have long been realized to be indicators of microglial heterogeneity in vivo (Flaris et al., 1993; Pedersen et al., 1997; McCluskey and Lamp-son, 2001). To date, careful analysis of how microglial function varies by brain region has not been done, primarily due to the difficulties of isolating sufficient numbers from each brain region.
Furthermore, recent experiments indicate that mechanical and ischemic damage may alter the homeostatic balance of microglia within the CNS parenchyma in ways not seen after an autoimmune or viral insult to the CNS. Using irradiation bone marrow chimeric mice, bone marrow derived cells have been found to take up long-term residence near the site of injury in mice with facial axotomies or ischemic damage (Flugel et al., 2001; Priller et al., 2001). This response is in stark contrast to what occurs in healthy mice or in mice after EAE, or virus-induced demyelinating disease (Hickey and Kimura, 1988; Sedgwick et al., 1991). In these situations, bone marrow-derived cells fail to contribute to the parenchymal microglial population. While the bone marrow-derived parenchymal cells in the ischemia studies have been noted to reduce their expression of CD45, additional studies will be needed to explore whether these cells are phenotypically different from other parenchymal microglia. Specifically, do these cells display an activated CD45intermediate phenotype or a CD45low phenotype? Most macrophage populations are short lived in comparison with microglia. Therefore does the CNS environment extend the life span of these cells? If reactivated, do these cells convert back to a full CD45high macrophage phenotype? Upon activation or antigen-capture, can these cells leave the CNS and travel to the draining lymph nodes? Answers to these questions could have important implications for the propensity to generate and regulate CNS-specific immune responses.
WHY DON’T ALL INFLAMMATORY EVENTS WITHIN THE CNS LEAD TO MS-LIKE SYNDROMES?
Finally, it must be noted that not all pathogenic anti-myelin immune responses result in relapsing/remitting CNS demyelination. Indeed, many murine models of EAE are models of acute, self-terminating immune responses. Relapsing/remitting EAE may represent an exaggerated unbalanced immune response. For example, the onset and relapse phases of EAE are associated with Th1 responses (IFN-γ, TNF-α) and the remitting phases with Th2 responses (IL-10, IL-5, IL-4) (Miller et al., 2001). The toxic pro-inflammatory actions of Th1 responses have been well documented, yet both cytokines have also been implicated in the termination of immune responses in vivo (Spanaus et al., 1998). Recent studies further reveal that IFN-γ stimulates microglia in vitro to produce TNF-α, but that simultaneous production of IGF-2 by IFN-γ-treated microglia prevents TNF-α-induced oligodendrocyte death (Nicholas et al., 2002). Conversely, Th2 responses in of themselves are not protective and can even promote the production of pathogenic antibodies. For example, transforming growth factor-β (TGF-β) induces micro-glial expression of FLICE, rendering microglia insensitive to FAS-mediated apoptosis. Thus, rather than being immunosuppressive, TGF-β could actually prolong a CNS inflammatory response (Schlapbach et al., 2000). Adoptive transfer of polarized Th2 myelin-specific T cells into RAG−/− mice leads to clinical EAE and the addition of antibodies directed against the myelin protein, MOG increases the severity of some forms of EAE (Lafaille et al., 1997). Interestingly, some IgM antibodies reactive for oligodendrocyte surface antigens actually promote oligodendrocyte proliferation and myelin gene expression and therefore may promote neuroprotection in vivo (Rodriquez et al., 1996). Thus, moderated production of Th2 cytokines may limit the function of antigen-presenting cells, and may even lead to the production of a therapeutic antibody response. A too robust production of Th2 responses may promote pathogenic “allergic” responses (mast cell and neutrophil recruitment, IgG antibody production).
STRIKING THE PERFECT BALANCE
Experiments from several research groups have shown that T-cell-mediated immune responses may be beneficial and even necessary to optimally limit neurodegeneration within the CNS. The beneficial nature of these responses is apparently dependent on antigen-dependent interactions between T cells and antigen-presenting cells within the CNS. For, example a robust proinflammatory response may be neuroprotective because it rapidly clears a pathogen from the CNS and thus minimizes the exposure of the CNS to both the pathogen and the pro-inflammatory response (Marten et al., 2000). A weak or attenuated response may result in much more severe clinical disease by allowing the pathogen and an inflammatory response to linger and thus actually increase the exposure of the CNS to the neurotoxic elements.
