Abstract
Cell migration is critically important for the repair of chronic wounds, which cost billions of dollars each year to treat and can lead to serious complications, including amputation and death. Growth factors, including epidermal growth factor (EGF) and insulin-like growth factor (IGF-1), are known to be deficient in chronic wounds; unfortunately, traditional delivery of soluble growth factors to wounds is expensive and complicated by their degradation. We have previously shown that directed and accelerated keratinocyte migration could be achieved by creating immobilized gradients of EGF. In this work, we have optimized EGF gradients for cell migration, synthesized and characterized gradient patterns of IGF-1, and tested for migration synergy upon combination of EGF and IGF-1 patterns. An optimal EGF concentration and pattern were identified, resulting in migration that was almost 10-fold that achieved on unpatterned controls. Immobilization of IGF-1 gradients also accelerated and directed keratinocyte migration (p<0.05), however, no difference in migration was found across various IGF-1 concentrations or gradient patterns. Although combining EGF with IGF-1 patterns did not accelerate migration beyond levels achieved using EGF alone, these methods can be applied to create other types of multi-component gradients that will ultimately be utilized to create 3-D bioactive wound dressings.
Key Terms: biomolecule tethering, epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), keratinocytes, wound healing
Introduction
Several billion dollars are spent each year treating chronic wounds with expensive, painful and often unsuccessful medical procedures.1, 2 Chronic wounds are characterized by their failure to heal in a timely manner, and they often lead to serious complications, including bone infection, amputation, sepsis, and even death. In the United States, approximately 82,000 lower-limb amputations were performed on diabetics in 2002, and currently at least 20.9% of all people age 60 or older have diabetes.3 As the world's population grows and life expectancy steadily increases, issues such as immobility, weakened immune systems, impaired vascular systems, and diseases that accompany wound healing deficiencies (such as diabetes) have become more prevalent, thus increasing the incidence of chronic wounds.4, 5
Cell migration is a critical component of normal wound healing in all tissues throughout the body.6-8 During normal dermal wound healing, keratinocytes migrate from the wound edge in order to reepithelialize the wound. The epidermis is a vital protective layer that not only prevents infection and tissue fluid loss, but also provides molecular cues, such as growth factors, to a variety of cells in the underlying wound bed to stimulate further wound healing. In contrast, chronic wounds are characterized by a failure to reepithelialize, and in turn, they lack the normal cell-cell communication that occurs via cues (i.e., growth factors) within the wound bed. Growth factor deficiencies lead to impaired wound healing, as reduced levels of epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and several other growth factors have been observed in chronic wounds when compared with normal acute wounds.9-11 Many of these chronic wounds fail to heal completely, exhibiting exceptionally slow healing accompanied by infection, scarring and serious complications.
Currently, the most common treatment for chronic wounds involves debridement, cleansing, and application of moist dressings on a daily basis – this is painful, often ineffective, and has a high long-term cost.12 Advances in chronic wound care include application of soluble growth factor-infused gels to the wound dressing. However, gels are rapidly washed away by wound exudate or absorbed by the wound dressing, and soluble growth factors are rapidly degraded when in contact with the body.13-15 Thus, soluble growth factors are not available for prolonged periods as is usually necessary to elicit the desired wound healing response.16
Our group has focused on developing a more sophisticated approach to enhance wound healing via precise spatial control of immobilized biomolecules in wound dressing materials. Immobilizing growth factors and other biomolecules would minimize the amount of expensive material lost to premature degradation, as well as prolong exposure of the cells in the wound bed to the growth factor stimuli. Gradients of soluble and matrix-bound biomolecules exist extensively in vivo, directing cells for various tissue functions.17 Thus, we proposed that immobilized concentration gradients of growth factors would induce accelerated and directed cell migration. In our previous publication, we described the synthesis and characterization of immobilized gradients of epidermal growth factor (EGF) in order to create a system that enabled directed migration. This work showed that immobilized EGF gradients did indeed induce accelerated and directed keratinocyte migration in vitro.18
Here we describe the optimization of EGF-patterned substrates for directed cell migration as well as the subsequent exploration of the potential migration synergy that may be achieved via immobilization of multiple growth factor patterns. Insulin-like growth factor-1 (IGF-1) is a fibroblast-derived growth factor that regulates cell cycle progression and cellular differentiation,19 promotes angiogenesis, stimulates keratinocyte and fibroblast proliferation, increases collagen synthesis, regulates cell metabolism, and protects against apoptosis.20 Most importantly, with respect to wound healing, the actions of IGF-1 are complementary to those of EGF. IGF-1 enhances keratinocyte migration through a mechanism that is distinct from that of EGF;21, 22 specifically, IGF-1 stimulates cell membrane protrusion and spreading,22 which may be particularly useful for sensing immobilized biomolecules. Moreover, the possible additive effects of IGF-1+EGF on wound epithelialization make this growth factor combination attractive for incorporation into our existing EGF-patterned system. Herein, we describe our synthesis and characterization of immobilized gradients of IGF-1, as well as exploration of their potential synergy with EGF.
Ultimately, our goal is to create a 3-D gradient patterned wound dressing designed to work with the patient's body to accelerate wound healing, decrease the duration and cost of treatment, and significantly minimize scarring, infection, and serious complications associated with delayed healing.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Cell Culture
Immortalized human keratinocytes (HaCaTs, courtesy of N. Fusenig, DKFZ, Heidelberg, Germany) were cultured and maintained in DMEM, 10% FBS, L-glutamine (2 mg/ml) penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C, 5% CO2.
Growth Factor Modification
Recombinant human EGF and IGF-1(Peprotech, Inc., Rocky Hill, NJ) were separately rendered photoactive via conjugation to Sulfo-SANPAH (sulfosuccinimidyl-6-[4′-azido-2′-nitrophenylamino]hexanoate; Pierce Biotechnology, Inc., Rockford, IL). Sulfo-SANPAH (SS) is a heterobifunctional crosslinker containing a photosensitive phenyl azide group on one end and an amine-reactive N-hydroxysuccinimide on the other (Figure 1). Photoreactive growth factors were synthesized via reaction of primary amine groups of the growth factors with the N-hydroxysuccinimide functionality of SS. The coupling reaction of growth factor with SS was performed in HEPES-buffered saline, pH 8.4, for 8 hours at room temperature with gentle shaking and a 50-fold molar excess of SS in order to ensure that a maximal amount of growth factor was rendered photoactive. All synthesis and purification steps were performed in the dark to preserve the photoactive moiety on SS. The resulting products of this conjugation will be referred to throughout this paper as SS-EGF, SS-IGF-1, or, in a general sense, SS-GF.
Figure 1.
