Abstract
Nitric oxide (NO) and hydrogen peroxide (H2O2) have emerged as essential small molecules for cellular signal transduction owing in large part to their ability to mediate oxidative posttranslational modifications (PTMs). Inventing new ways to track these small, diffusible, and reactive species with spatial and temporal resolution is a key challenge in elucidating their chemistry in living systems. Recent progress in the development of fluorescent probes that respond selectively to NO and H2O2 produced at cell signaling levels offers a promising approach to interrogating their physiological production, accumulation, trafficking, and function.
Introduction
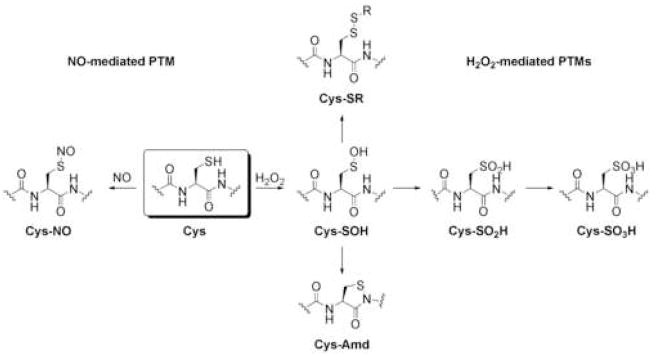
Chemical diversification of proteins by posttranslational modifications (PTMs) can expand the functional proteomes of living organisms by one to two orders of magnitude beyond their original genome sizes [1]. By decorating their proteins with precise combinations of removable phosphates, sulfates, lipids, and sugars, PTMs endow humans and other complex species with a higher level of dynamic control for disseminating genetic and biochemical information. Owing to their physiological importance and molecular diversity, PTMs offer a rich frontier of study at the chemistry/biology interface. In this regard, an emerging class of these essential in vivo reactions are broadly defined as oxidative PTMs. Unlike most PTMs, which require large enzymes to catalyze covalent sidechain modification, oxidative PTMs are mediated by small oxygen and/or nitrogen metabolites termed reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively, that are produced and destroyed on demand within all cells. Although traditionally thought of as harbingers of oxidative stress and damage, newer data supports the growing notion that ROS and RNS are also signaling molecules required for normal cellular function. The archetypal RNS/ROS second messenger is nitric oxide (NO), which is implicated in a wide range of physiological processes ranging from vasodilation in the heart and circulatory system to neurotransmission in the brain and central nervous system. In addition to binding the heme of soluble guanylate cyclase (sGC) to affect downstream function, the molecular actions of NO as a redox modulator also include the S-nitrosylation of cysteine residues on a variety of downstream protein targets [2]. Another important small signaling molecule is hydrogen peroxide (H2O2), which until recently was viewed only as an oxidative stress marker in aging and disease or as a defense agent in response to pathogen invasion. The directed molecular production of H2O2 occurs in cells throughout the body by activation of NADPH oxidase complexes (NOX) during cellular stimulation with peptide growth factors [3,4], cytokines[5], hormones [6], and neurotransmitters [7]. Once produced, H2O2 can diffuse and reversibly oxidize cysteines [8,9,10], histidines [11], or methionines [12] on protein targets that ultimately control cellular processes ranging from protein phosphorylation and gene expression. In this context, Figure 1 displays representative oxidative PTMs at cysteine modulated by either NO or H2O2.
Figure 1.
Oxidative PTMs of cysteine. Oxidation of cysteine residues by H2O2 results in the formation of cysteine sulfenic acids (Cys-SOH) that can be subsequently trapped in a variety of ways. Reactions with intra- or intermolecular thiols can form disulfides, ultimately leading back to the cysteine sulfhydryl. Further oxidation results in formation of cysteine sulfinic (Cys-SO2H) and sulfonic acids (Cys-SO3H), respectively. Intramolecular capture of Cys-SOH with the nitrogen atom of the neighboring amide backbone forms the intriguing cyclic sulfenyl amide (Cys-Amd).
