Abstract
We have generated genetically engineered mice that are uniquely susceptible to lipopolysaccharide (LPS)-induced and mechanical ventilation-induced lung injury in a sex-specific and age-specific manner. These mice express a nonmuscle isoform of the myosin light chain kinase gene (nmMLCK2) targeted to the endothelium. Homozygous mice have significantly reduced fecundity and litter survival until weaning, and they are initially growth delayed but eventually exceed the size of wild-type littermates. Mice at all ages show increased protein transport across the lung barrier; however, the phenotype is most discernible in 8-12-week-old male mice. When subjected to a clinically relevant LPS-induced lung injury model, 8-12-week-old young females and 30-36-week-old males seem to be the most significantly injured group. In contrast, 30-36-week-old males remain the most significantly injured group when mechanically ventilated at high tidal volumes, which is a clinically relevant model of mechanical stress lung injury. These data reveal that nmMLCK2 overexpression in the endothelium exacerbates lung injury in vivo in a sexually dimorphic and age-dependent manner.
Actin microfilaments generate force by virtue of their ability to contract away from their site of attachment to the plasma membrane, and they do so via traction of the myosin light chain head groups attached to actin filaments by cross-bridges. The cross-bridge pulling action of myosin involves a conformational change in the molecule initiated by energetic phosphorylation of Ser19 on the myosin light chains.1 The key enzyme involved in this phosphorylation event is myosin light chain kinase (MLCK), which exists in different tissues, and it may be characterized broadly as skeletal/cardiac, smooth muscle, and nonmuscle myosin light chain kinase (nmMLCK) isoforms. Although biochemical evidence for the existence of a Ca2+/calmodulin-dependent nonmuscle isoform in cultured endothelial cell (EC) was suggested earlier,[2] and [3] the controversy as to whether this enzymatic activity is identical to the 110-kDa protein found abundantly in smooth muscle preparations was resolved when we provided conclusive proof of a larger MLCK protein isoform (210 kDa) by cloning the gene (MYLK) from a human EC-derived cDNA library.4 The gene was subsequently mapped to chromosome 3q21.5
Background
All inflammatory disorders are marked by the disruption of the semi-permeable endothelial barrier that results in increased vascular permeability. The chief mechanism involved is dynamic perturbation of the actomyosin assembly that is governed largely by the enzyme myosin light chain kinase (MLCK). Knockout of the nonmuscle MLCK (nmMLCK) results in protection from lung injury.
Translational Significance
We demonstrate that overexpression of a splice variant of the same nmMLCK protein isoform (nmMLCK2) in the endothelium results in increased lung injury. This converse result confirms the criticality of nmMLCK in the endothelium, reveals a role of age and gender in the process, and allows us to direct potential therapies in a tissue-specific manner.
Detailed characterization of the gene revealed that a single 217-kb gene, which spans 31 exons (upgraded to 272 kb and 33 exons in RefSeq build 36; all exon numbers in this manuscript are denoted according to build 36) produces both the 1914 amino acids (210 kDa) nonmuscle and the 1091 amino acids (108 kDa) smooth muscle isoforms.6 The gene also encodes a ~19-kDa protein known as kinase-related protein (KRP) or telokin that is transcribed from the C-terminal exons 30-33, and it serves to stabilize actin filaments.7 In addition to smooth muscle myosin light chain kinase (smMLCK) and KRP, 5 splice variants were identified that use the start codon of the 210-kDa isoform, but they have internal exon deletions compared with the longest variant (nmMLCK1).6 Both smMLCK and nmMLCK isoforms contain catalytic domains (eg, calmodulin binding motif) and structural domains (myosin light chain binding motif), and both isoforms exhibit robust MLC kinase activity. A major distinguishing feature is the 922 amino acids N-terminal stretch unique to nmMLCK1, which exhibits distinct cellular functions through unique interactions with other contractile proteins.[8] and [9]
