Abstract
Aspirin exerts its unique pharmacological effects by irreversibly acetylating a serine residue in the cyclooxygenase site of prostaglandin-H2-synthases (PGHSs). Despite the irreversibility of the inhibition, the potency of aspirin varies remarkably between cell types, suggesting that molecular determinants could contribute to cellular selectivity. Using purified enzymes, we found no evidence that aspirin is selective for either of the two PGHS isoforms, and we showed that hydroperoxide substrates of the PGHS peroxidase inhibited the rate of acetylation of PGHS-1 by 68%. Using PGHS-1 reconstituted with cobalt protoporphyrin, a heme devoid of peroxidase activity, we demonstrated that reversal by hydroperoxides of the aspirin-mediated acetylation depends upon the catalytic activity of the PGHS peroxidase. We demonstrated that inhibition of PGHS-2 by aspirin in cells in culture is reversed by 12-hydroperoxyeicosatetraenoic acid dose-dependently (ED50 = 0.58 ± 0.15 μM) and that in cells with high levels of hydroperoxy-fatty acids (RAW264.7) the efficacy of aspirin is markedly decreased as compared to cells with low levels of hydroperoxides (A549; IC50s = 256 ± 22 μM and 11.0 ± 0.9 μM, respectively). Together, these findings indicate that acetylation of the PGHSs by aspirin is regulated by the catalytic activity of the peroxidase which yields a higher oxidative state of the enzyme.
1. Introduction
Irreversible inhibition of the prostaglandin H synthases (PGHSs) is central to the unique pharmacology of aspirin [1, 2]. Aspirin covalently acetylates a serine in the catalytic pocket of the PGHSs (S530 in PGHS-1, and S516 in PGHS-2) [3, 4]. PGHSs thus acetylated are unable to synthesize PGH2, the precursor for biosynthesis of all prostaglandins and thromboxanes. Because anuclear platelets are unable to synthesize new PGHS-1 in normal conditions, irreversible inhibition of the enzyme persists for the lifespan of the platelet. Thus, the cumulative inhibitory effect of daily administration of small doses of aspirin (e.g. 40–81 mg) will yield marked inhibition of biosynthesis of thromboxane A2 by platelets [5, 6], whereas achieving this extent of inhibition by a single dose requires 160–325 mg. This selectivity for platelets resulting from accumulation of irreversible PGHS-1 inhibition during chronic administration of aspirin is amplified by exposure of platelets to the higher concentrations of aspirin in the portal circulation as compared with the systemic circulation in which concentrations of the drug are lower due to presystemic clearance [7] and dilution into its volume of distribution.
In contrast to the mechanistic insights into the relative selectivity of aspirin for the platelet, the reason why aspirin exerts little or no anti-inflammatory effect at antipyretic and analgesic doses has not been understood. The relative ineffectiveness of aspirin as an anti-inflammatory drug is mirrored at the cellular level by the demonstration that aspirin is 166 times more potent in inhibiting PGHS activity in the platelet than in the J774.2 macrophage cell line [8], leading to consideration that the action of aspirin might be selective for the PGHS-1 isoform. However, the potency of inhibition of PGHS-1 by aspirin ex vivo observed in normal platelets is lost in platelets of patients after coronary artery bypass grafting [9], and PGHS-1 from platelet homogenates is poorly acetylated by aspirin [10], suggesting differences in acetylation that can not be attributed to isoform selectivity. Collectively, these observations reflect considerable variability in acetylation of the PGHSs by aspirin, a variability that implies a mechanistic basis for regulating the action of the drug.
The bifunctional nature of the PGHSs provides a conceptual framework for consideration of the regulation of acetylation of the enzyme by aspirin. The PGHSs contain both a heme-containing peroxidase and a cyclooxygenase catalytic site. Reduction of a hydroperoxide by the peroxidase oxidizes the heme to a protoporphyrin radical cation. This initial event increases the overall oxidative state of the enzyme by generating radical species in the protein [11, 12] through intramolecular electron transfer. Generation of the Tyr385 radical in the cyclooxygenase catalytic pocket [13] leads to abstraction of an hydrogen from arachidonic acid, which initiates the oxygenation that leads to formation of prostaglandin G2 (PGG2). We previously found that hydroperoxide substrates that drive the peroxidase activity antagonize the inhibition of PGHSs by sodium salicylate [14]. This evidence led to the hypothesis that acetylation of the PGHSs by aspirin also could be dependent on the catalytic activity of their peroxidases. The present report describes investigations that provide evidence in support of that hypothesis.
2. Materials and Methods
2.1. Materials
PGHS-1 was purified from ram seminal vesicles as described previously [15]. Wild type murine PGHS-2 was expressed in SF-9 cells (Novagen) and purified as previously described [16]. Ovine COX-1 is readily available from sheep seminar vesicles and is the closest species in amino acid sequence to human COX-1. The latter is poorly expressed in insect cells and exhibits low specific activity, which makes it unsuitable for routine inhibitor assays. In contrast, mouse and human COX-2 are highly expressed in insect cells and exhibit excellent specific activity. Recombinant mouse COX-2 crystallizes readily and a number of crystal structures of mouse-COX-2-inhibitor complexes have been determined. The availability of such structural information makes mouse COX-2 a good choice for inhibitor studies. A-549 cells were a generous gift from Dr. Robert Coffey (Vanderbilt University) and HUVECs were gifted by Dr. Douglas E. Vaughan (Vanderbilt University). RAW 264.7 cells were purchased from American Type Culture Collection (Rockville, MD). Human blood was obtained following a protocol approved by the Institutional Review Board of Vanderbilt University. Washed platelets were prepared as described previously [17]. 12-Hydroperoxyeicosatetraenoic acid (12-HPETE), 12-hydroxyeicosatetraenoic acid (12-HETE), 5-phenyl-4-pentenyl hydroperoxide (PPHP) and 5-phenyl-4-pentenyl alcohol (PPA) were purchased from Cayman Chemicals (Ann Arbor, MI). Medium 199, Hanks’ Balanced Salt Solution (HBSS), Dulbecco’s modified Eagle’s medium (DMEM), foetal bovine serum (FBS), aspirin (acetyl salicylic acid), salicylic acid, butylated hydroxyanisole, iron protoporphyrin IX (Heme), phenol and Tris(hydroxymethyl) aminomethane (Tris), lipopolysaccharide (LPS), INF-γ and IL-1β were purchased from Sigma-Aldrich (St. Louis, MO). Cobalt protoporphyrin IX was obtained from Porphyrin Products (Logan, UT). Diethyl ether was purchased from Aldrich Chemical Co. (Milwaukee, WI). Silica gel 60A thin layer chromatography plates were purchased from Whatman (Clifton, NJ). [14C] Arachidonic acid was from Perkin Elmer Life Sciences (Boston, MA). [Acetyl-1-14C] aspirin was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO).