The far-ranging effects of NO and H2O2 have prompted interest in devising new ways to study their cellular formation and migration, but their small size, high reactivity, and transient nature makes tracking NO and H2O2 in complex living systems a difficult task. Fluorescence microscopy with synthetic dyes that respond selectively to NO, H2O2, or related ROS/RNS offers an attractive technique for studying ROS and RNS in a non-invasive way with the ability to spatially and temporally resolve cellular events. This approach has revolutionized the field of calcium biology [13] and holds much promise for ROS/RNS biology. Aside from issues of biological compatibility and optical optimization, a key chemical challenge to overcome is disentangling the roles of individual, transient ROS and RNS in complex oxidation biology cascades. As an example, dihydrodichlorofluorescein (DCFH) is a useful and widely employed fluorescent dye for identifying global oxidative stress and signaling, yet this molecule is non-discriminant and responds fluorescently to a variety of oxidants. Accordingly, advances in the chemical design and development of selective probes for specific ROS and RNS will further our understanding of the precise molecular players and their contributions to the oxidation biology of the cell. This review will summarize design criteria for and recent progress in the development of chemoselective NO and H2O2 fluorescent probes. More comprehensive reviews for fluorescence NO [14,15] and ROS/RNS [16,17] detection have appeared in the literature.
Designing effective chemical imaging probes for cellular nitric oxide and hydrogen peroxide
Probes suitable for fluorescence imaging of NO and H2O2 in living systems meet several criteria. First and foremost is the ability to elicit a direct, selective fluorescence response to the analyte of interest without reaction with other ROS or RNS competitors. Fluorescence signaling by a turn-on emission increase or shift in excitation or emission wavelength provides spatial information that is largely lost by turn-off detection approaches. Biological constraints require water solubility, permeability to extracellular and/or intracellular membranes, and minimal toxicity to living samples. Other requirements include optical properties tailored toward use in biological environments, including sizable extinction coefficients and quantum yields in aqueous media, and visible or near-IR excitation and emission profiles to reduce or eliminate sample damage and autofluoresence from endogenous chromophores. Ultimately, selectivity remains the chief criterion by which the utility of these probes for monitoring specific analytes will be judged. With these issues in mind, we present a brief survey of recently-developed fluorescent indicators for NO and H2O2.
Small molecule fluorescent probes for nitric oxide
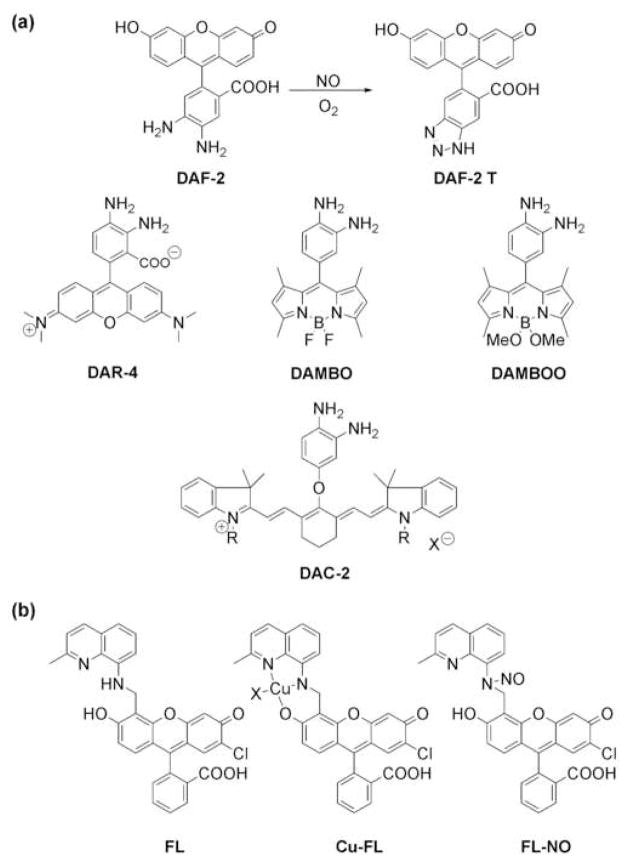
The development of fluorescent probes that can track NO signaling remains an active area of research [14,17]. Of the many strategies developed for fluorescence NO sensing [14,15,17,18], the most commonly employed NO-responsive motif is the o-phenylenediamine scaffold, which in the presence of NO and air oxidizes to the corresponding aryl triazole. The electronic differences between the electron-rich diamine and electron-poor triazole groups provide a robust switch for NO detection. For example, Nagano and co-workers have prepared a wide variety of elegant diamine probes appended to fluorescein [19], rhodamine [20], BODIPY [21,22], and cyanine [23**] chromophores for cellular NO imaging (Figure 2). Because of the greater penetration of near-IR light, the cyanine probe DAC is capable of identifying NO production in isolated whole organs as demonstrated by experiments with intact rat kidneys [23**]. In all cases, the electron-rich diamine quenches the intrinsic fluorescence of the fluorophore core by photoinduced electron transfer (PET), resulting in a weakly fluorescent species without NO. Reaction of the diamine switch with NO in the presence of oxygen generates the electron-poor triazole species and triggers a fluorescence turn-on of the dye by alleviating PET quenching. A critical feature contributing to the success of these diamine-based probes is their high selectivity for NO under aerated conditions, as the fluorescent triazole product is not formed by reaction with superoxide, hydrogen peroxide, or peroxynitrite.