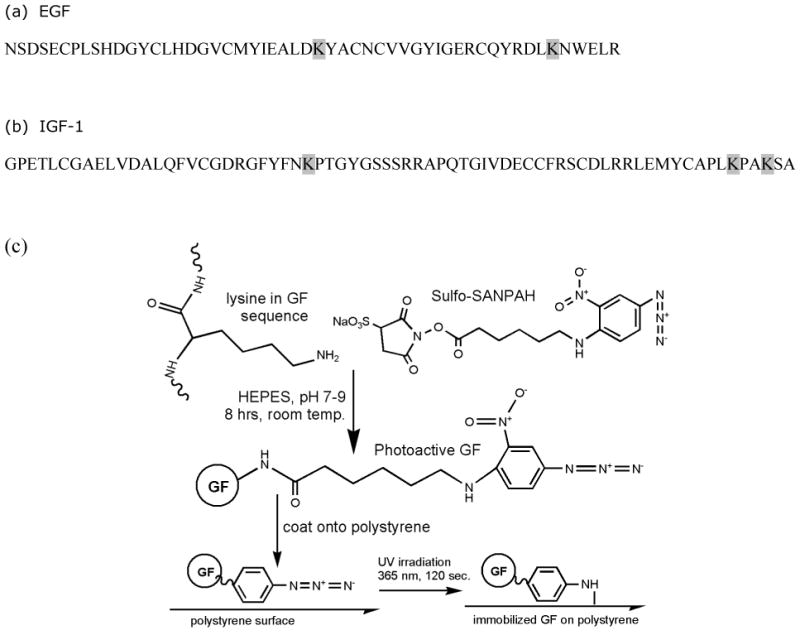
(a) Human epidermal growth factor (hEGF) amino acid sequence. Each hEGF contains 53 amino acids, including 2 lysines (K) at positions 28 and 48. (b) Human insulin-like growth factor 1 (hIGF-1) amino acid sequence. Each hIGF-1 contains 70 amino acids, including 3 lysines (K) at positions 27, 65, and 68. (c) Reaction scheme of SS with primary amines of EGF or IGF-1 to form photoactive growth factors (GFs), and subsequent photoimmobilization of photoactive GF onto a polystyrene surface.
Validation of SS-IGF-1 Bioactivity
To confirm bioactivity of IGF-1 post-conjugation to SS, an apoptosis assay was performed (TdT-FragEL DNA fragmentation detection kit, Calbiochem). Keratinocytes were seeded at 28 × 103 cells/cm2 (96 well plate) in DMEM with 10% FBS and allowed to attach overnight. The media was aspirated and replaced with 0.2 μM methotrexate (a potent apoptosis-inducing agent) in DMEM with 0.5% FBS and incubated for 24 hours with one of the following conditions: 10 ng/ml SS-IGF-1, 100 ng/ml SS-IGF-1, 10 ng/ml IGF, 100 ng/ml IGF, or no IGF/SS-IGF-1 (n=3 per condition). The cells were fixed in 10% neutral buffered formalin for five minutes, permeabilized with proteinase K (2 mg/ml in 10 mM Tris, pH 8), followed by inactivation of endogenous peroxidases. Then, the labeling reaction mix (TdT, Biotin-dNTP, unlabeled-dNTP) was added to label breaks in DNA. Streptavidin-HRP in combination with DAB was used for detection of apoptotic cells (indicated by brown staining), and DAPI (1 μg/ml) was then added to facilitate total cell number quantification. Photomicrographs were taken of each well in each condition at 200× magnification. Positively-stained cells were counted and divided by total cell count, yielding percent apoptotic cells. The bioactivity of EGF following modification with SS was confirmed in a previous publication.18
Creation of Growth Factor Gradients
In an adaptation of the methods used by Ito et al,23 growth factors (EGF, IGF-1) were photoimmobilized onto polystyrene plates via the phenyl azide functionality of the coupled SS. The optimal reaction conditions (i.e., light intensity and exposure time) for this surface-immobilization process were defined in a previous publication by our lab.18 In order to create 2-D surfaces patterned with gradients of growth factor/s, standard photoimmobilization techniques were used in combination with a gradient-patterned photomask film. Several 3×18 mm gradient images were created in Adobe Illustrator 10, and were then used to print photomask transparency films (Imagesetter, Madison, WI). The slope of the gradient was controlled via alterations in photomask pattern design; the gradient patterns generated for the migration studies described in this manuscript are labeled g1 (quadratic), g2 (power law), g3 (linear), and g5 (logarithmic) and are described mathematically in Table 1. The g4 (power law) gradient was applied in our previous work18 and was not used in the present study because it was already shown to not be an optimal pattern.
Table 1.
Pictorial and mathematical descriptions of gradient patterns (grayscale intensity vs. pixels) involved in migration experiments. *pattern described in previous publication18
| Pattern | Photomask | Description | Equation | R2 Value |
|---|---|---|---|---|
| g1 |
|
Quadratic | y = 0.0439x2 - 1.0567x +9.7232 | 0.9979 |
| g2 |
|
Power Law | y = 0.3092x1.5608 | 0.9991 |
| g3 |
|
Linear | y = 3.4732x - 1.8789 | 0.9998 |
| g4* |
|
Power Law | y = 4.5806x0.9869 | 0.9764 |
| g5 |
|
Logarithmic | y = 75.365Ln(x) – 55.355 | 0.9594 |
Silicone isolators (Grace Bio-Labs, Inc., Bend, OR) were placed onto tissue culture polystyrene (TCPS) dishes, and 110 μl of SS-growth factor (GF) solution (0.15 μg/μl) was pipetted into one isolator on each dish and allowed to dry in an oven at 40°C. The dried SS-GF was then covered with film photomasks with various gradient slopes. Unpatterned controls consisted of unmodified TCPS, wherein an outline the same size as the GF patterns was traced on the plate. All samples, including TCPS controls with no SS-GF, were exposed to ultraviolet light at 365 nm wavelength and 90 mW/cm2 for 120 seconds (Omnicure 2000, EXFO America, Inc., Plano, TX). Upon UV exposure, the phenyl azide group enables immobilization of the SS-GF to the dish as pictured in Figure 1 and described previously. All plates were rinsed twice with deionized water (diH2O) and then filled with diH2O to rinse overnight on an orbital shaker (30 rpm). When creating samples with one growth factor patterned onto another, all steps through rinsing overnight were completed before applying the second growth factor to be patterned. When creating the first pattern, an outline is drawn framing the area the solution is dried, followed by an additional drawn outline of the patterned area. These framed areas are used to line up the second GF patterned area with the first. To prepare for cell seeding, the plates were UV-sterilized (254 nm) in a laminar flow hood for 1 hour.