2.2. Acetylation of PGHS-1 and PGHS-2 with [1-14C] acetyl salicylic acid
Ovine PGHS-1 (1.5 μM; specific activity: oxygenation of 202 mol of AA/min/mol of enzyme) or wild-type murine PGHS-2 (2.7μM; specific activity: oxygenation of 109 mol of AA/min/mol of enzyme) was reconstituted in 100 mM Tris-HCl buffer, pH 8.0 containing 500 μM phenol in presence of 2 molar equivalents of hematin (Fe-PPIX) or cobalt protoporphyrin Co-PPIX at 4°C for 30 minutes. This solution was warmed for 2 min at 37°C. [Acetyl-1-14C] aspirin was added to a final concentration of 90 μM with or without the designated concentration of 12-HPETE or 12-HETE or PPHP or PPA. At different time intervals, aliquots were taken out, mixed with SDS-PAGE sample loading buffer and the proteins were denatured by heating at 70°C for 10 minutes. Samples were loaded on 10 % SDS-PAGE gel for electrophoresis followed by staining with Coomassie blue. The gels were dried and the radioactivity associated with the proteins was determined by autoradiography using PhosphorScreen (Amersham Biosciences, Piscataway, NJ). The data was analyzed on Typhoon 9400 using the software Typhoon Scanner Control (Amersham Biosciences, Piscataway, NJ). The radioactivity associated with each band was quantified using the software ImageQuant 5.2 (Amersham Biosciences, Piscataway, NJ) and was expressed as a percentage of the intensity of the band at 2, 4, or 8 min in control conditions (no hydroperoxide added to the enzymatic reaction). Preincubation with indomethacin prior to addition of aspirin prevented acetylation of PGHS as measured by this method.
2.3. Activity assay of PGHSs
Ovine PGHS-1 (1.5 μM) was reconstituted as described above and incubated at 37°C in the absence (control) or presence of 12-HPETE (2 and 5 μM). At the indicated times, aliquots were taken out from the reaction mixture and diluted with Tris-Phenol buffer so as to have 5.4 nM final concentration of PGHS-1. Then, [14C] arachadonic acid (4.8 nCi, 0.5 μM final concentration) was added and the activity of PGHS-1 was assayed as described previously [14].
2.4. Preparation of washed Platelets
50 ml of human blood was drawn with a syringe containing 5 ml of 3.8% sodium citrate and centrifuged at 300 × g for 10 min at room temperature. Platelet-rich supernatant plasma was acidified to pH 6.4 with 0.15 M citric acid followed by centrifugation at 1,000 × g for 10 min at room temperature. The pellet was resuspended with 24.4 mM sodium phosphate buffer, pH 6.5/0.113 M NaCl/5.5 mM glucose and purified on a Sepharose 2B column equilibrated with the same buffer to obtain the washed platelets [17].
2.5. Cell culture
A-549 and murine RAW 264.7 macrophage cells were cultured in 6-well plates at 37°C in a humid atmosphere containing 5% CO2. Cells were grown in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B.
2.6. Western blot analysis
A549 cell lysates containing 30 μg of protein were mixed with NuPAGE lithium dodecyl sulphate protein sample buffer and heated at 70°C for 10 min. Samples were run on NuPAGE 10% Bis-Tris gels. Proteins were electrophoretically transferred from gels to polyvinylidene difluoride membranes for one hour at 30 V. Membranes were then blocked in blocking buffer consisting of 10 % non-fat dry milk (Bio-rad) in TBST (20 mM Tris-HCl, pH 7.5, 137 mM containing 0.2 % Tween-20) for one hour at room temperature. After blocking, membranes were incubated at 4°C with COX-2 goat polyclonal IgG (sc-1747, Santa Cruz, CA). Blots were reprobed with goat IgG polyclonal antibodies conjugated to HRP (sc-2020, Santa Cruz, CA).
2.7. Prostaglandin synthesis in A-549 and RAW264.7 cells
At 90 % confluence, A-549 cells were activated with 1ng/ml IL-1β and RAW264.7 cells were activated with 10 μg/ml LPS and 10 units/ml INF-γ overnight in serum free DMEM containing penicillin, streptomycin and amphotericin B. 2.0 μM of [2H8] AA was added, cells were incubated for 15 min, and the medium was harvested for analysis of PGD2 and PGE2 by gas chromatography (GC)/negative ion chemical ionization mode (NICI)/mass spectrometry (MS) as described later.
2.8. Effect of Aspirin on Prostaglandin Formation in Cells
After the addition of fresh medium to the activated cells, aspirin was added in desired concentrations and incubated at 37°C for 30 min. 2.0 μM [2H8] AA was added. Cells were further incubated for 15 min and the medium was harvested for GC/NICI/MS analysis.
2.9. Effect of 12-HPETE and 12-HETE on Aspirin Mediated Inhibition of Prostaglandin Synthesis in A-549 cells
Two series of experiments were performed in parallel: in one set 0.0, 0.1, 0.5, 1.0, 2.0 and 5.0 μM 12-HPETE or 12-HETE was added along with 20 μM aspirin and in the second set the same concentrations of 12-HPETE or 12-HETE were added to the cells with no aspirin. Cells were then incubated at 37°C for 30 minutes, following with 2.0 μM [2H8] AA was added and after 15 min, the biosynthesis of PGE2 was analyzed by GC/NICI/MS as described below.
2.10. GC/MS Analysis
[2H4] Prostaglandins (2 ng) or [2H4] thromboxane B2 (6 ng) were added to samples as internal standards. Prostaglandins were isolated and derivatized for analysis by GC/NICI/MS monitoring selected ions as described previously [18]. The signals for different molecules are : TxB2, m/z = 614; and internal standards [2H4] TxB2, m/z = 618; [2H4] PGD2 and PGE2, m/z = 528. To account for the deuterium-protium exchange at the position C12 of [2H8] PGE2, the summation of the signals obtained at m/z = 530, 531 and 532 was performed.
2.11. HPETE synthesis in A-549 and RAW264.7 cells
Activated A-549 cells and RAW264.7 cells were taken as described in section 2.7. The medium was collected to analyze production of HETEs by liquid chromatography (LC)/atmospheric pressure chemical ionization (APCI)/MS. No exogenous arachidonic acid was added.
2.12. 12-HPETE synthesis in human washed platelets
Washed platelets (100 μl at 600,000 platelets/μl) were sonicated (broken cells) or kept as such (whole cells) and incubated at 37°C for 45 min to mimic the experimental conditions used to study the effect of aspirin. After this time, the cells were centrifuged at 2,000 × g for 10 min and the supernatant was collected for analysis of 12-HETE by LC/APCI/MS/MS. No exogenous arachidonic acid was added.
2.13. LC/APCI/MS/MS Analysis
50 μl aliquots of the platelet supernatants or cell culture media were analyzed by liquid chromatography LC/APCI/MS/MS. 2 ng of [2H8] 12-HETE was added to the samples as internal standard. The carboxylic acid of 12-HETE was derivatized to pentafluorobenzyl ester and analyzed by LC/APCI/MS/MS as described previously [19].