Figure 2.
Fluorescent probes for detection of NO. (a) Probes based on the o-phenylenediamine scaffold give highly fluorescent triazole products upon reaction with nitric oxide in the presence of oxygen. Diaminofluorescein-2 (DAF-2), Diaminorhodamine-4 (DAR-4), Diaminobenzene-BODIPY (DAMBO), DAMBO-4,4-diOMe (DAMBOO), and diaminocyanine (DAC) are representative examples using this switch. (b) Cu(II) complexes of fluorescein derivatives, such as Cu-FL, allow for the direct detection of NO by release of the highly fluorescent nitrosylated FL ligand (FL-NO) upon reduction of Cu(II).
Despite their broad utility for cell, tissue, and organ imaging, probes that employ the o-phenylenediamine oxidation strategy leave room for improvement. One potential limitation of this switch is the indirect detection of NO, as molecular oxygen is required to react with NO to produce an intermediate RNS capable of forming the ultimate fluorescent triazole product. Accordingly, the development of tactics to directly identify NO is an important current goal of NO sensing research [15]. Lippard and co-workers recently provided the first metal-based approach for direct fluorescence detection of NO in living cells using copper(II)-fluorescein complexes [24**,25]. A prototypical example is FL, which is comprised of an 8-aminoquinaldine ligand attached to the 4′ position of the fluorescein xanthene ring (Figure 2). Formation of the 1:1 metal:ligand Cu(II) complex (CuFL) provides a dim species (ΦFL=0.077) owing to quenching by the paramagnetic Cu(II) ion. Treatment of CuFL with NO generates the bright nitrosylated fluorophore FL-NO (ΦFL-NO=0.58), presumably through a sequence involving NO-mediated reduction of Cu(II) to Cu(I) followed by nitrosylation of FL via NO+ and subsequent dissociation of the Cu(I):FL-NO complex. The system responds rapidly to NO and shows selectivity over other RNS and ROS, including H2O2, HNO, NO2−, NO3−, and ONOO−. Further experiments establish the utility of CuFL for real-time detection of endogenous NO in living cells, as this probe can visualize NO produced by both cNOS and iNOS in human neuroblastoma cells and murine macrophages, respectively. Taken together, the representative organic (diamine/triazole) and inorganic (Cu-mediated nitrosylation) approaches described here provide complementary methods for chemospecific NO and RNS detection in living systems.