Gradient Pattern Analysis
Immobilized gradients of IGF-1 were created (as described above) and immunostained to verify successful patterning. Immobilized IGF-1 was detected via standard immunochemical methods using monoclonal anti-hIGF-1 antibody (R&D Systems, Minneapolis, MN), followed by Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (Invitrogen, Eugene, OR). Photomicrographs were taken at eleven equally-spaced intervals at 200× magnification (Olympus IX51 microscope with epi-fluorescence, Hamamatsu 285 digital camera, and Simple PCI digital imaging software (Compix, Inc. Imaging Systems, Cranberry Township, PA)). NIH ImageJ software was used to measure fluorescence intensity along the length of each photomicrograph of the gradient at 2 μm intervals, and then plotted against gradient path length. The physical characterization of EGF gradient patterns was previously reported by our group.18
A modified ELISA for human IGF-1 (Quantikine, R&D Systems Inc., Minneapolis, MN) was used to quantify the total average immobilized IGF-1 (ng/ml) on the gradient patterns created using different starting concentrations (0, 75, 150, or 250 μg/ml SS-IGF-1) to verify that different increased starting concentrations corresponded to increased density of immobilized growth factors. Briefly, immobilized IGF-1 was detected via standard immunochemical methods using polyclonal antibody against IGF-1 conjugated to horseradish peroxidase, and tetramethylbenzidine as the chromogenic substrate, followed by reading absorbance at 405 nm (Synergy HT, BioTek Instruments Inc., Winooski, VT). A calibration curve was constructed using the capture antibody-coated plate (anti-hIGF-1) and untethered recombinant human IGF-1 provided with the kit, in combination with the same detection molecules described above. The ELISA was also performed on negative controls consisting of unpatterned TCPS without IGF-1.
Migration Studies and Analysis
Keratinocytes were seeded at 5 × 105 cells/ml in 1 cm2 removable wells in reduced-serum medium (5% FBS) at the start (low concentration end) of each gradient pattern. Unpatterned controls consisted of unmodified TCPS, wherein an outline the same size as the EGF patterns was traced on the plate, and keratinocytes were seeded within 1 cm2 removable wells at the base of the ‘empty’ outline under identical conditions as patterned samples (5 × 105 cells/ml, 5% FBS medium). Grid-patterned transparencies were attached underneath the patterned surfaces in order to facilitate tracking cell movement. After allowing 24 hours for cell attachment, the reduced serum medium was replaced with serum-free medium and the seeding fences were removed, thus allowing the cells to access the gradient patterns. Photomicrographs were taken of the leading edge of cell migration at 12.5× and 40× magnification every 24 hours for 7 days.
Net cell edge displacement in a single direction was measured by overlaying time-course images (Adobe Photoshop Elements 2.0), then quantifying migration distance (NIH ImageJ) via measurement of advancement of the leading cell edge. Using ImageJ software, the leading edge of keratinocytes was carefully traced and copied, along with portions of the micrograph underneath, onto the next time point micrograph. Grid lines were used to precisely line up the micrographs. The migration distance of the advancing cell sheet was measured at five separate locations on each sample by drawing perpendicular lines between the previous leading edge and new leading edge at equally spaced intervals, with a minimum sample size of three per condition.
Fluorescence Microscopy
After seven days of migration on immobilized gradient patterns of IGF-1, EGF, EGF+IGF-1, and TCPS control, keratinocytes were fixed in 10% formalin for 5 min and rinsed twice with PBS. The cells were then permeabilized in 0.1% Triton-X-100 for 5 min followed by two rinses with PBS. Phalloidin (5 units/ml), to visualize cytoskeletal f-actin, and DAPI (1 μg/ml), a nuclear counterstain, were both added to the samples and incubated at 37° C for 20 min in the dark, followed by four rinses with PBS. Photomicrographs (200×) were taken of the cells along the leading edge of migration (Olympus IX71 microscope with 3i spherical aberration correction (Intelligent Imaging Innovations), Hamamatsu ORCA-ER monochromatic camera, and SlideBook 4.2 image acquisition software).
Statistics
All experiments were performed a minimum of three separate times, with n ≥ 3. Data were compared using two-tailed, unpaired t-tests. P values less than or equal to 0.05 were considered statistically significant. Data are presented as mean ± standard deviation.
Results
Photoactive GF Bioactivity
IGF-1 is known to protect against apoptosis;20 thus, to verify the retention of bioactivity following chemical modification of IGF-1 (SS-IGF-1), an apoptosis assay was performed (Figure 2). Treatment of cell culture samples with IGF-1 (10 or 100 ng/ml) or SS-IGF-1 (10 or 100 ng/ml) in combination with a potent apoptosis-inducing agent (methotrexate) resulted in a significantly lower percentage of apoptotic cells than treatment with methotrexate alone (p<0.001), thus verifying SS-IGF-1 bioactivity. Specifically, 10ng/ml soluble IGF-1 had 3.5% (+/- 6.8%) apoptosis, 100ng/ml soluble IGF-1 had 0.8% (+/- 1.6%) apoptosis, 10ng/ml SS-IGF had 4.8% (+/- 6.8%) apoptosis, 100 ng/ml SS-IGF-1 had 3.1% (+/- 4.6%) apoptosis, and the positive control with no growth factor had 56.9% (+/- 12.8%) apoptosis. IGF-1 samples (10 or 100 ng/ml) and SS-IGF-1 samples (10 or 100 ng/ml) were not significantly different from one another. Previous studies confirmed the bioactivity of EGF following immobilization.18, 24