2.14. Effect of 12-HPETE on the hydrolysis of aspirin
Reconstituted ovine PGHS-1 (1.5 μM) was incubated with aspirin (90 μM) in the absence (control) and presence of 12-HPETE (5 μM) for 45 min at 37°C. The samples were then kept at −80°C until they were analyzed by LC/ESI/MS/MS. Deacetylation of aspirin was determined by injecting 20 μl of the reaction medium in a reversed phase HPLC (Phenomenex Prodigy ODS 5μ, 1.0 × 150 mm C18 column, Torrence, CA). The flow rate was set at 0.1 ml/min with a gradient starting with 100 % solvent A (95:5, H2O:MeOH containing 10 mM ammonium acetate), holding for 2 min followed by a linear gradient increasing to 100 % solvent B (5:95, H2O:MeOH containing 10 mM ammonium acetate) in 10 min, holding for 1 min subsequently coming back to the initial conditions in 12 min followed by a hold for 3 min. In these conditions, aspirin was eluted in 5.1 min and salicylic acid was eluted in 7.1 min. ESI/MS/MS analysis was carried out on a Finnigan TSQ 7000 system (Finnigan Corp., San Jose, CA). The mass spectrometer was operated in a positive ion mode. Nitrogen was used as the sheath gas (70 psi) and auxiliary gas (10 psi) to assist the nebulization. The heated capillary was operated at 200°C and 20 V, and the tube lens voltage was set at 37 V for aspirin and 51 for salicylic acid (hydrolyzed aspirin). The mass spectrometer was operated in the selected reaction monitoring (SRM) mode. Ions were subjected to collision-induced dissociation at an argon pressure of 1.5 mTorr. Mass transitions were monitored as follows: m/z = 137.1 to 93.1 at −22 eV for salicylic acid and m/z = 179.2to 92.1 at −26 eV for aspirin. Data acquisition and analysis were performed using Xcaliber software, version 1.3.
2.14. Statistical Methods
Statistical analysis was performed using Prism 4 from GraphPad Software, Inc (San Diego, CA). The significance of differences between the IC50 values of aspirin for the different cell lines was analyzed by two-tailed unpaired Student’s t-test. The significance of differences between control and aspirin-treated experiments at each concentration of 12-HPETE or 12-HETE was analyzed by paired two-tailed Student’s t-test.
3. Results
3.1. Analysis of the selectivity of aspirin for the two isoforms of PGHS
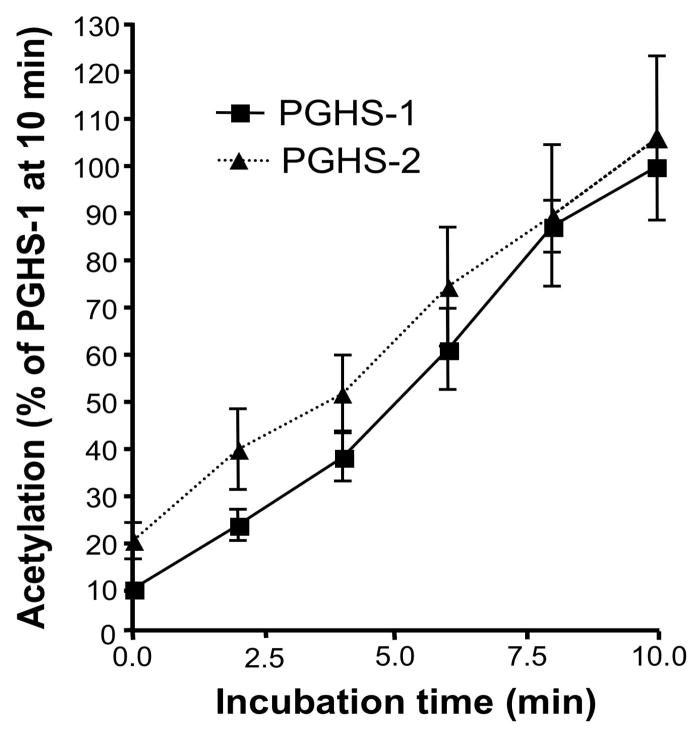
To determine whether the rate of acetylation of the two isoforms by aspirin differs, we examined the acetylation profiles of purified ovine PGHS-1 and murine recombinant PGHS-2 reconstituted with iron protoporphyrin IX (Fe3+-PPIX). The amount of the two isoforms had equal catalytic activity. After incubation with [acetyl-1-14C] aspirin, the extent of acetylation of the enzyme was determined by measuring the radioactivity bound to the protein isolated on SDS-PAGE. We found that the two isoforms were acetylated by aspirin to the same extent (Fig. 1).
Fig. 1.
Comparision of acetylation of PGHS-1 and PGHS-2 by aspirin. Equipotent amounts of ovine PGHS-1 (1.5 μM) and murine PGHS-2 (2.7 μM) were reconstituted in 100 mM Tris HCl, pH 8.0, 500 μM phenol with two molar equivalents of hematin at 4°C for 30 minutes and [acetyl-1-14C] aspirin was added to a final concentration of 90 μM. At 0, 2, 4, 6, 8 and 10 minutes, aliquots were analyzed by electrophoresis. The radioactivity associated with the proteins was determined by autoradiography. Each data point represents the mean ± S.E.M of five values.
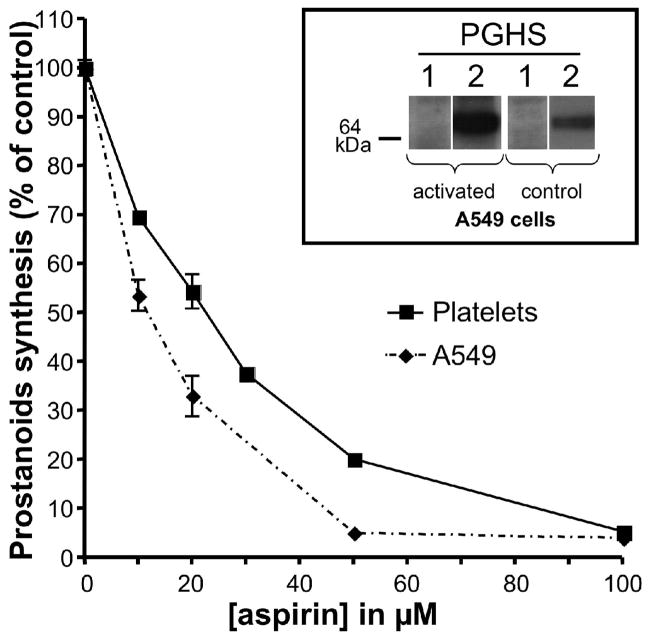
The finding that the action of aspirin is not selective for the purified PGHS isoforms was extended with experiments in cells. Inhibition of prostanoid biosynthesis by aspirin was compared in washed human platelets (PGHS-1) and in A549 cells which are a human lung cancer cell line that expresses PGHS-2 when activated with interleukin-1β (IL-1β) [20]. These cells were chosen because each selectively expresses a different one of the two isoforms and both are sensitive to aspirin. As depicted in figure 2, the IC50 for A549 cells (11.0 ± 0.9 μM) is significantly less than that for platelets (19.8 ± 0.3 μM). These results in concert indicate that both isoforms can be inhibited by aspirin with similar IC50s, suggesting that the isoform selectivity is unlikely to be a major determinant of the cellular selectivity of PGHS inhibition by aspirin.