Small molecule fluorescent probes for hydrogen peroxide
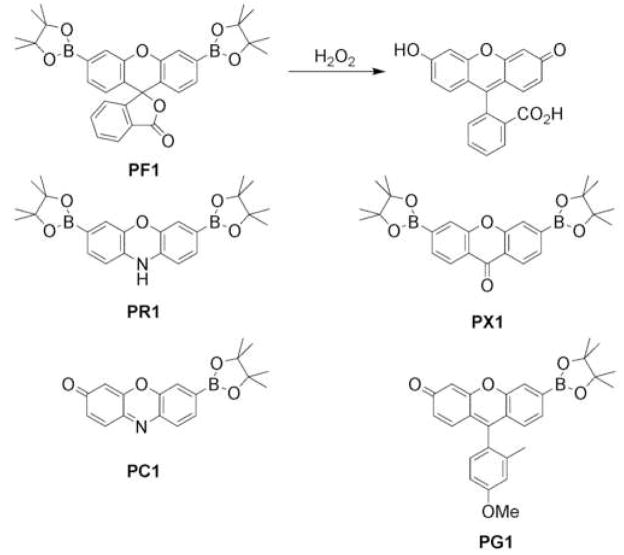
As is the case with NO, devising effective fluorescent probes for imaging cellular H2O2 offers the same global challenge of identifying this specific oxygen metabolite in complex mixtures of other ROS and RNS. Several types of H2O2-responsive fluorophores have been reported, including those that employ hydrolysable protecting groups [26,27,28], oxidizable phosphines [29,30,31], and lanthanide complexes [32,33]. We have focused on creating new chemospecific probes for H2O2 with properties amenable for imaging applications in living biological systems. In a departure from traditional ROS indicators like dihydrodichlorofluorescein and dihydrorhodamine that report global oxidant indices by nonspecific dye oxidation, our general strategy for selective H2O2 detection is to employ bond-making and bond-breaking chemical reactions on a fluorescent scaffold that are specifically triggered by H2O2. This reactivity approach can, in principle, provide specificity for any particular analyte of interest as dictated by chemistry. We have found recently that the H2O2-mediated deprotection of boronic esters to phenols provides a useful reaction-based approach to H2O2 detection (Figure 3). For example, installation of boronic esters at the 3′ and 6′ positions of a xanthenone fluoran core produces Peroxyfluor-1 (PF1), where the boronates force this platform to adopt a closed, colorless, and non-fluorescent lactone form [34]. Addition of H2O2 to PF1 triggers chemospecific, hydrolytic deprotection of the pendant boronates to generate the open, colored, and fluorescent fluorescein product with up to a >1000-fold increase in green fluorescence. Because this probe relies on a chemoselective switch, PF1 displays a >500-fold response to H2O2 over such ROS as superoxide, tert-butylhydroperoxide (TBHP), NO, and hypochlorite. Furthermore, PF1 is membrane-permeable and can image changes in intracellular H2O2 pools within living cells. We have expanded this singular example to provide Peroxyresorufin-1 (PR1) and Peroxyxanthone-1 (PX1) analogs that respond specifically to H2O2 by increases in red and blue fluorescence, respectively [35].
Figure 3.
Boronate-based optical probes for H2O2. Treatment of colorless, non-fluorescent Peroxyfluor-1 (PF1) with hydrogen peroxide yields bright green fluorescein. Peroxyresorufin-1 (PR1) and Peroxyxanthone-1 (PX1) give red and blue fluorescent compounds upon treatment with hydrogen peroxide. Mono-boronate analogs Peroxycrimson-1 (PC1) and Peroxygreen-1 (PG1) are minimally fluorescent compounds that give turn-on responses to physiological levels of hydrogen peroxide.
PR1, PF1, and PX1 offer a unique family of red-, green-, and blue-fluorescent probes that in living cells at oxidative stress levels. However, initial attempts to selectively respond to H2O2 use these diboronate reagents to identify H2O2 under oxidative signaling conditions were unsuccessful. Seeking to develop new chemical tools that were sensitive enough to report H2O2 production at physiological signaling levels while maintaining H2O2 specificity, we targeted dyes that could be activated by a single boronate deprotection. Peroxygreen-1 (PG1) and Peroxycrimson-1 (PC1) are second-generation probes that achieve both of these goals [36**]. Because of their enhanced turn-on responses to H2O2, these new chemical tools are capable of detecting endogenous bursts of H2O2 produced by growth factor signaling in living cells (Figure 4). Specifically, imaging studies using PG1 in EGF-stimulated A431 epidermoid carcinoma cells afford the first direct experiments for selectively monitoring H2O2 produced for cell signaling as well as mechanistic information on its molecular production pathway through a PI3K/Nox pathway. Moreover, analogous results obtained in primary rat hippocampal neurons stimulated with EGF indicate that the cellular machinery for generating H2O2 via an EGFr/PI3K/Nox pathway exists in brain systems as well, opening the possibility of probing similarities and differences in H2O2 signaling across different organ systems.