Figure 2.
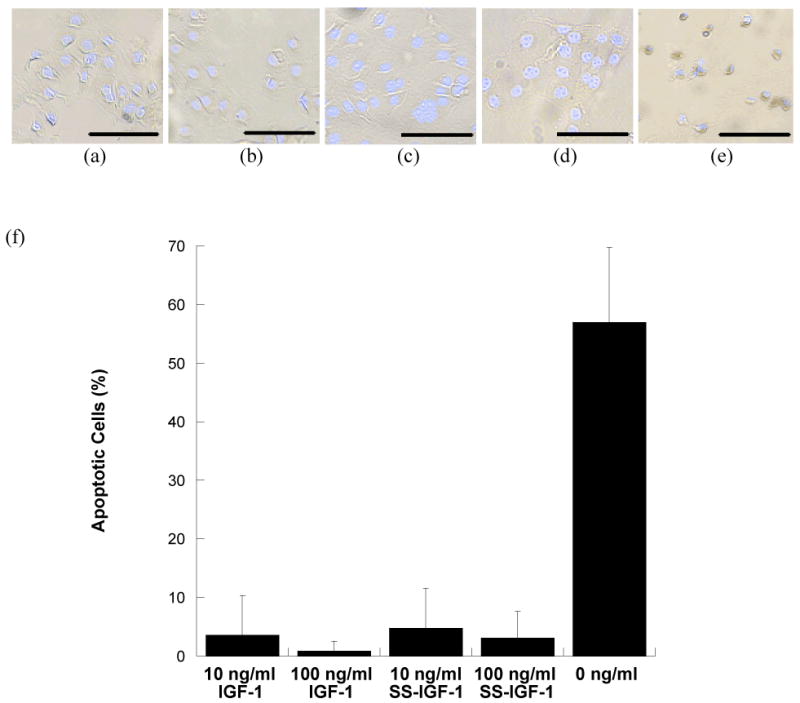
The bioactivity of SS-IGF-1 was evaluated by measuring apoptosis of keratinocytes following addition of methotrexate (0.2 μM) to samples in the presence or absence of IGF-1 or SS-IGF-1. Photomicrographs of: (a)10 ng/ml IGF-1 + methotrexate (b)100 ng/ml IGF-1+methotrexate (c)10 ng/ml SS-IGF-1+methotrexate (d)100 ng/ml SS-IGF-1+methotrexate, and (e) methotrexate alone (dark brown pigment indicates apoptotic cells). (f) Average percent apoptotic cells following treatment with methotrexate and 10 ng/ml IGF-1, 100 ng/ml IGF-1, 10 ng/ml SS-IGF-1, 100 ng/ml SS-IGF-1, and no IGF-1. n=3, p<0.001 for all IGF-1 and SS-IGF-1 samples when compared to control with no IGF-1/SS-IGF-1. (Scale bar represents 100μm)
Optimization of Immobilized EGF Gradients
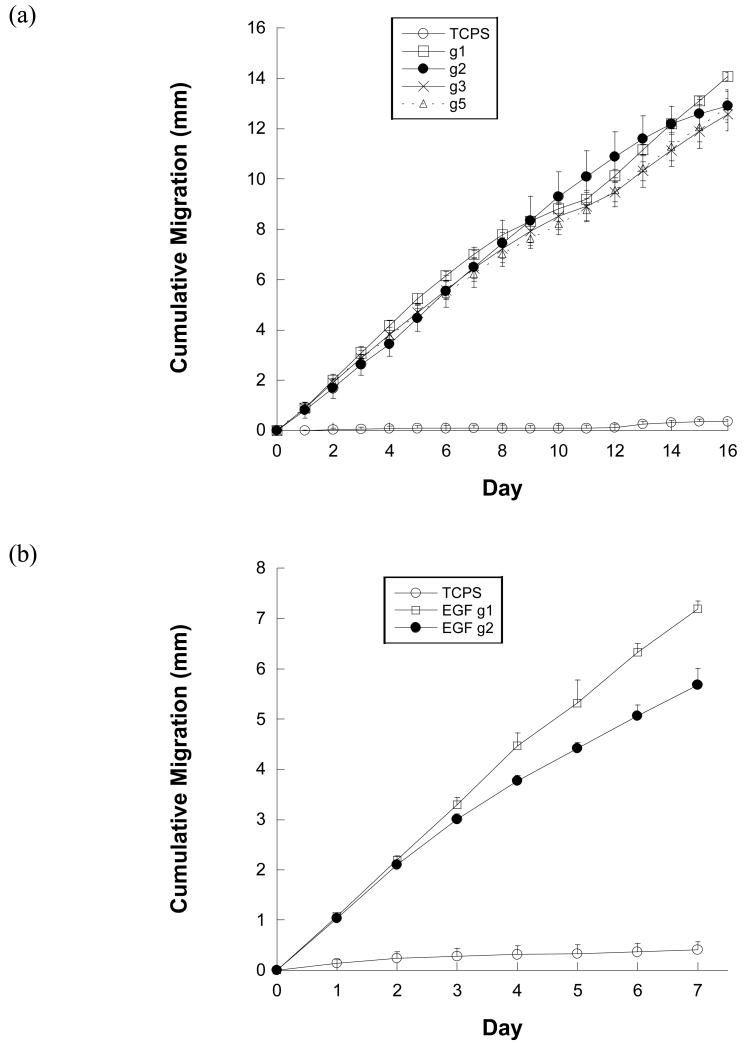
In an effort to identify a gradient pattern that elicited the greatest keratinocyte migratory response to immobilized EGF, several different gradients patterns, g1(quadratic), g3 (linear), g5 (logarithmic) (Table 1) were created and evaluated. As described in Table 1, the slope of g1 is initially low, and then increases (a quadratic fit); g2 follows a power law equation; g3 is linear; and g5 initially has a steep, increasing slope, followed by a decreasing slope (a log fit). All EGF gradients elicited significantly greater average cumulative migration than TCPS controls at every time point (p<0.001, n= 5), with a generally linear trend (Figure 3a). These trends are similar to previously published results in which cumulative migration was analyzed for linearity on EGF patterns and showed a linear trend with an R2 value greater than 0.99.18 Throughout the 16-day migration experiments, the total average cumulative migration on g1 (quadratic) gradients tended to be higher than migration on all other gradient patterns (p<0.05 at final time point). In previously published experiments, keratinocyte migration on g2 (power law) gradients of immobilized EGF was statistically higher than on g4 (power law) gradients when averaged over days 1-13.18 Therefore, we chose to compare the “best” gradient pattern from previous experiments, g2 (power law), with the “best” gradient pattern of our current experiments, g1 (quadratic). Due to the fact that trends emerged within the first week of most migration experiments, the experimental migration time was reduced to 7 days. Through this comparison (Figure 3b), it was found that the average cumulative migration of keratinocytes on g1 (quadratic) gradients of EGF was significantly higher than g2 (power law) gradients on days 3-7 (p<0.05).
Figure 3.
(a) Average cumulative migration of keratinocytes on gradients g1 (quadratic), g2 (power law), g3 (linear), g5 (logarithmic) of immobilized SS-EGF (167 μg/ml) and TCPS over 7 days. (b) Average cumulative migration of keratinocytes on gradients g1 (quadratic), g2 (power law) of immobilized SS-EGF (167 μg/ml) and TCPS over 7 days. (c) Average cumulative migration of keratinocytes on immobilized SS-EGF g1 (quadratic) gradients of 0, 83, 167, 333, and 667 μg/ml SS-EGF solutions and TCPS over 7 days.
To further optimize keratinocyte migration on the aforementioned g1 (quadratic) EGF gradient pattern, various concentrations of EGF solutions (83, 167, 333, 667μg EGF/ml) were patterned and evaluated for average cumulative migration. As shown in Figure 3c, all EGF gradients elicited significantly greater average cumulative migration than TCPS controls at every time point (p<0.001). The 83, 167 and 333 μg EGF/ml conditions resulted in linear average cumulative migration trends similar to earlier experiments. Average cumulative migration of keratinocytes on gradient patterns using 167 μg EGF/ml was significantly higher than 83 μg EGF/ml on days 2-7 (p<0.05). On days 6 and 7, the average cumulative migration distance of keratinocytes on the 167 μg EGF/ml condition was significantly higher than that found when using 667 μg EGF/ml (p<0.05). The highest concentration, 667 μg EGF/ml, exhibited a downward trend in cumulative migration, indicating a slowing migration speed after day 3. There was no significant difference in cell migration when comparing surfaces modified using 167 vs. 333 μg EGF/ml. Thus, the 167 μg EGF/ml condition emerged as the optimal concentration, as it represented the lowest EGF amount at which the highest level of migration could still be achieved.
IGF-1 Gradient Characterization
To verify successful photo-immobilization of SS-IGF-1 to tissue culture polystyrene, a g1 quadratic pattern was created, immunostained, and analyzed. The SS-IGF-1 was successfully photo-immobilized in the desired gradient pattern (Figure 4). The plot profile of the IGF-1 gradient (as measured by image quantification) correlated well with the plot profile of the photomask. Immobilized IGF-1 increased steadily and slowly initially, then steeply, along the length of the gradient path (along the x-axis), while remaining homogeneous across the width of the path (along the y-axis), as desired (Figure 4).
Figure 4.