Fig. 2.
Comparision of inhibition of PGHS-1 and PGHS-2 by aspirin. Human cells expressing PGHS-1 (platelets) or PGHS-2 (A-549) were pre-incubated with varying concentrations of aspirin for 30 min at 37°C followed by addition of 2 μM [2H8] arachidonic acid. After 15 min at 37°C, the medium was collected, and TxB2 (platelet) and PGE2 (A549) were quantified by analysis by GC/NICI/MS as described previously [18]. Each data point represents the mean ± S.E.M. of four values. The significance of difference between the IC50 values of aspirin for the two cell lines was analyzed by two-tailed unpaired Student t-test (p<0.0001). The expression of PGHS-1 and -2 before and after activation of the A-549 cells was assessed by Western blot analysis and is shown in the inset.
3.2. Effect of hydroperoxide on the rate of acetylation of PGHS-1 by aspirin
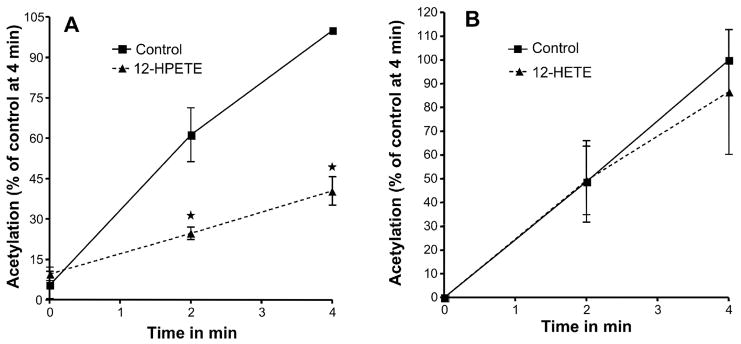
To determine the effect of hydroperoxides on the acetylation of PGHS, we assessed the effect of 12-HPETE on acetylation of PGHS-1 by aspirin. 12-HPETE is the product of oxygenation of AA by 12-lipooxygenase and is a hydroperoxide substrate for the PGHS-peroxidase. When 12-HPETE was added to incubations of PGHS-1 with phenol along with [acetyl-1-14C] aspirin, the acetylation was markedly inhibited (p=0.012 at 2 min and p<0.001 at 4 min) (Fig. 3, A).
Fig. 3.
Effect of 12-HPETE and 12-HETE on the acetylation of PGHS-1 by aspirin. The PGHS activity is expressed as a percentage of the control activity at 4 minutes. (A) Tris HCl (control) or 5 μM 12-HPETE were mixed separately with 90 μM [acetyl -1-14C] aspirin just before being added to the heme-reconstituted ovine PGHS-1 (1.5 μM). (B) Tris HCl (control) or 5 μM 12-HETE were mixed separately with 90 μM [acetyl-1-14C] aspirin just before being added to the heme reconstituted ovine PGHS-1. In both experiments, aliquots were taken at 0, 2, and 4 min, and PGHS-1 was analyzed as described for figure 1. Each data point represents the mean ± S.E.M of four values for 12-HPETE and five values for 12-HETE experiments. The significance of differences between the control and the experiments done for each concentration of 12-HPETE or 12-HETE was analyzed by paired two-tailed Student’s t-test (*: p<0.05; NS: non significant).
As the higher oxidative state of the enzyme with this experimental intervention is transitory, we determined how long acetylation would be inhibited after a single pulse of hydroxperoxide before the acetylation rate returned toward that of the enzyme in its reduced state. During the first 4 minutes, the rate of acetylation was decreased by 12-HPETE to 32% of the control (control = 13.5 ± 1.6 %.min−1, 12-HPETE = 4.3 ± 0.5 %.min−1, p<0.005, n=4) (Fig. 3A and Supplemental Fig. S1). Following this, during the 4–8 min period, the rate of acetylation of PGHS-1 in presence of 12-HPETE increased toward that of the control enzyme (control = 10.7 ± 1.5 %.min−1, 12-HPETE = 8.0 ± 1.4 %.min−1, p>0.2, n=4) (Supplemental Fig. S1). Accordingly, experiments to evaluate the initial rate were performed during the first 4 min unless otherwise indicated.
As 12-HPETE conceivably could compete with aspirin for access to the cyclooxygenase active site, we also examined the effect of the corresponding alcohol 12-HETE, which has a similar eicosanoid structure but lacks the hydroperoxide moiety, to compare their effects on the PGHS-1 acetylation by aspirin. 12-HETE did not alter the acetylation of PGHS-1 by aspirin (Fig. 3, B) suggesting that the effect of 12-HPETE on acetylation of PGHS by aspirin is mediated by the peroxidase activity. To ascertain that the effect was not due to the inactivation of the enzyme by 12-HPETE, the enzyme activity was tested under the same conditions in a parallel experiment. Our results indicate that in presence of 500 μM phenol, the reducing co-substrate used in all experiments to protect the enzyme against inactivation, 12-HPETE did not inactivate PGHS-1 when used at 5 μM and up to 10 min of pre-incubation (Supplemental Fig. S2).
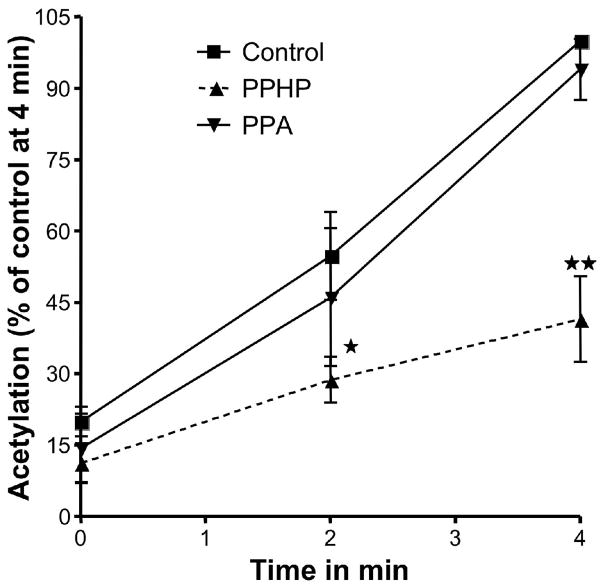
To further ascertain whether this effect was due to catalytic turnover of the peroxidase of PGHS-1, we repeated the experiments with another hydroperoxide, 5-phenyl-pentenyl hydroperoxide (PPHP), a non-eicosanoid hydroperoxide, which is a substrate of the peroxidase site but does not access the cyclooxygenase site. In this experiment, PPHP significantly inhibited the acetylation of PGHS-1 by aspirin (p<0.05 at 2 min and p<0.001 at 4 min) (Fig. 4, A). 5-Phenyl-pentenyl alcohol (PPA), the product of reduction of PPHP, did not alter acetylation of the enzyme by aspirin (Fig. 4, B). Again, under these experimental conditions, the activity of the enzyme was tested in a parallel experiment and it was found that PPHP did not inactivate the enzyme. In contrast, a reducing co-substrate, phenol, enhanced the rate of acetylation of the Fe3+ PPIX enzyme (data not shown). Taken together these results suggest that hydroperoxides can prevent the acetylation of Ser530 in the PGHS-cyclooxygenase site by increasing the peroxidase activity of the enzyme.