However, T-cell-mediated responses can be actively neuroprotective, even in the absence of pathogens. Less CNS regeneration is observed after mechanical trauma and more severe dysfunction results after cytokine induced injury in mice deficient for T cells or antigen-presenting cells (Stalder et al., 1998; Kassiotis et al., 1999; Serpe et al., 1999). More recent studies in rats and mice have even shown that T-cell responses directed against myelin could prevent secondary neurodegeneration associated with spinal cord injury (Schwartz et al., 1999; Hauben et al., 2000).
Although the mechanisms of T-cell-mediated neuroprotection are as yet undefined, they are likely to rely in part on microglia as antigen-presenting cells and as effector cells. Lymphocytes need to be anchored to the sites of CNS damage by local antigen-presentation, potentially provided by trauma-activated microglia. In vitro, antigen-stimulation causes human lymphocytes to produce BDNF and NGF (Heese et al., 1998; Kerschensteiner et al., 1999). BDNF-positive cells morphologically resembling lymphocytes have been found within human MS lesions, indicating the potential for local antigen-presentation to promote growth factor production at the site of damage. Autoimmune T cells may then recruit microglia and macrophages to the vicinity of tissue damage by antigen-specific and nonspecific mechanisms. Once recruited these cells have two different functions: the removal of CNS tissue too damaged to survive and the production of growth factors to support the survival of the remaining nerves. Two types of observations are consistent with this suggestion. First, the greater potential for motor neurons than rubrospinal neurons to recover from axotomy correlates with the greater microgliosis and microglial ensheathment of axotomized motor neurons (Raivich et al., 1998; Moalem et al., 1999a, b). Second, activated micro-glia with CD45intermediate phenotypes, can be induced to produce neurotrophins and other growth factors that promote neuronal and oligodendrocyte survival in culture. Whether activated microglia produce high levels of growth factors or neurotoxic molecules such as NO and glutamate will depend on the summed signals received from healthy and damaged neurons, T cells, glia, and macrophages.
LOSS OF BALANCE AND MS
In sum, whether myelin-specific inflammation results in demyelination and increased axonal transection or recovery and remyelination may simply be due to the extent that the destruction of unsalvageable tissue is balanced with the nurture of salvageable tissue. In this equation, microglia influence outcome in three distinct roles: as initiators of immune responses, as effectors of immune responses, and as responders/amplifiers of ongoing inflammation. Whether their responses are beneficial or deleterious for CNS function is likely to depend on the context of “activating” signals. For example, microglia are capable of restimulating both Th1 and Th2 T cells (Aloisi et al., 1999). However, antigen-specific interactions with CD40L-expressing Th1 T cells, but not Th2 T cells, promote microglial expression of IL-12, while interactions with both Th1 and Th2 cells promote microglial production of prostaglandin PGE2. Interestingly, blocking the CD40L-CD40 interactions between microglia and T cells blocks microglial production of IL-12, but not PGE2. Consequently, microglial presentation of the identical myelin antigen could promote immunosuppression (prostaglandin production) if presented to CD40L-negative T cells or to Th2 cells but would promote inflammation and T-cell production of IFN-γ if presented to CD40L-expressing Th1 cells. The precise balance of responses to pathogenic insult is therefore moderated not only by the physiological responses of neurons, glia, and CNS-infiltrating immune cells, but by the stepwise differentiation and activation of microglia.
Thus, a pathogenic insult may induce inflammatory CNS demyelination, but under ideal circumstances the induced inflammation and demyelination is acute and self-resolving. This is apparently the situation observed in many human CNS diseases and murine models of EAE. In an idealized scenario, antigen presentation by microglia promotes T-cell production of cytokines and perhaps neurotrophins. T-cell-aided production of antibodies may stimulate microglia to clear toxic or damaged material. These events would, in turn, induce microglia to produce pro-inflammatory molecules, as well as chemoattractants for macrophages, dendritic cells, and lymphocytes. These factors would serve to speed the destruction of pathogens. The production of factors such as NO and prostaglandins may be neurotoxic, but would also limit T-cell proliferation and subsequent T-cell activation by other antigen-presenting cells in the vicinity (Minghetti et al., 1999; Willenborg et al., 1999). Phagocytosis of apoptotic lymphocytes would further reduce the ability of microglia to present antigen and thus also lead to reduced inflammation (Chan et al., 2001; Magnus et al., 2001). However, the propensity to phagocytose apoptotic lymphocytes is in itself regulated by the cytokine environment.