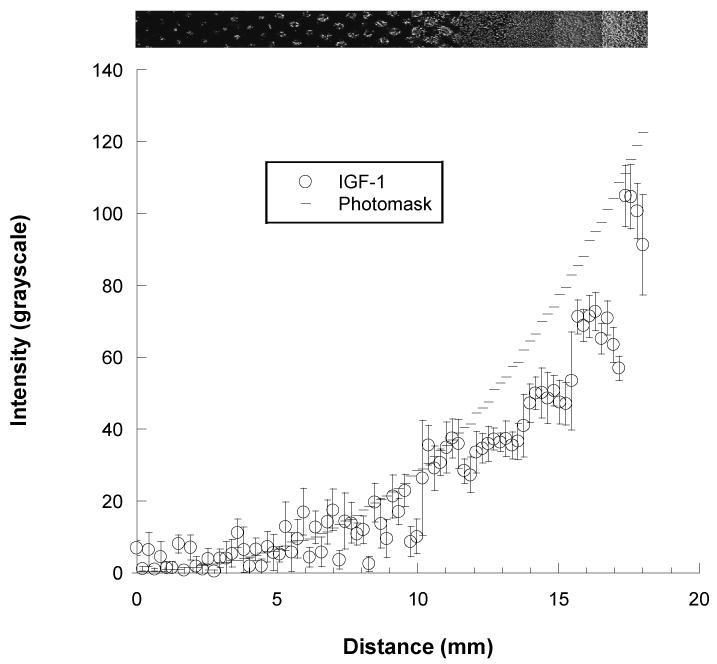
Top: Photomicrograph of fluorescently labeled SS-IGF-1 (150 μg/ml) patterned onto a polystyrene surface using a g1 (quadratic) gradient photomask (200× magnification), and Bottom: Corresponding graph of the increasing concentration gradient of immobilized SS-IGF-1 (open circles). Graph of photomask pattern represents expected values (dashes).
An IGF-1 ELISA was performed to quantify total immobilized IGF-1 patterned using 75, 150, or 250 μg/ml SS-IGF-1 as the starting solution to verify that the total patterned IGF-1 increased with increasing concentration of the starting solution. As the starting solution increased, the total immobilized IGF-1 also increased, with all concentrations significantly different from one another (data not shown).
Optimization of Immobilized IGF-1 Gradients
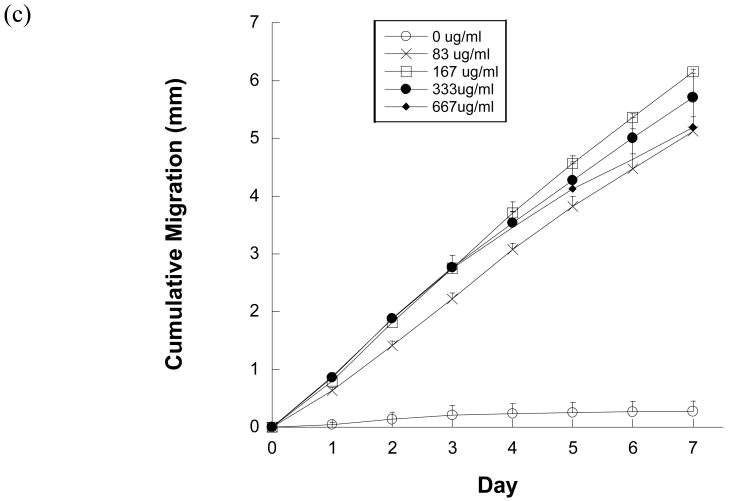
A 7 day pilot migration study was first performed to evaluate whether immobilized IGF-1 could elicit a migratory response in keratinocytes. The average cumulative migration of keratinocytes on IGF-1 g1 (quadratic) gradients (150 μg/ml IGF-1) was, in fact, significantly higher than TCPS controls at every time point (p<0.05, n=5; data not shown). However, the average keratinocyte migration rate on all gradients of IGF-1 slowed dramatically by day 3.
Given that gradients of immobilized SS-IGF-1 did induce accelerated and directed keratinocyte migration, optimization experiments were performed, including a comparison between g1 (quadratic) and g2 (power law) patterns and various concentrations of IGF-1. As shown in Figure 5a, the average cumulative migration on both g1 (quadratic) and g2 (power law) gradients of immobilized IGF-1 was significantly higher than TCPS controls at every time point for 7 days (p<0.05), although there was no significant difference between the g1 (quadratic) and g2 (power law) gradient patterns.
Figure 5.
(a) Average cumulative migration of keratinocytes on gradients g1 (quadratic), g2 (power law) of immobilized SS-IGF-1 (150 μg/ml) and TCPS over 7 days. (b) Average cumulative migration of keratinocytes on immobilized SS-IGF-1 g1 (quadratic) gradients of 0, 75, 100, 150, and 250 μg/ml SS-IGF-1 solutions and TCPS over 7 days.
To determine an optimal range of IGF-1 concentrations, as with EGF, various concentrations of IGF-1 solutions (75, 150, 200, 250 μg/ml IGF-1) were patterned and seeded with keratinocytes to evaluate their effects on directed migration. The average cumulative migration of keratinocytes seeded on all concentrations of immobilized IGF-1 was significantly greater than TCPS controls at every time point (p<0.05; Figure 5b), but there were no significant differences between the various concentrations. Interestingly, similar to the EGF concentration results, the trend of average cumulative migration of cells on the highest concentration (250 μg/ml IGF-1) gradient was consistently below the average cumulative migration on 75, 150, and 200 μg/ml IGF-1gradients. Again, the average keratinocyte migration rate on all gradients of IGF slowed significantly by day 3.