Fig. 4.
Effect of PPHP and PPA on the acetylation of PGHS-1 by aspirin. The PGHS activity is expressed as a percentage of the control activity at 4 minutes. Tris HCl (control,), 2μM PPHP or 2μM PPA were mixed separately with 90 μM [acetyl-1-14C] aspirin just before being added to the heme-reconstituted ovine PGHS-1 (1.5 μM). At 0, 2 and 4 minutes, aliquots were taken and analyzed as described for figure 1. Each data point represents the mean ± S.E.M of four values for PPHP and three values for PPA experiments. The significance of differences between the control and the experiments done for each concentration of PPHP or PPA was analyzed by paired two-tailed Student’s t-test (*: p<0.05; **: p<0.01; NS: non significant).
3.3. Analysis of the role of the peroxidase activity in the effects of hydroperoxides
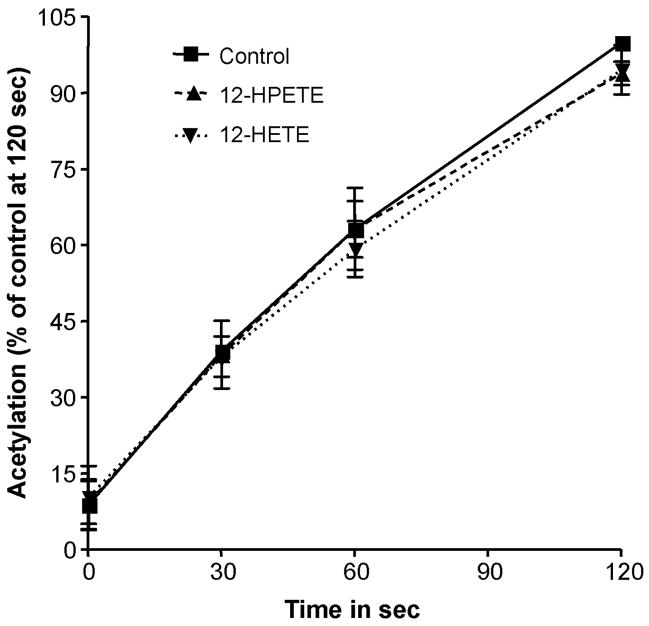
To assess whether the effect of hydroperoxides on the acetylation of PGHS is mediated by reduction of these peroxides by the PGHS peroxidase, we examined the effects of 12-HPETE and 12-HETE on the acetylation by aspirin of Cobalt-PGHS-1. In this case, the enzyme was reconstituted with cobalt protoporphyrin IX (Co3+-PPIX) instead of iron protoporphyrin IX (Fe3+-PPIX). Co3+-PGHS-1 folds in the conformation of the native enzyme but is devoid of peroxidase as well as cyclooxygenase catalytic activities. It, however, binds ligands such as the enzyme substrate, arachidonic acid, and enzyme inhibitors such as aspirin [21]. We found that the rate of acetylation of this enzyme was not affected by either 12-HPETE or 12-HETE (Fig. 5). These results, in contrast with the inhibitory effects of hydroperoxides on acetylation by aspirin of PGHSs reconstituted with Fe3+-PPIX, indicate that inhibition of acetylation of Ser530 by hydroperoxides results from reduction of the hydroperoxide by the PGHS peroxidase with the resultant 2-electron oxidation that leads to formation of the ferryloxo protoporphyrin radical.
Fig. 5.
Acetylation of Co3+-PGHS-1 by Aspirin in presence of 12-HPETE and 12-HETE. The PGHS activity is expressed as a percentage of the control activity at 120 seconds. Tris HCl (control,), 5 μM 12-HPETE and 5 μM 12-HETE were mixed separately with 90 μM [acetyl-1-14C] aspirin just before being added to the ovine PGHS-1 (1.5 μM) reconstituted with cobalt protoporphyrin. At 0, 30, 60 and 120 seconds, aliquots were taken and analyzed as described for figure 1. Each data point represents the mean ± S.E.M of three values. The acetylation profiles are not statistically different.
3.4. Effect of hydroperoxides on aspirin hydrolysis
Aspirin is known to spontaneously hydrolyze in aqueous solution and the rate of hydrolysis is dependent on the experimental conditions. To assess whether addition of hydroperoxides to the buffer increased the rate of deacetylation of aspirin, we analyzed the extent of formation of salicylic acid in the reaction mixture. Using LC/ESI/MS/MS, we found that 14.6 ± 1.6 % of aspirin was deacetylated to salicylic acid in absence of 12-HPETE, and that addition of the hydroperoxide did not significantly modify this value (14.6 ± 1.3%, n = 4).
3.5. Prevention by hydroperoxides of the inhibition of PGHS-2 by aspirin in cells
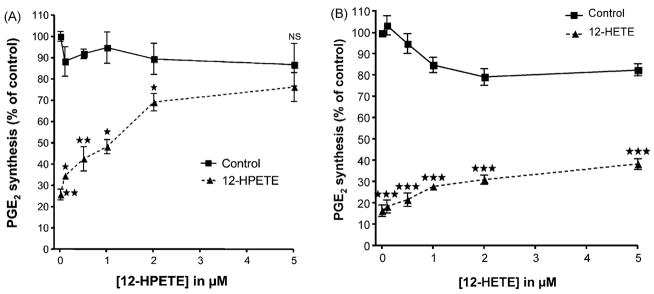
The effect of hydroperoxide on the inhibition of PGHS by aspirin was examined in A-549 cells. In preliminary experiments, we determined that in these cells, PGE2 biosynthesis could be inhibited by aspirin in a dose-dependent manner. Subsequently, we used aspirin at the final concentration of 20 μM because it inhibited PGE2 synthesis by ~80% in our experimental conditions. As aspirin can be deacetylated in these cells to form salicylic acid [22], we performed the same experiment with equivalent concentrations of salicylic acid and demonstrated that the inhibition observed with aspirin was not due to its deacetylated product. A range of concentrations of 12-HPETE was added to IL-1β activated A549 cells that immediately were exposed to 20 μM aspirin. A concentration-related reversal of the inhibition of PGHS activity was produced by 12-HPETE (Fig. 6). However, no significant effect (P>0.05) was observed when 12-HETE was used. These experiments indicate that a hydroperoxide added exogenously can antagonize inhibition of PGHS-2 in cells by aspirin.