Humans rarely live in specific-pathogen free environments. Therefore, in addition to genetic predispositions, the homeostatic balance of microglial effector functions present within the CNS is likely to be altered by the various environmental challenges encountered (head injury, abscess, neuronal dysfunction, peripheral immune status). In MS, viruses, toxins, and other changes in the CNS microenvironment may further alter the balance of how microglia (and macrophages) interact with immune cells, neurons, and glia. Only slight changes in the balance between these components may be sufficient to generate a downward spiral into severe neurodegenerative disease, characterized by local inflammation (too much or too little), causing demyelination and axonal transection, which may in turn may induce further inflammation and chronic demyelination. The revelation that MS pathogenesis is heterogeneous within populations but fairly homogeneous within an individual indicates that some parameters globally affect the progression of disease (differences in genetic predisposition, initiating event, previous immune encounters). The unpredictability of the rate of relapse and remission also suggests that transient and local parameters are important (local injury, infections, alterations in microglial subsets or activation). In the end, our understanding of MS pathogenesis and the role of microglia in this disease will depend on the development and use of markers distinguishing microglia from CNS-infiltrating immune cells in MS tissue, precise identification of microglial activation states in correlation with MS pathology, and direct tests of microglial function in vivo.
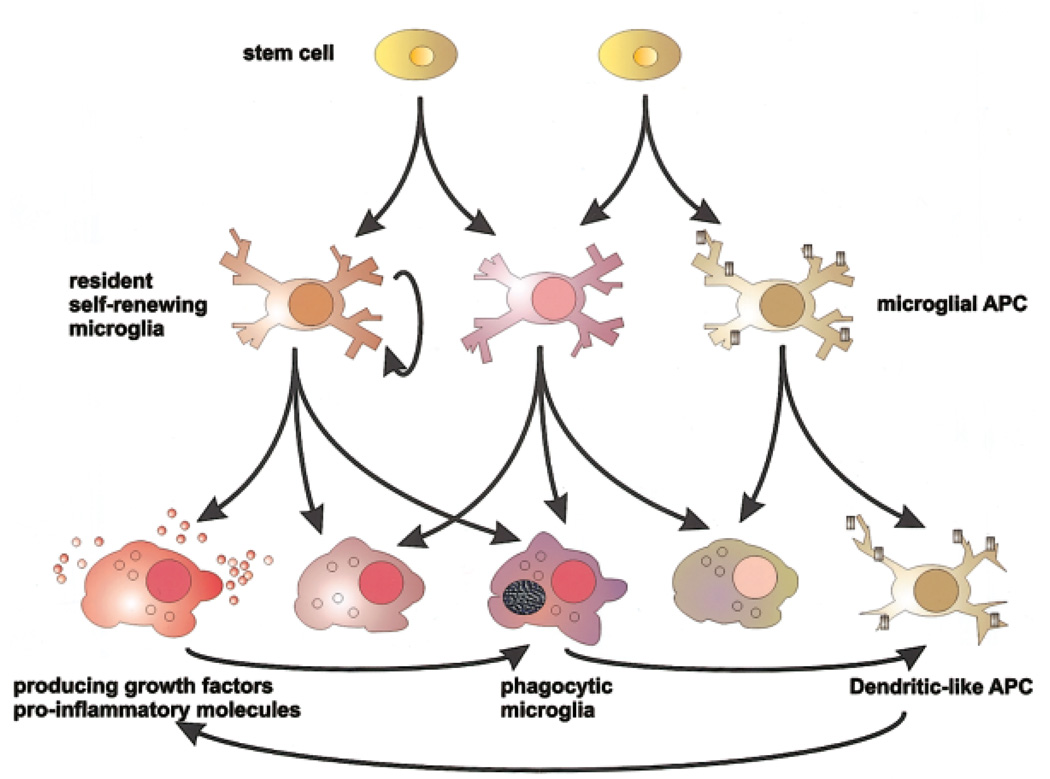
Fig. 1.
Knowing when to stop. Microglia are capable of developing a broad range of effector functions, determined in part by environmental signals from neurons, glia, and CNS-infiltrating immune cells and in part to their prior activation/developmental state. Their great plasticity and sensitivity to external cues allow for fine control of microglial effector function but also predispose microglia to develop maladaptive functions in response to dysregulated environmental signals. APC, antigen-presenting cell.
ACKNOWLEDGMENT
The author thanks Dr. David Lo for helpful comments during the preparation of this manuscript.