IGF-1 and EGF Synergy Experiments
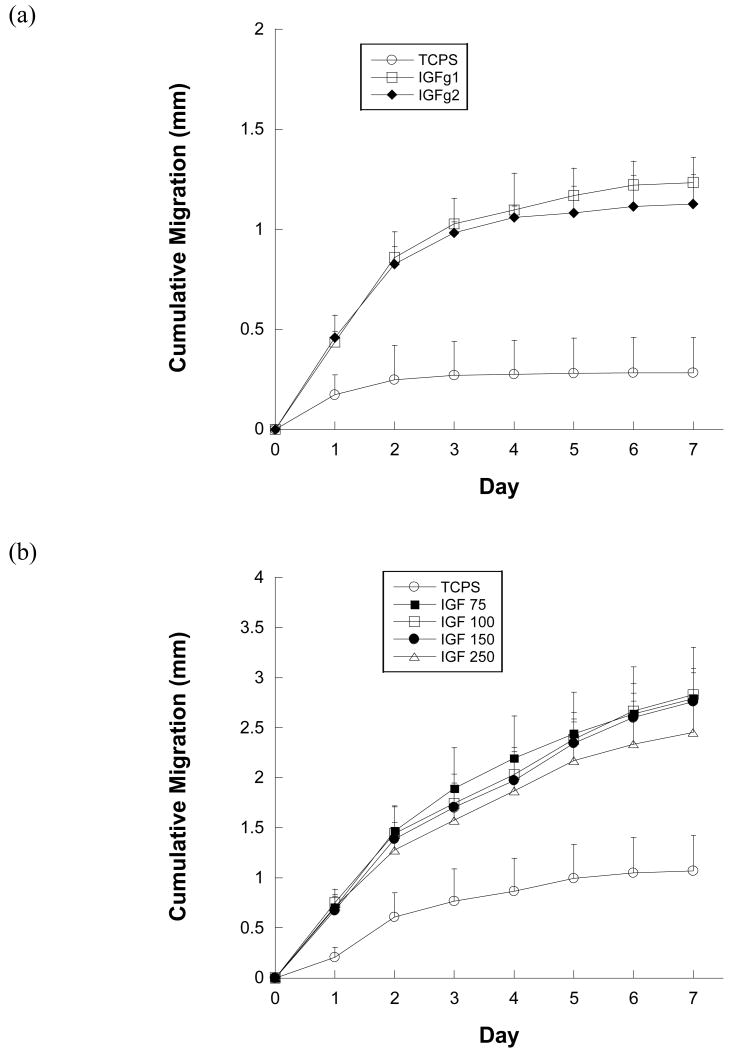
The success of EGF and IGF-1 gradients in stimulating directed cell migration led to the exploration of possible synergy upon gradient combination, and previous studies have shown synergistic migration and receptor upregulation when combining soluble EGF and IGF-1.25-28 Therefore, several migration experiments were performed to evaluate a possible synergistic effect when patterning EGF and IGF-1 together. To distinguish doubling the total growth factor concentration from true synergy, the first experiment compared IGF-1 patterned onto already-patterned EGF at 150 μg/ml each (total growth factor concentration of 300 μg/ml), and IGF-1 patterned onto already-patterned EGF at 75 μg/ml each (total growth factor concentration of 150 μg/ml). These conditions were compared against patterned EGF alone (total growth factor concentration 150 μg/ml) to determine if a synergistic effect was present. As seen in Figure 6a, the average cumulative migration of keratinocytes on all patterned gradients of growth factors was significantly higher than TCPS controls, however, there was no significant difference between IGF-1 patterned onto already-patterned EGF at 150 μg/ml each (total of 300 μg/ml) and patterned EGF alone (150 μg/ml). However, the average cumulative migration on IGF-1 patterned onto already-patterned EGF (150 μg/ml each) and patterned EGF alone (150 μg/ml) was significantly higher than IGF-1 patterned onto already-patterned EGF at 75 μg/ml each on days 5-7 (p<0.05).
Figure 6.
Average cumulative migration of keratinocytes on gradients of immobilized SS-EGF, SS-IGF-1, and SS-IGF-1+SS-EGF and TCPS over 7 days. (a) g2 (power law) gradient patterns of SS-EGF (150 μg/ml), SS-IGF-1 patterned on SS-EGF (each 75 μg/ml and denoted 1/2), SS-IGF-1 patterned on SS-EGF (each 150 μg/ml) (b) g1 (quadratic) gradients of SS-EGF (150 μg/ml), SS-EGF patterned on SS-IGF-1 (each 150 μg/ml)
To determine if patterning one growth factor on top of another altered effectiveness, migration experiments were conducted with IGF-1 and EGF (150 μg/ml each) mixed prior to patterning compared to IGF-1 patterned onto already patterned EGF (150 μg/ml each). Again, these conditions were compared to patterned EGF alone (150 μg/ml). The average cumulative migration values obtained when patterning the premixed IGF-1 + EGF, IGF-1 patterned onto EGF, and patterned EGF alone were not statistically different from each other (data not shown).
In order to determine if the order of growth factor patterning affected the observed migration outcomes, another synergy experiment was conducted comparing EGF patterned onto IGF-1 (150 μg/ml each) and patterned EGF alone (150 μg/ml). While Figure 6b shows that the average cumulative migration on both growth factor conditions was significantly greater than TCPS controls (p<0.05), there was no significant difference between these conditions.
Lamellipodia and Actin Organization Analysis
Lamellipodia and actin organization of keratinocytes were qualitatively analyzed using fluorescence microscopy. Photomicrographs of leading edge cells were compared from migration studies on TCPS controls, and patterned gradients of EGF, IGF-1, and EGF+IGF-1. Keratinocytes that migrated on TCPS controls exhibited a spread, flat morphology typical of a non-migrating cell. These cells contained significant actin stress fibers anchoring the cell in place, and little to no lamellipodia. Cells on IGF-1 exhibited the morphology of moderately migrating cells with rounded morphology, diffuse actin with stress fibers along each side of the cell, and many small filopodia branching off one or two lamellipodia. Keratinocytes migrating on EGF gradients exhibited the morphology of actively migrating cells with rounded morphology and diffuse actin, but in contrast to IGF-1, generally had one broad lamellipodium. Keratinocytes migrating on gradients of EGF+IGF-1 showed a combination of EGF and IGF-1 morphology. These cells contained diffuse actin with stress fibers along the sides and one broad lamellipodium with ruffling and tiny filopodia branching off the leading edge of the lamellipodia. Representative photomicrographs are shown in Figure 7.
Figure 7.
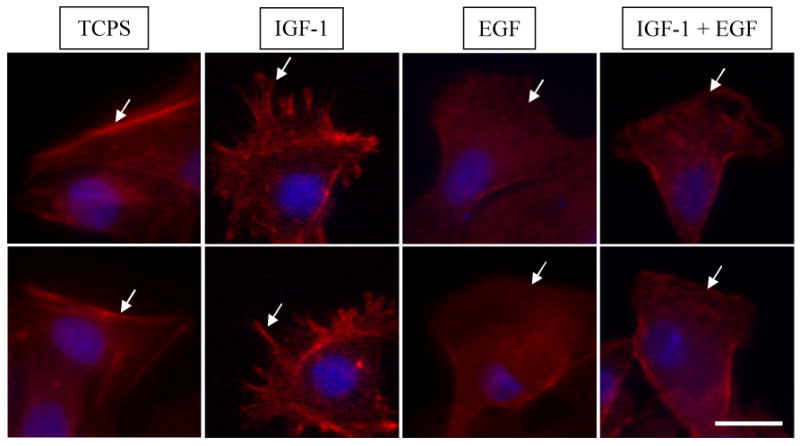
Lamellipodia morphology and actin organization. TCPS: spread, flat morphology of non-migrating cell with significant actin stress fibers anchoring cell in place and little to no lamellipodia. SS-IGF-1: moderately migrating cell with rounded morphology, diffuse actin, actin stress fibers along sides, one or two lamellipodia and many small filopodia. SS-EGF: actively migrating cell with rounded morphology, very diffuse actin and generally one broad lamellipodia. SS-IGF-1 + SS-EGF: actively migrating cell with rounded cell morphology, diffuse actin and one broad lamellipodia with ruffling and filopodia along leading edge. (Scale bar represents 30 μm, arrows denote representative areas of interest.)