Fig. 6.
Effect of 12-HPETE and 12-HETE on Aspirin mediated inhibition of PGHS-2 in IL-1β activated Human Lung Cancer Cell line (A-549 cell). Control experiments were conducted with 12-HPETE or 12-HETE without aspirin. 12-HPETE (panel A) or 12-HETE (panel B) were added to cells in culture at the indicated concentrations immediately before 20 μM aspirin was added. After 30 minutes pre-incubation, 2.0 μM arachidonic acid was added and kept at 37°C for 15 min. Prostanoids were analyzed by GC/NICI/MS as previously described [18]. The concentration of PGE2 present in the medium is represented as a percentage of the control to which no aspirin was added. Experiments were performed three times in duplicate. Each data points indicate mean ± S.E.M of six values. The significance of differences between the control and the experiments done for each concentration of 12-HPETE or 12-HETE was analyzed by paired two-tailed Student’s t-test (*: p<0.05; **: p<0.01 and ***p<0.0001).
3.6. Relation between aspirin efficacy and cellular hydroperoxide production
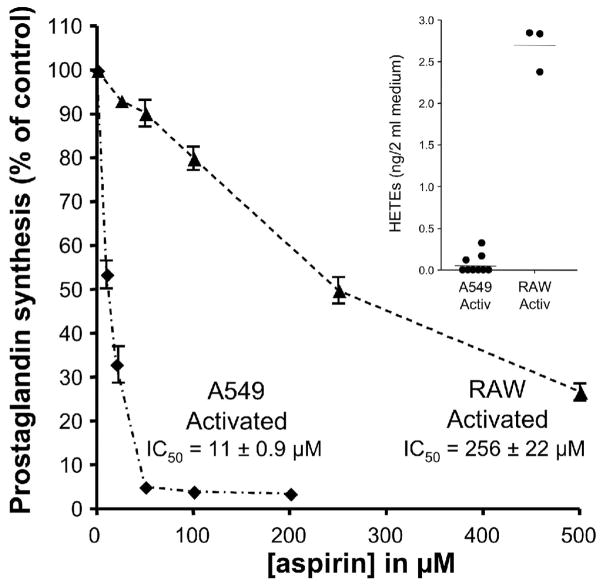
Based on the findings with purified PGHSs and in the A549 cells, we hypothesized that aspirin would be a weaker inhibitor of PGHS activity in cells with higher production of the hydroperoxides that are effective substrates for the PGHSs. The PGHS peroxidase exhibits a preference for alkyl hydrophobic peroxides as substrates, with highest activity seen with hydroperoxy-polyunsaturated fatty acids (such as HPETEs) which are slightly more active than PGG2 [23]. The rate constant for an HPETE is approximately 1000-fold greater than for H2O2 [24]. To evaluate the relation of aspirin’s effect to hydroperoxide levels in cells, the inhibition of PGHS by aspirin in activated A549 cells (PGHS-2) was compared with that in activated RAW264.7 cells (PGHS-2) which are a monocyte-macrophage cell line. HPETE formation by A549 and RAW264.7 cells was determined by analysis of the HPETE products, HETEs, with LC/APCI/MS/MS. The levels of total HETEs in activated RAW264.7 cells (1.35 ± 0.07 ng/ml) were markedly greater than in activated A549 cells (0.03 ± 0.02 ng/ml) (Fig. 7 inset). Moreover, a larger quantity of the PGHS products, PGE2 plus PGD2, was formed in the RAW264.7 cells (8 ± 0.1 ng/ml) than in the A549 cells (0.4 ± 0.05 ng/ml), indicating formation of more of the hydroperoxide intermediate, PGG2, in RAW264.7 cells. Aspirin is far more potent as an inhibitor of PGHS activity in the A549 cells (IC50 = 11.0 ± 0.9 μM) than in the RAW264.7 cells (IC50 = 256 ± 22 μM) which generate much higher levels of HPETEs and PGG2 (Fig. 7).
Fig. 7.
Inhibition of PGHS-2 by aspirin inversely correlates with HPETE production by activated human cell lines. Human cells expressing PGHS-2 were activated with IL-1β (A549) or LPS and INF-γ (RAW264.7) for 18 h. The cells were then pre-incubated with varying concentrations of aspirin for 30 min at 37°C followed by addition of 2 μM [2H8] arachidonic acid. After 15 min at 37°C, the medium was collected, and the sum of PGD2 and PGE2 was quantified by analysis by GC/NICI/MS as described previously [18]. Each data point represents the mean ± S.E.M. of four or six values. The significance of difference between the IC50 values of aspirin for the two cell lines was analyzed by two-tailed unpaired Student t-test (p<0.0001). Inset: Production of HPETEs by A549 and RAW264.7 cells. No exogenous arachidonic acid was added. After activation of the cells, the medium was collected and HETEs, the products of reduction of HPETEs, were extracted and derivatized as described in “Materials and Methods”. 11-HETE represented 98% of total HETEs. The amounts of HETEs were determined by LC/APCI/MS/MS analysis using [2H8] 12-HETE as an internal standard.
4. Discussion
The results indicate that acetylation of the PGH synthases by aspirin is regulated by the catalytic activity of the PGHS peroxidase. Acetylation occurs most efficiently when the concentration of the hydroperoxide substrates of the PGHS peroxidase are low, and is inhibited by high peroxide concentrations that generate formation of the ferryloxo protoporphyrin radical cation. This conclusion is derived from the evidence that two structurally different hydroperoxide substrates of the PGHS peroxidase both antagonize acetylation of the enzyme by aspirin, whereas the corresponding alcohol products of peroxidase catalysis do not. That the hydroperoxide effect on acetylation is mediated by the catalytic activity of the PGHS peroxidase is demonstrated in the experiments in which PGHS-1 is reconstituted with Co3+-heme. With the catalytically inactive PGHS peroxidase of the Co3+-heme enzyme, hydroperoxide is ineffective in blocking acetylation by aspirin.