REFERENCES
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Polazzi E, Minghetti L, Adorini L. CD40-CD154 interaction and IFN-gamma are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol. 1999;162:1384–1391. [PubMed] [Google Scholar]
- Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000a;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000b;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- Aschner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ. Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol. 1999;39:151–173. doi: 10.1146/annurev.pharmtox.39.1.151. [DOI] [PubMed] [Google Scholar]
- Aw SE. Autoimmune disease—pathogenesis through molecular mimicry at the tripeptide level. Ann Acad Med Singapore. 1986;15:546–554. [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- Bauer J, Rauschka H, Lassmann H. Inflammation in the nervous system: the human perspective. Glia. 2001;36:235–243. doi: 10.1002/glia.1112. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Becher B, Fedorowicz V, Antel JP. Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res. 1996;45:375–381. doi: 10.1002/(SICI)1097-4547(19960815)45:4<375::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- Bonetti B, Pohl J, Gao YL, Raine CS. Cell death during autoimmune demyelination: effector but not target cells are eliminated by apoptosis. J Immunol. 1997;159:5733–5741. [PubMed] [Google Scholar]
- Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Lo D. Immunology. The push-me pull-you of T cell activation. Science. 2001;293:618–619. doi: 10.1126/science.1063516. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG. The role of microglia in CNS inflammatory disease: Friend or foe? In: Campbell A, editor. Inflammatory events in neurodegeneration. Scottsdale AZ: Prominent Press; 2001. pp. 1–14. [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999a;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999b;154:481–494. doi: 10.1016/S0002-9440(10)65294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash E, Rott O. Microglial cells qualify as the stimulators of unprimed CD4+ and CD8+ T lymphocytes in the central nervous system. Clin Exp Immunol. 1994;98:313–318. doi: 10.1111/j.1365-2249.1994.tb06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash E, Zhang Y, Rott O. Microglia present myelin antigens to T cells after phagocytosis of oligodendrocytes. Cell Immunol. 1993;147:129–138. doi: 10.1006/cimm.1993.1053. [DOI] [PubMed] [Google Scholar]
- Chan A, Magnus T, Gold R. Phagocytosis of apoptotic inflammatory cells by microglia and modulation by different cytokines: mechanism for removal of apoptotic cells in the inflamed nervous system. Glia. 2001;33:87–95. doi: 10.1002/1098-1136(20010101)33:1<87::aid-glia1008>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Chen SC, Leach MW, Chen Y, Cai XY, Sullivan L, Wiekowski M, Dovey-Hartman BJ, Zlotnik A, Lira SA. Central nervous system inflammation and neurological disease in transgenic mice expressing the CC chemokine CCL21 in oligodendrocytes. J Immunol. 2002;168:1009–1017. doi: 10.4049/jimmunol.168.3.1009. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–933. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- Delgado R, Carlin A, Airaghi L, Demitri MT, Meda L, Galimberti D, Baron P, Lipton JM, Catania A. Melanocortin peptides inhibit production of proinflammatory cytokines and nitric oxide by activated microglia. J Leukoc Biol. 1998;63:740–745. doi: 10.1002/jlb.63.6.740. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121(Pt 8):1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP, Matthews PM, Arnold DL. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- Evans CF, Horwitz MS, Hobbs MV, Oldstone MB. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med. 1996;184:2371–2384. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- Flaris NA, Densmore TL, Molleston MC, Hickey WF. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993;7:34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996;184:1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fressinaud C, Vallat JM, Pouplard-Barthelaix A. Platelet-derived growth factor partly prevents chemically induced oligodendrocyte death and improves myelin-like membranes repair in vitro. Glia. 1996;16:40–50. doi: 10.1002/(SICI)1098-1136(199601)16:1<40::AID-GLIA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gobin SJ, Montagne L, Van Zutphen M, Van Der Valk P, Van Den Elsen PJ, De Groot CJ. Upregulation of transcription factors controlling MHC expression in multiple sclerosis lesions. Glia. 2001;36:68–77. doi: 10.1002/glia.1096. [DOI] [PubMed] [Google Scholar]