Discussion
It is well known that numerous growth factors are deficient in chronic wounds.9-11 During normal wound healing, keratinocytes are drawn into the wound bed via a variety of factors and cytokines, including EGF and IGF-1.12 Unfortunately, application of wound dressings containing soluble growth factors has been met with only limited success.14, 15 These soluble growth factors degrade rapidly when in physiological conditions and are easily washed away by wound exudate; therefore a new strategy to prolong growth factor availability is warranted. Our method incorporates important wound healing factors into immobilized physiologically-relevant gradient patterns that are stable for weeks in vitro.
Current methods of generating gradients include complex microfluidics29, 30 (i.e., microfluidic chemotaxis chamber) using soluble growth factors, and various gel techniques such as an electrophoresis gradient maker31, 32. Soluble growth factors in combination with microfluidics are generally not practical for application to wounds, and the growth factors degrade rapidly. Gradient makers can only generate one gradient pattern that is difficult to precisely control, and most of the literature reports these gradients as linear.32, 33 Still others utilizing different gradient patterning techniques can only achieve approximate descriptions of gradients (i.e., linear or non-linear). Our methods allow precise control and the ability to create complex patterns with multiple factors using simple chemistry and methods. These gradient patterns are easily mathematically characterized to better understand cellular response to particular patterns, factors, and combinations of factors.
With the gradient patterning approach described herein, one can easily create different gradient patterns and combine multiple factors to study cellular responses to particular patterns, concentrations and combinations. These methods enable precise control over gradient pattern formation; thus, a variety of patterns were fabricated, characterized, and utilized in cell migration experiments to optimize migration on immobilized gradients of EGF and IGF-1 and to investigate possible synergy when combining EGF and IGF-1. Ultimately, the goal is a multi-functional wound dressing with many bioactive components, and a significant step toward this goal has been made by the successful demonstration of growth factor co-immobilization described in this report.
One limitation of the photopatterning immobilization method is that the tethering process may alter growth factor bioactivity. Thus, analysis of photoactive growth factors post-conjugation was performed to verify bioactivity prior to migration experiments. We have shown that both EGF and IGF-1 can be modified with a photoactive moiety without adversely affecting bioactivity. Moreover, these modified growth factors can then be covalently tethered in a gradient pattern, and used to induce accelerated and directed migration of keratinocytes. Although the immobilization of EGF has been previously described by our group18 and others,23, 34-36 the present communication is the first report to describe immobilization of IGF-1.
Different gradient patterns of immobilized EGF showed different trends in average cumulative migration, with the g1 (quadratic) gradient showing the best overall trend in cumulative migration of keratinocytes. As outlined in Table 1, the slope of g1 is initially low, and then increases (a quadratic fit); g2 follows a power law equation; g3 is linear; and g5 initially has a steep, increasing slope, followed by a decreasing slope (a log fit). The g1 (quadratic) and g2 (power law) gradient patterns most closely resemble an exponential fit in shape, and both of these appeared to perform the best of all the patterns tested. Exponential gradient patterns make physiological sense because, in wound healing, the clot in the wound acts as a point source of growth factors diffusing outward, likely following exponential decay outward from the wound. When tested in subsequent migration experiments, g1 (quadratic) gradient patterns of immobilized EGF consistently induced statistically significantly greater cumulative migration than g2 (power law) patterns. The differences between g1 (quadratic) and g2 (power law) may be due to the initial linearity of the g2 (power law) pattern at low concentrations. Supporting our findings, Kreuger et al found that the migration response of endothelial cells was greater when exposed to exponential gradients of soluble growth factors compared to linear soluble gradients.37
To further optimize keratinocyte migration on g1 (quadratic) EGF gradient patterns, various concentrations of EGF solutions (83, 167, 333, 667 μg/ml EGF) were patterned and evaluated for their ability to direct keratinocyte migration. Keratinocytes migrated significantly further on immobilized gradients of 167 μg/ml EGF than 83 μg/ml, 667 μg/ml EGF and TCPS controls. There was no significant difference in migration between 167 μg/ml and 333 μg/ml EGF samples. These results indicate that 167 μg/ml EGF is within the optimal concentration range for these conditions, and in fact, there is no migratory advantage to using twice this concentration. Also interesting is that the gradient patterns prepared with 667 μg/ml EGF showed a downward trend in migration speed after day 3. It is possible that the cells reached a prohibitively high concentration of EGF; high concentrations of growth factors can saturate receptors and initiate down-regulation.38
No significant difference or obvious trend was seen between g1 (quadratic) and g2 (power law) gradient patterns of immobilized IGF-1. This could be due to the fact that keratinocytes only appear to migrate consistently for the first 3-4 days on the IGF-1 patterns, and then slow down significantly; the differences in migration on g1 (quadratic) and g2 (power law) EGF patterns did not appear until day 3. Alternatively, it is also possible that the optimal gradient pattern simply was not tested. Only two patterns were tested and further studies would be necessary to determine if another pattern may be optimal for inducing keratinocyte migration on immobilized gradients of IGF-1.
Immobilized IGF-1 gradients did induce accelerated keratinocyte migration, however migration speed slowed dramatically by day 3 in all experiments. This is consistent with Wehrmann's findings that IGF-1 is released in phases during normal wound healing, peaking at 12 hours and rapidly diminishing by day 3.39 Perhaps keratinocytes respond initially, but are later inhibited by the increasing IGF-1 as it is not normally present during the entire wound healing process.
Although no significant differences in average cumulative migration were found between immobilized gradients of IGF-1+EGF and EGF alone, there appeared to be combinatorial effects in lamellipodial formation. As shown in Figure 7, there were notable differences in cell morphology, lamellipodia/filopodia, and actin organization among keratinocytes that migrated on TCPS controls and gradients of immobilized EGF, IGF-1, and EGF+IGF-1. These results are consistent with the general knowledge that EGF stimulates motility, cell rounding, actin polymerization, membrane ruffling, and lamellipodial extension,40, 41 and IGF-1 signals through PI-3K which regulates actin reorganization and in turn induces membrane protrusion and lamellipodial extension.42
In previous migration studies, soluble IGF-1 and EGF combined induced additive effects.25 Whereas, in our experiments, the migration rate of keratinocytes on co-immobilized IGF-1 and EGF was roughly equal to that seen on EGF alone. Adjusting the concentration of the EGF demonstrated that EGF acted as the dominant force in guiding cell migration speed on combined IGF-1/EGF patterns. Immobilized patterns of IGF-1+EGF may not allow the proper conformation necessary to elicit a migratory synergistic effect. It is possible that growth factor or growth factor receptor clustering is necessary and this could be hindered by immobilizing the growth factors. Other factors and components may be available that are not included in the in vitro environment tested. It is also possible that IGF-1 would accelerate wound healing via mechanisms other than cell motility and show synergy with EGF in overall wound healing in vivo. IGF-1 is known to increase neovascularization and collagen synthesis43, both of which are important components in wound healing as well. Therefore, in vivo studies would be necessary for a more thorough exploration of the existence of EGF+IGF-1 synergy in wound healing.