The mechanism by which acetylation of serine 530 by aspirin is regulated by catalytic activity of the PGHS peroxidase may not be inferred from the evidence currently available. Present knowledge does, however, provide a conceptual framework for hypotheses. Catalytic activity of the PGH synthases is initiated by 2 electron reduction of a hydroperoxide in the PGHS peroxidase site, with resulting oxidation of the heme to the ferryloxo state with a protoporphyrin radical cation. Intramolecular electron transfer to this radical generates the Tyr385 radical that then abstracts the 13-pro(S) hydrogen from arachidonic acid. Redox cycling of the peroxidase also generates a Tyr504 radical [11], and oxidation of other amino acid residues may be inferred as well [12]. In the presence of PGHS cyclooxygenase inhibitors, however, peroxidase catalysis does not lead to formation of a Tyr385 radical in PGHS-1 [25, 26] or in PGHS-2 [27]. This suggests that these reversible inhibitors either inhibit the formation of the Tyr385 radical or markedly accelerate its reprotonation, and implies an interaction of these inhibitors in the region of Tyr 385. The effect of aspirin itself (before deacetylation) on the Tyr385 radical is not known; after acetylation by aspirin has taken place, the acetylated PGHS-1 does not exhibit the peroxide-induced Tyr385 radical, but this radical is generated by peroxide in acetylated PGHS-2, consistent with the ability of acetylated PGHS-2 to abstract a hydrogen from carbon 13 of arachidonic acid [28]. Acetylation of the enzyme by aspirin is almost completely abrogated in the Tyr385Phe mutant [29]. Crystallographic analysis indicates that Tyr348 is hydrogen bonded to Tyr385 [30] and there is evidence that this hydrogen bond affects the conformation of Tyr385 [31]. It is therefore of interest that acetylation of the enzyme also is reduced in the Tyr348Phe mutant [29]. These findings have elicited the hypothesis that a Tyr348-Tyr385-aspirin hydrogen bond network facilitates the acetylation reaction by positioning the acetyl group prior to nucleophilic attack [27, 29]. In this case, aspirin would replace the water molecule that bridges by hydrogen bonding between Tyr385 and Ser530 [32]. The present evidence that acetylation of the PGHS by aspirin is inhibited by the catalytic activity of the peroxidase complements these previous findings on the consequences of PGHS-1 peroxidase activity and on the determinants of acetylation.
The catalysis of hydroperoxide by the PGHS peroxidase in the presence of a reducing co-substrate is very rapid [33], and therefore the higher oxidative state of the enzyme is only short-lived. Accordingly, our data indicate that the reduction in acetylation rate persists for approximately 4 minutes following addition of hydroperoxide and subsequently increases back to a rate similar to that of control conditions (Supplemental figure S1). Several hypothetical mechanisms could be considered to explain the persistence of inhibition of acetylation even after the ferryloxo heme radical has returned to its reduced state. Acetylation of S530 by aspirin is an enzymatic reaction, requiring the contact of aspirin with residues that contribute to positioning and binding the substrate for catalytic efficiency. Formation of the ferryloxo heme radical cation by peroxidase activity can cause conformational change in the cylooxygenase catalytic channel [31], and can oxidize residues in this site in addition to Tyr385 [11, 12]. A rate of restitution of catalytically important perturbations in conformation or oxidative state that was slower than the rate of return of the heme to its reduced state could account for persistence of inhibition of acetylation longer than the higher oxidative states of the heme.
The demonstration of similar rates of acetylation of purified PGHS-1 and PGHS-2 does not support a selectivity of aspirin for PGHS-1. This evidence for lack of isoform selectivity was obtained with enzymes incubated with phenol as a reductant of the PGHS peroxidase in order to minimize the possible confounding effect of autoinactivation of the PGHSs by hydroperoxides. Similarly, the equal potency of aspirin on cells expressing selectively PGHS-1 (platelets) or PGHS-2 (A-549) confirms lack of isoform selectivity in a cellular environment. These findings are consistent with the similar t1/2 for inhibition of murine PGHS-1 and PGHS-2 expressed in COS-1 cells by aspirin reported by Meade et al. [34].
Oxidation of Tyr385 by peroxidase catalysis is more efficient for PGHS-2 than for PGHS-1 [35, 36], and therefore it is conceivable that hydroperoxide concentrations below the threshold for oxidation of Tyr385 in PGHS-I could engender Tyr385 formation in PGHS-2. Whether this could produce some isoform selectivity for aspirin at low concentrations of hydroperoxide deserves investigation. Certainly, however, isoform selectivity in humans can not be great in normal physiologic conditions because the clear antipyretic effect of aspirin indicates an inhibition of PGHS-2, based on the evidence that fever is a consequence of PGHS-2 derived PGE2 biosynthesis [37–39].
The concept that hydroperoxide concentration regulates PGHS acetylation by aspirin provides a basis for a hypothesis that the concentration of hydroperoxides in a cell could regulate the ability of aspirin to irreversibly inhibit the PGHSs and thereby confer a cellular selectivity on the action of aspirin. Consistent with this hypothesis is our finding that, in A549 cells which produce little of the hydrophobic hydroperoxides that are preferred substrates for the PGHS peroxidase, the potency of aspirin is more than 20 fold greater than in the RAW 264.7 cells in which these hydroperoxides are formed in much greater abundance. The resistance of this “inflammatory” cell to aspirin’s action is of interest in light of the fact that aspirin’s anti-inflammatory effect in humans is achieved only at very high doses.
The previous observation that aspirin is less effective in sonicated than in intact platelets [10] also is likely to be related to the formation of PGG2 and 12-HPETE from the arachidonic acid released on cell disruption.
Competitive inhibition of the PGHS synthases by salicylic acid also is determined by the catalytic activity of the PGHS peroxidase. This is a likely explanation for cell selectivity in the action of salicylic acid. It will be of interest to determine whether the action of any of the other competitive PGHS inhibitors is influenced by PGHS peroxidase redox cycling.
In summary, these findings provide a molecular basis for regulation of acetylation of the PGH synthases by aspirin. Acetylation occurs most efficiently when PGHS peroxidase activity is low, and acetylation is antagonized by hydroperoxides that are substrates for the PGHS peroxidase.