Figure 4.
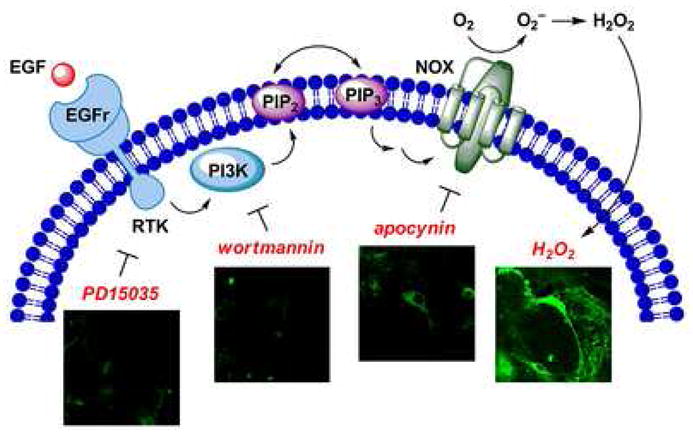
H2O2-mediated EGF signaling in the brain. H2O2 production in live rat hippocampal neuron cultures can be assayed using PG1. Binding of EGF to its cognate receptor results in upregulation of receptor tyrosine kinase activity and downstream activation of PI3K. Phosphorylation of PIP2 to PIP3 ultimately results in assembly of an active NADPH oxidase complex (NOX) to produce H2O2, which is detected by PG1. Addition of small molecule inhibitors such as PD153035, wortmannin, or apocynin results in loss of H2O2 production, as indicated by a relative decrease in PG1 fluorescence.
Conclusions and outlook
Progress in the development of new chemical tools capable of tracking NO and H2O2 with molecular fidelity in complex living systems offers a host of new opportunities for studying oxidative signaling and PTMs mediated by these small molecule second messengers. With specific regard to peroxide signaling, further investigations of the underlying molecular mechanisms of H2O2 production and trafficking promise to uncover new paradigms of funneling and/or crosstalk of H2O2 signals with various cell stimulants as well as with other cell signals, including NO. In turn, the possibilities of performing new biological experiments provide motivation to create new chemical reagents for probing living systems. A partial wishlist of new features include probes that respond by a shift in excitation and/or emission wavelength for quantitation of H2O2 and other ROS bursts by ratiometric imaging, indicators that can be targeted to specific subcellular regions to study localized signals, reagents that can respond to multiple oxidation and reduction cycles to visualize redox signaling and/or stress dynamics, and agents that can push toward imaging in whole organisms. We have made initial progress in the development of small-molecule ratiometric probes [37] and reversible redox sensors [38]. Finally, although this review has focused specifically on NO and H2O2 indicators, new fluorogenic reagents for peroxynitrite [39], hypochlorous acid [40], superoxide [41], highly reactive oxygen species [42], and global nitrative stress [43] have also been reported recently. The continuing interplay between chemical design and biological inquiry presages a rich future for studying the oxidation biology of the cell and its contributions to human health and disease.