- Gran B, Hemmer B, Martin R. Molecular mimicry and multiple sclerosis—a possible role for degenerate T cell recognition in the induction of autoimmune responses. J Neural Transm. 1999;55 suppl:19–31. doi: 10.1007/978-3-7091-6369-6_3. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Heppner FL, Haas D, Nitsch R. Fluorescent dye prelabelled microglial cells migrate into organotypic hippocampal slice cultures and ramify. Eur J Neurosci. 1997;9:863–866. doi: 10.1111/j.1460-9568.1997.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese K, Hock C, Otten U. Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem. 1998;70:699–707. doi: 10.1046/j.1471-4159.1998.70020699.x. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Bauer J, Akassoglou K, Lassmann H, Kollias G, Probert L. A tumor necrosis factor-induced model of human primary demyelinating diseases develops in immunodeficient mice. Eur J Immunol. 1999;29:912–917. doi: 10.1002/(SICI)1521-4141(199903)29:03<912::AID-IMMU912>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20:3622–3630. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SC, Stagg AJ. Antigen-presenting cell types. Curr Opin Immunol. 1993;5:374–382. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- Krakowski ML, Owens T. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol. 2000;30:1002–1009. doi: 10.1002/(SICI)1521-4141(200004)30:4<1002::AID-IMMU1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immu-nodeficient hosts rather than protect them from the disease. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liblau R, Gautam AM. HLA, molecular mimicry and multiple sclerosis. Rev Immunogenet. 2000;2:95–104. [PubMed] [Google Scholar]
- Lo D, Feng LL, Li L, Carson MJ, Crowley M, Pauza M, Nguyen A, Reilly CR. Integrating innate and adaptive immunity in the whole animal. Immunol Rev. 1999;169:225–239. doi: 10.1111/j.1600-065x.1999.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Grauer O, Toyka KV, Gold R. Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J Immunol. 2001;167:5004–5010. doi: 10.4049/jimmunol.167.9.5004. [DOI] [PubMed] [Google Scholar]
- Marten NW, Stohlman SA, Atkinson RD, Hinton DR, Fleming JO, Bergmann CC. Contributions of CD8+ T cells and viral spread to demyelinating disease. J Immunol. 2000;164:4080–4088. doi: 10.4049/jimmunol.164.8.4080. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987;17:71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Matyszak MK. Inflammation in the CNS: balance between immunological privilege and immune responses. Prog Neurobiol. 1998;56:19–35. doi: 10.1016/s0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. Eur J Immunol. 1999;29:3063–3076. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- McCluskey LP, Lampson LA. Local immune regulation in the central nervous system by substance P vs. glutamate. J Neuroimmunol. 2001;116:136–146. doi: 10.1016/s0165-5728(01)00295-8. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- Miller SD, Olson JK, Croxford JL. Multiple pathways to induction of virus-induced autoimmune demyelination: lessons from Theiler’s virus infection. J Autoimmun. 2001;16:219–227. doi: 10.1006/jaut.2000.0489. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Polazzi E, Nicolini A, Greco A, Levi G. Possible role of microglial prostanoids and free radicals in neuroprotection and neurodegeneration. Adv Exp Med Biol. 1999;468:109–119. doi: 10.1007/978-1-4615-4685-6_9. [DOI] [PubMed] [Google Scholar]
- Moalem G, Monsonego A, Shani Y, Cohen IR, Schwartz M. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. FASEB J. 1999a;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999b;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Myers KJ, Dougherty JP, Ron Y. In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J Immunol. 1993;151:2252–2260. [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Neumann H, Misgeld T, Matsumuro K, Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci U S A. 1998;95:5779–5784. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Benveniste EN. Critical role of TNF-alpha and NF-κB in IFN-gamma-induced CD40 expression in microglia/macrophages. J Biol Chem. 2002;277:13796–13803. doi: 10.1074/jbc.M111906200. [DOI] [PubMed] [Google Scholar]
- Nicholas RS, Stevens S, Wing MG, Compston DA. Microglia-derived IGF-2 prevents TNFalpha induced death of mature oligodendrocytes in vitro. J Neuroimmunol. 2002;124:36–44. doi: 10.1016/s0165-5728(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399(6738) suppl:A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. J Virol. 2001a;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001b;108:311–318. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen EB, McNulty JA, Castro AJ, Fox LM, Zimmer J, Finsen B. Enriched immune-environment of blood-brain barrier deficient areas of normal adult rats. J Neuroimmunol. 1997;76:117–131. doi: 10.1016/s0165-5728(97)00038-6. [DOI] [PubMed] [Google Scholar]
- Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36:137–144. doi: 10.1002/glia.1103. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Ploix C, Lo D, Carson MJ. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J Immunol. 2001;167:6724–6730. doi: 10.4049/jimmunol.167.12.6724. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting genemodified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Mechanisms of inflammation in MS tissue: adhesion molecules and chemokines. J Neuroimmunol. 1999;98:57–68. doi: 10.1016/s0165-5728(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Miller DJ, Lennon VA. Immunoglobulin reactive with myelin basic protein promotes CNS remyelination. Neurology. 1996;46:538–545. doi: 10.1212/wnl.46.2.538. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapbach R, Spanaus KS, Malipiero U, Lens S, Tasinato A, Tschopp J, Fontana A. TGF-beta induces the expression of the FLICE-inhibitory protein and inhibits Fas-mediated apoptosis of microglia. Eur J Immunol. 2000;30:3680–3688. doi: 10.1002/1521-4141(200012)30:12<3680::AID-IMMU3680>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Schmidt S. Candidate autoantigens in multiple sclerosis. Mult Scler. 1999;5:147–160. doi: 10.1177/135245859900500303. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Moalem G, Leibowitz Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- Scolding N. The differential diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;71:ii9–ii15. doi: 10.1136/jnnp.71.suppl_2.ii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc Res Tech. 2001;54:81–94. doi: 10.1002/jemt.1123. [DOI] [PubMed] [Google Scholar]
- Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xL. Eur J Immunol. 1998;28:4398–4408. doi: 10.1002/(SICI)1521-4141(199812)28:12<4398::AID-IMMU4398>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sriram S, Rodriguez M. Indictment of the microglia as the villain in multiple sclerosis. Neurology. 1997;48:464–470. doi: 10.1212/wnl.48.2.464. [DOI] [PubMed] [Google Scholar]
- Stalder AK, Carson MJ, Pagenstecher A, Asensio VC, Kincaid C, Benedict M, Powell HC, Masliah E, Campbell IL. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am J Pathol. 1998;153:767–783. doi: 10.1016/S0002-9440(10)65620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman SA, Hinton DR. Viral induced demyelination. Brain Pathol. 2001;11:92–106. doi: 10.1111/j.1750-3639.2001.tb00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglial response to brain injury: a brief synopsis. Toxicol Pathol. 2000;28:28–30. doi: 10.1177/019262330002800104. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Sun D, Hu X, Liu X, Whitaker JN, Walker WS. Expression of chemokine genes in rat glial cells: the effect of myelin basic protein-reactive encephalitogenic T cells. J Neurosci Res. 1997;48:192–200. [PubMed] [Google Scholar]
- Tan J, Town T, Saxe M, Paris D, Wu Y, Mullan M. Ligation of microglial CD40 results in p44/42 mitogen-activated protein kinase-dependent TNF-alpha production that is opposed by TGF-beta 1 and IL-10. J Immunol. 1999;163:6614–6621. [PubMed] [Google Scholar]
- Tran EH, Hoekstra K, vanRooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- Trapp BD, Ransohoff RM, Fisher E, Rudick RA. Neurodegeneration in multiple sclerosis: relationship to neurological disability. Neuroscientist. 1999a;5:48–57. [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999b;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52:460–470. doi: 10.1097/00005072-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 4. Applications to planning and interpretation of clinical therapeutic trials. Brain. 1991;114:1057–1067. doi: 10.1093/brain/114.2.1057. [DOI] [PubMed] [Google Scholar]
- Wekerle H. CD4 effector cells in autoimmune diseases of the central nervous system. In: Keane RW, Hickey WF, editors. Immunology of the nervous system. Oxford: Oxford University Press; 1997. pp. 460–492. [Google Scholar]
- Willenborg DO, Staykova MA, Cowden WB. Our shifting understanding of the role of nitric oxide in autoimmune encephalomyelitis: a review. J Neuroimmunol. 1999;100:21–35. doi: 10.1016/s0165-5728(99)00212-x. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Weinshenker BG. Multiple sclerosis: epidemiology, genetics, classification, natural history, and clinical outcome measures. Neuroimaging Clin North Am. 2000;10:611–624. [PubMed] [Google Scholar]
- Wolf SA, Gimsa U, Bechmann I, Nitsch R. Differential expression of costimulatory molecules B7-1 and B7-2 on microglial cells induced by Th1 and Th2 cells in organotypic brain tissue. Glia. 2001;36:414–420. doi: 10.1002/glia.1127. [DOI] [PubMed] [Google Scholar]