Conclusion
In this study, we have reported several advancements that will ultimately facilitate the creation of multi-faceted bioactive wound dressings. Namely, we have optimized gradient patterns of EGF and shown that the slope of the gradient can significantly impact cell migration; we have covalently tethered IGF-1 to surfaces, which has not previously been documented, and shown that this tethered IGF-1 also induces directed cell migration; lastly, we have demonstrated co-immobilization of multiple growth factor patterns on the same surface. It is anticipated that the 2-D growth factor immobilization techniques described herein can eventually be translated to fabricate radial 3-D gradient patterns on materials that are suitable as wound dressings.
Acknowledgments
The authors would like to thank Anika Y. Lohrentz (University of Wisconsin-Madison) for her technical assistance and acknowledge the NIH (NIBIB) for funding support (1R21-EB005440).
Abbreviations
- EGF
Epidermal growth factor
- FBS
Fetal bovine serum
- IGF-1
Insulin-like Growth Factor 1
- SS
Sulfo-SANPAH (sulfosuccinimidyl-6-[40-azido-20-nitrophenylamino]hexanoate)
- TCPS
Tissue culture polystyrene
References
- 1.Petrie NC, Yao F, Eriksson E. Gene therapy in wound healing. Surg Clin of N Am. 2003;83(3):597–616. doi: 10.1016/S0039-6109(02)00194-9. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM. Diabetic Foot Disorders: A Clinical Practice Guideline (2006 Revision) J Foot Ankle Surg. 2006;45(5S):1–66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Diabetes Digestive Kidney Diseases (NIDDK) National diabetes statistics fact sheet. 2005. [Google Scholar]
- 4.Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower-extremity ulcers. Diabetes Care. 2000;23(9):1333–1338. doi: 10.2337/diacare.23.9.1333. [DOI] [PubMed] [Google Scholar]
- 5.Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26(4):306. doi: 10.1097/01.bcr.0000169887.04973.3a. [DOI] [PubMed] [Google Scholar]
- 6.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 7.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 9.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 10.Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190(5):589. doi: 10.1002/(SICI)1096-9896(200004)190:5<589::AID-PATH553>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DM, Yu EZ, Hennessy P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219:688. doi: 10.1097/00000658-199406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound healing: the role of growth factors. Drugs Today. 2003;39(10):787. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 13.Finetti G, Farina M. Recombinant human basic-fibroblastic growth factor: different medical dressings for clinical application in wound healing. Pharmaco. 1992;47:967. [PubMed] [Google Scholar]
- 14.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90(2):133. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 15.Gu DL, Nguyen T, Phillips ML, Chandler LA, Sosnowski B. Matrix-immobilized growth factor gene therapy enhances tissue repair. Wounds. 2004;16:34. [Google Scholar]
- 16.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 17.Schneider IC, Haugh JM. Mechanisms of gradient sensing and chemotaxis: conserved pathways, diverse regulation. Cell Cycle. 2006;5(11):1130–1134. doi: 10.4161/cc.5.11.2770. [DOI] [PubMed] [Google Scholar]
- 18.Stefonek TJ, Masters KS. Immobilized gradients of epidermal growth factor promote accelerated and directed keratinocyte migration. Wound Repair Regen. 2007;15(6):847–855. doi: 10.1111/j.1524-475X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones JI. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 20.Dunn SE. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 1997;57(13):2687–2693. [PubMed] [Google Scholar]
- 21.Ando Y, Jensen P. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J Invest Dermatol. 1993;100:633–639. doi: 10.1111/1523-1747.ep12472297. [DOI] [PubMed] [Google Scholar]
- 22.Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116(Pt 15):3227. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Hayashi M, Imanishi Y. Gradient micropattern immobilization of heparin and its interaction with cells. J Biomater Sci Polymer Edn. 2001;12:367. doi: 10.1163/156856201750195270. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Ito Y, Imanishi Y. Photo-immobilization of epidermal growth factor enhances its mitogenic effect by artificial juxtacrine signaling. Biochim Biophys Acta. 1997;1358:200–208. doi: 10.1016/s0167-4889(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 25.Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116(15):3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 26.Lin JJ, Cybulsky AV, Goodyer PR, Fine RN, Kaskel FJ. Insulin-like growth factor-1 enhances epidermal growth factor receptor activation and renal tubular cell regeneration in postischemic acute renal failure. J Lab Clin Med. 1995;125(6):724–733. [PubMed] [Google Scholar]
- 27.Pietrzkowski Z, Sell C, Lammers R, Ullrich A, Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF Receptor Mediates IGF-1-stimulated Shc Phosphorylation and ERK1/2 Activation in COS-7 Cells. J Biol Chem. 2000;275(29):22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 29.Jeon NL, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM. Generation of solution and surface gradients using microfluidic systems. Langmuir. 2000;16(22):8311–8316. [Google Scholar]
- 30.Wu H, Huang B, Zare RN. Generation of complex, static solution gradients in microfluidic channels. J Am Chem Soc. 2006;128(13):4194–4195. doi: 10.1021/ja058530o. [DOI] [PubMed] [Google Scholar]
- 31.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J Biomed Mater Res. 2004;68(2):235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 33.Moore K, Macsween M, Shoichet M. Immobilized Concentration Gradients of Neurotrophic Factors Guide Neurite Outgrowth of Primary Neurons in Macroporous Scaffolds. Tissue Eng. 2006;12(2):267–278. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- 34.Ito Y. Regulation of cell functions by micropattern-immobilized biosignal molecules. Nanotechnology. 1998;9(3):200. [Google Scholar]
- 35.Ito Y. Surface micropatterning to regulate cell function. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 36.Ito Y, Chen G, Imanishi Y. Micropatterned immobilization of epidermal growth factor to regulate cell function. Bioconj Chem. 1998;9(2):277. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- 37.Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, Johansson H, Jarvius J, Park JW, Jeon NL, Kreuger J. Endothelial cell migration in stable gradients of VEGFA and FGF2: Effects on chemotaxis and chemokinesis. J Biol Chem. 2008 doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- 38.Helin K, Beguinot L. Internalization and down-regulation of the human epidermal growth factor receptor are regulated by the carboxyl-terminal tyrosines. J Biol Chem. 1991;266(13):8363–8368. [PubMed] [Google Scholar]
- 39.Wagner W, Wehrmann M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. J Cell Mol Med. 2007;11(6):1342–1351. doi: 10.1111/j.1582-4934.2007.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaya A, Ohba Y, Kurokawa K, Matsuda M. RalA Activation at Nascent Lamellipodia of Epidermal Growth Factor-stimulated Cos7 Cells and Migrating Madin-Darby Canine Kidney Cells. Mol Biol Cell. 2004;15(6):2549–2557. doi: 10.1091/mbc.E03-11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan AY, Bailly M, Zebda N, Segall JE, Condeelis JS. Role of Cofilin in Epidermal Growth Factor-stimulated Actin Polymerization and Lamellipod Protrusion. J Cell Biol. 2000;148(3):531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobes CD, H A. Rho. rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein RH. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology. 1989;124(2):964–970. doi: 10.1210/endo-124-2-964. [DOI] [PubMed] [Google Scholar]