Acknowledgments
We thank the NIH (GM079465), the University of California, the American Federation for Aging Research, and the Dreyfus, Beckman, Packard and Sloan foundations for research support. EWM was supported by a Chemical Biology Training Grant from the NIH (T32 GM066698) and a Stauffer fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Walsh CT. Posttranslational Modification of Proteins. 1. Englewood, Colo.: Roberts and Co. Publishers; 2006. [Google Scholar]
- 2.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 3.Sundaresan M, Yu Z-X, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 4.Bae YS, Kang SW, Seo MS, Baines IC, Takle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 5.Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994;126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura T, Okajima F, Sho K, Kobayashi I, Kondo Y. Thyrotropin-induced hydrogen peroxide production in FRTL-5 thyroid cells is mediated not by adenosine 3′,5′-monophosphate, but by Ca2+ signaling followed by phospholipase-A2 activation and potentiated by an adenosine derivative. Endocrinology. 1995;136:116–123. doi: 10.1210/endo.136.1.7828520. [DOI] [PubMed] [Google Scholar]
- 7.Mukhin YV, Garnovskaya MN, Collinsworth G, Grewal JS, Pendergrass D, Nagai T, Pinckney S, Greene EL, Raymond JR. 5-Hydroxytryptamine1A receptor/Gibetagamma stimulates mitogen-activated protein kinase via NAD(P)H oxidase and reactive oxygen species upstream of src in chinese hamster ovary fibroblasts. Biochem J. 2000;347:61–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 9.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 10.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 12.Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. BBA-Proteins Proteom. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 14.Nagano T, Yoshimura T. Bioimaging of nitric oxide. Chem Rev. 2002;102:1235–1270. doi: 10.1021/cr010152s. [DOI] [PubMed] [Google Scholar]
- 15.Lim MH, Lippard SJ. Metal-based turn-on fluorescent probes for sensing nitric oxide. Acc Chem Res. 2007;40:41–51. doi: 10.1021/ar950149t. [DOI] [PubMed] [Google Scholar]
- 16.Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem. 2006;386:532–543. doi: 10.1007/s00216-006-0366-9. [DOI] [PubMed] [Google Scholar]
- 17.Gomes A, Fernandes E, Lima J. Use of fluorescence probes for detection of reactive nitrogen species: A review. J Fluoresc. 2006;16:119–139. doi: 10.1007/s10895-005-0030-3. [DOI] [PubMed] [Google Scholar]
- 18.Meineke P, Rauen U, de Groot H, Korth HG, Sustman R. Nitric oxide detection and visualization in biological systems. Applications of the FNOCT method. Biol Chem. 2000;381:575–582. doi: 10.1515/BC.2000.074. [DOI] [PubMed] [Google Scholar]
- 19.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 20.Kojima H, Hirotani M, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Hirata Y, Nagano T. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal Chem. 2001;73:1967–1973. doi: 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- 21.Gabe Y, Ueno T, Urano Y, Kojima H, Nagano T. Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Anal Bioanal Chem. 2006;386:621–626. doi: 10.1007/s00216-006-0587-y. [DOI] [PubMed] [Google Scholar]
- 22.Gabe Y, Urano Y, Kikuchi K, Kojima H, Nagano T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore-rational design of potentially useful bioimaging fluorescence probe. J Am Chem Soc. 2004;126:3357–3367. doi: 10.1021/ja037944j. [DOI] [PubMed] [Google Scholar]
- 23**.Sasaki E, Kojima H, Nishimatsu H, Urano Y, Kikuchi K, Hirata Y, Nagano T. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J Am Chem Soc. 2005;127:3684–3685. doi: 10.1021/ja042967z. This communication utilizes the classic diamine/triazole switch with a near-IR reporter to provide a probe for imaging NO in isolated organs. [DOI] [PubMed] [Google Scholar]
- 24**.Lim MH, Xu D, Lippard SJ. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nat Chem Biol. 2006;2:375–380. doi: 10.1038/nchembio794. This paper describes the first metal-based fluorescent probe system for direct NO detection in living cells. [DOI] [PubMed] [Google Scholar]