Supplementary Material
Acknowledgments
We thank Tina Masterson for expert assistance in cell culture. This work was supported in part by National Institutes of Health Grant GM15431 and by a grant from Merck-Frosst Canada & Company. J. A. O. is the Thomas F. Frist, Sr. Professor of Medicine. This work was presented in part at the 9th international conference “Eicosanoids and other bioactive lipids in cancer, inflammation and related disease”, San Francisco, September 11 - 14, 2005
Abbreviations
- PGHS
prostaglandin H2 synthase
- PG
prostaglandin
- 12-HPETE
12-hydroperoxyeicosatetraenoic acid
- 12-HETE
12-hydroxyeicosatetraenoic acid
- PPHP
5-phenyl-4-pentenyl hydroperoxide
- PPA
5-phenyl-4-pentenyl alcohol
- DMEM
Dulbecco’s modified Eagle’s medium (DMEM)
- FBS
Foetal Bovine Serum
- Tris
Tris(hydroxymethyl) aminomethane
- FePPIX
Iron Protoporphyrin IX
- AA
arachidonic acid
- LPS
Lipopolysaccahride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I Acetylation of a particulate fraction protein. J Clin Invest. 1975;56:624–32. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith WL, Lands WE. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971;246:6700–2. [PubMed] [Google Scholar]
- 3.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–15. [PubMed] [Google Scholar]
- 4.Roth GJ, Machuga ET, Ozols J. Isolation and covalent structure of the aspirin-modified, active-site region of prostaglandin synthetase. Biochemistry. 1983;22:4672–5. doi: 10.1021/bi00289a010. [DOI] [PubMed] [Google Scholar]
- 5.FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–88. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–72. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984;311:1206–11. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann N, Wenk A, Kim U, Kienzle P, Weber AA, Gams E, et al. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation. 2003;108:542–7. doi: 10.1161/01.CIR.0000081770.51929.5A. [DOI] [PubMed] [Google Scholar]
- 10.Burch JW, Baenziger NL, Stanford N, Majerus PW. Sensitivity of fatty acid cyclooxygenase from human aorta to acetylation by aspirin. Proc Natl Acad Sci U S A. 1978;75:5181–4. doi: 10.1073/pnas.75.10.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogge CE, Liu W, Wu G, Wang LH, Kulmacz RJ, Tsai AL. Identification of Tyr504 as an alternative tyrosyl radical site in human prostaglandin H synthase-2. Biochemistry. 2004;43:1560–8. doi: 10.1021/bi035717o. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa T, Kulmacz RJ, DeWitt DL, Smith WL. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J Biol Chem. 1990;265:20073–6. [PubMed] [Google Scholar]
- 13.Karthein R, Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur J Biochem. 1988;171:313–20. doi: 10.1111/j.1432-1033.1988.tb13792.x. [DOI] [PubMed] [Google Scholar]
- 14.Aronoff DM, Boutaud O, Marnett LJ, Oates JA. Inhibition of prostaglandin H2 synthases by salicylate is dependent on the oxidative state of the enzymes. J Pharmacol Exp Ther. 2003;304:589–95. doi: 10.1124/jpet.102.042853. [DOI] [PubMed] [Google Scholar]
- 15.Marnett LJ, Siedlik PH, Ochs RC, Pagels WR, Das M, Honn KV, et al. Mechanism of the stimulation of prostaglandin H synthase and prostacyclin synthase by the antithrombotic and antimetastatic agent, nafazatrom. Mol Pharmacol. 1984;26:328–35. [PubMed] [Google Scholar]
- 16.Rowlinson SW, Crews BC, Lanzo CA, Marnett LJ. The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2). A putative L-shaped binding conformation utilizing the top channel region. J Biol Chem. 1999;274:23305–10. doi: 10.1074/jbc.274.33.23305. [DOI] [PubMed] [Google Scholar]
- 17.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc Natl Acad Sci U S A. 2002;99:7130–5. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutaud O, Brame CJ, Salomon RG, Roberts LJ, 2nd, Oates JA. Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry. 1999;38:9389–96. doi: 10.1021/bi990470+. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Williams MV, DuBois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2168–76. doi: 10.1002/rcm.1170. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol Pharmacol. 1997;51:907–12. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 21.Malkowski MG, Theisen MJ, Scharmen A, Garavito RM. The formation of stable fatty acid substrate complexes in prostaglandin H(2) synthase-1. Arch Biochem Biophys. 2000;380:39–45. doi: 10.1006/abbi.2000.1906. [DOI] [PubMed] [Google Scholar]
- 22.Higgs GA, Salmon JA, Henderson B, Vane JR. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci U S A. 1987;84:1417–20. doi: 10.1073/pnas.84.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemler ME, Cook HW, Lands WE. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys. 1979;193:340–5. doi: 10.1016/0003-9861(79)90038-9. [DOI] [PubMed] [Google Scholar]
- 24.Lu G, Tsai AL, Van Wart HE, Kulmacz RJ. Comparison of the peroxidase reaction kinetics of prostaglandin H synthase-1 and -2. J Biol Chem. 1999;274:16162–7. doi: 10.1074/jbc.274.23.16162. [DOI] [PubMed] [Google Scholar]
- 25.Kulmacz RJ, Palmer G, Tsai AL. Prostaglandin H synthase: perturbation of the tyrosyl radical as a probe of anticyclooxygenase agents. Mol Pharmacol. 1991;40:833–7. [PubMed] [Google Scholar]
- 26.Lassmann G, Odenwaller R, Curtis JF, DeGray JA, Mason RP, Marnett LJ, et al. Electron spin resonance investigation of tyrosyl radicals of prostaglandin H synthase. Relation to enzyme catalysis. J Biol Chem. 1991;266:20045–55. [PubMed] [Google Scholar]
- 27.Rogge CE, Ho B, Liu W, Kulmacz RJ, Tsai AL. Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2. Biochemistry. 2006;45:523–32. doi: 10.1021/bi051235w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao G, Tsai AL, Palmer G, Boyar WC, Marshall PJ, Kulmacz RJ. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 1997;36:1836–45. doi: 10.1021/bi962476u. [DOI] [PubMed] [Google Scholar]
- 29.Hochgesang GP, Jr, Rowlinson SW, Marnett LJ. Tyrosine-385 is critical for acetylation of cyclooxygenase-2 by aspirin. J Am Chem Soc. 2000;122:6514–5. [Google Scholar]
- 30.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–43. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 31.Dorlet P, Seibold SA, Babcock GT, Gerfen GJ, Smith WL, Tsai AL, et al. High-field EPR study of tyrosyl radicals in prostaglandin H(2) synthase-1. Biochemistry. 2002;41:6107–14. doi: 10.1021/bi015871f. [DOI] [PubMed] [Google Scholar]
- 32.Selinsky BS, Gupta K, Sharkey CT, Loll PJ. Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry. 2001;40:5172–80. doi: 10.1021/bi010045s. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald ID, Graff G, Anderson LA, Dunford HB. Optical spectra and kinetics of reactions of prostaglandin H synthase: effects of the substrates 13-hydroperoxyoctadeca-9,11-dienoic acid, arachidonic acid, N,N,N′,N′-tetramethyl-p-phenylenediamine, and phenol and of the nonsteroidal anti-inflammatory drugs aspirin, indomethacin, phenylbutazone, and bromfenac. Arch Biochem Biophys. 1989;272:194–202. doi: 10.1016/0003-9861(89)90210-5. [DOI] [PubMed] [Google Scholar]
- 34.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–4. [PubMed] [Google Scholar]
- 35.Capdevila JH, Morrow JD, Belosludtsev YY, Beauchamp DR, DuBois RN, Falck JR. The catalytic outcomes of the constitutive and the mitogen inducible isoforms of prostaglandin H2 synthase are markedly affected by glutathione and glutathione peroxidase(s) Biochemistry. 1995;34:3325–37. doi: 10.1021/bi00010a023. [DOI] [PubMed] [Google Scholar]
- 36.Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and -2. J Biol Chem. 1995;270:24019–23. doi: 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- 37.Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–72. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, Blatteis CM. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2(−/−), but not in cyclooxygenase-1(−/−) mice. Brain Res. 1999;825:86–94. doi: 10.1016/s0006-8993(99)01225-1. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Electron microscopic evidence for induction of cyclooxygenase-2 in brain endothelial cells. Ann N Y Acad Sci. 1998;856:278–80. doi: 10.1111/j.1749-6632.1998.tb08338.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.