- 25.Lim MH, Wong BA, Pitcock WH, Mokshagundam D, Baik M-H, Lippard SJ. Direct Nitric Oxide Detection in Aqueous Solution by Copper(II) Fluorescein Complexes. J Am Chem Soc. 2006;128:14364–14373. doi: 10.1021/ja064955e. [DOI] [PubMed] [Google Scholar]
- 26.Maeda H, Fukuyasu Y, Yoshida S, Fukuda M, Saeki K, Matsuno H, Yamauchi Y, Yoshida K, Hirata K, Miyamoto K. Fluorescent probes for hydrogen peroxide based on a non-oxidative mechanism. Andew Chem, Int Ed. 2004;43:2389–2391. doi: 10.1002/anie.200452381. [DOI] [PubMed] [Google Scholar]
- 27.Xu K, Tang B, Huang H, Yang G, Chen Z, Li P, An L. Strong red fluorescent probes suitable for detecting hydrogen peroxide generated by mice peritoneal macrophages. Chem Commun. 2005:5974–5976. doi: 10.1039/b512440a. [DOI] [PubMed] [Google Scholar]
- 28.Lo L-C, Chu C-Y. Development of highly selective and sensitive probes for hydrogen peroxide. Chem Commun. 2003:2728–2729. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 29.Onoda M, Uchiyama S, Endo A, Tokuyama H, Santa T, Imai K. First fluorescent photoinduced electron transfer (PET) reagent for hydroperoxides. Org Lett. 2003;5:1459–1461. doi: 10.1021/ol0342150. [DOI] [PubMed] [Google Scholar]
- 30.Onoda M, Tokuyama H, Uchiyama S, Mawatari K-i, Santa T, Kaneko K, Imai K, Nakagomi K. Fluorescence enhancement by hydroperoxides based on a change in the intramolecular charge transfer character of benzofurazan. Chem Commun. 2005:1848–1850. doi: 10.1039/b500419e. [DOI] [PubMed] [Google Scholar]
- 31.Soh N, Sakawaki O, Makihara K, Odo Y, Fukaminato T, Kawai T, Irie M, Imato T. Design and development of a fluorescent probe for monitoring hydrogen peroxide using photoinduced electron transfer. Bioorg Med Chem. 2005;13:1131–1139. doi: 10.1016/j.bmc.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Wolfbeis OS, Duerkop A, Wu M, Lin Z. A Europium-ion-based luminescent sensing probe for hydrogen peroxide. Angew Chem, Int Ed. 2002;41:4495–4498. doi: 10.1002/1521-3773(20021202)41:23<4495::AID-ANIE4495>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Kozhevnikov VN, Mandl C, Miltschitzky S, Duerkop A, Wolfbeis OS, Koenig B. Strong emission increase of a dicarboxyterpyridine europium (III) complex in the presence of citrate and hydrogen peroxide. Inorg Chim Acta. 2005;358:2445–2448. [Google Scholar]
- 34.Chang MCY, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol. 2007;3:263–267. doi: 10.1038/nchembio871. This letter presents the first chemoselective fluorescent probes for imaging physiological levels of hydrogen peroxide in living cells. [DOI] [PubMed] [Google Scholar]
- 37.Albers AE, Okreglak VS, Chang CJ. A FRET-based approach to ratiometric fluorescence detection of hydrogen peroxide. J Am Chem Soc. 2006;128:9640–9641. doi: 10.1021/ja063308k. [DOI] [PubMed] [Google Scholar]
- 38.Miller EW, Bian SX, Chang CJ. A fluorescent sensor for imaging reversible redox cycles in living cells. J Am Chem Soc. 2007;129:3458–3459. doi: 10.1021/ja0668973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang D, Wang HL, Sun ZN, Chung NW, Shen JG. A highly selective fluorescent probe for the detection and imaging of peroxynitrite in living cells. J Am Chem Soc. 2006;128:6004–6005. doi: 10.1021/ja0603756. [DOI] [PubMed] [Google Scholar]
- 40.Kenmoku S, Urano Y, Kojima H, Nagano T. Development of a Highly Specific Rhodamine-Based Fluorescence Probe for Hypochlorous Acid and Its Application to Real-Time Imaging of Phagocytosis. J Am Chem Soc. 2007;129:7313–7318. doi: 10.1021/ja068740g. [DOI] [PubMed] [Google Scholar]
- 41.Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, Yoshida S, Fukuda M, Fukuyasu Y, Yamauchi Y, et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J Am Chem Soc. 2005;127:68–69. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]
- 42.Koide Y, Urano Y, Kenmoku S, Kojima H, Nagano T. J Am Chem Soc. ASAP; 2007. Design and Synthesis of Fluorescent Probes for Selective Detection of Highly Reactive Oxygen Species in Mitochondria of Living Cells. [DOI] [PubMed] [Google Scholar]
- 43.Ueno T, Urano Y, Kojima H, Nagano T. Mechanism-based molecular design of highly selective fluorescence probes for nitrative stress. J Am Chem Soc. 2006;128:10640–10641. doi: 10.1021/ja061972v. [DOI] [PubMed] [Google Scholar]