Despite the uniqueness of the nmMLCK N-terminus, information is limited regarding the tissue distribution or physiologic roles of the splice variants that use the nmMLCK ATG. Northern and reverse transcription-polymerase chain reaction (RT-PCR) data6 suggest that splice variant 2 (nmMLCK2) is the most abundant isoform in many tissues, including the endothelium. In gastrointestinal epithelium, however, nmMLCK1 accounts for 97% of MLC kinase activity in the perijunctional actomyosin ring.10 Kinetically, nmMLCK1 or 2 do not significantly differ in Vmax or K0.5CaM, although only nmMLCK1 can undergo p60Src-catalyzed phosphorylation on Tyr464 and Tyr471, which are posttranslational modifications that significantly increases Vmax(3-fold increase). Interestingly, both tyrosine residues are encoded within the exon 11 that is spliced out in nmMLCK2.11
Complete targeted deletion of the nmMLCK (MLCK210) isoforms (but not smMLCK or KRP) resulted in protection of the mice from the combined stress of bacterial product lipopolysaccharide (LPS) challenge and mechanical ventilation,12 which raised the question as to the target tissue for nmMLCK protective effects in acute lung injury (ALI) (ie, epithelium, leukocytes, or vascular endothelium). In this study, we report the role of the nmMLCK2 splice variant in vascular barrier regulation, and we describe the consequences of overexpressing this isoform specifically in murine endothelium. Furthermore, as several acute and chronic inflammatory pulmonary disorders, which include ALI and acute respiratory distress syndrome (ARDS), are influenced by gender,[13], [14], [15], [16], [17], [18], [19], [20], [21] and [22] age, [23], [24], [25], [26] and [27] or gender-age interactions,[28], [29] and [30] we designed our study to evaluate the influence of these comodifiers on postulated nmMLCK2-mediated increases in vascular paracellular transport. This is supported by findings in mice, which like humans, have varying respiratory properties that differ between sexes[31], [32] and [33] and with age,[34] and [35] information often neglected when describing a pulmonary phenotype. We have further applied standard observational and pathologic approaches to initially phenotype this line—an approach that is now becoming de rigueur in the postgenomic era.[36] and [37]
Materials and methods
Construction of the tissue-specific transgenic vector
Restriction and DNA-modifying enzymes were purchased from New England Biolabs (Ipswich, Mass) or from Roche (Indianapolis, Ind). nmMLCK2 cDNA was initially subcloned from the pFastBacHta vector11 as a blunt (RsrII) -XbaI fragment into blunt (EcoRV)-XbaI digested pcDNA1.1Amp vector (Invitrogen, Carlsbad, Calif). This vector was opened at the BamHI site in the polylinker region, and a 111-bp double-stranded oligonucleotide (J27/J28) that had the features BglII-XhoI-AgeI-Optimal Kozak consensus-3XFLAG-SacII-XmaI-BamHI (in a 5′→ 3′ orientation) was inserted in this site. The nmMLCK2 open reading frame (ORF) in the resulting 3XFLAG-containing vector was brought in frame to the ATG of the tag, and the nmMLCK2-specific ATG was eliminated by deleting a fragment between XmaI and FseI (in exon 7 of nm-MLCK2 ORF) in this vector and by replacing with a 710-bp XmaI-FseI PCR product. This PCR product was amplified from the pcDNA1.1Amp subclone of the nmMLCK2 template by using the forward primer J29 (5′-ATCCCCGGGGATGTGAAGCTGGTTGCCTCG-3′) and the reverse primer J32 (5′-CTTCCCCGACCCGTTCACCAC-3′); and spanned between the 2nd amino acid (Gly; GGG) of exon 2 and the FseI site in exon 7 of the nmMLCK2 cDNA. The resulting plasmid (pJM2) was verified by sequencing both strands in the reconstructed N-terminal region, and they represent the CMV-promoter-driven mammalian expression vector for 3XFLAG-tagged nmMLCK2.
To replace the nonspecific CMV promoter with an endothelial-specific promoter, the plasmid pJM2 was digested with AseI and XhoI. This step not only released the CMV immediate-early promoter as an AseI-AseI fragment, but in addition disrupted the C-terminal part of the β-lactamase gene that was also released as an AseI-AseI fragment. This defect was repaired by a triple ligation in which the C-terminal part of the β-lactamase gene was replaced as an AseI-AscI PCR fragment (amplified from pcDNA1.1 template with forward primer J38 5′-GGCAACAATTAATAGACTGGATGGAGGCGG-3′, and reverse primer J49 5′-CGGCTCGAGGGCGCGCCTAATAACTAGTCAATAATC-3′), and ligated simultaneously to a 33-bp polylinker (AscI-NsiI-MluI-EheI-ClaI-XhoI) and the 9.4-kb AseI/XhoI digested pJM2 vector backbone. The resulting promoter-less plasmid pJM2.5 was sequence verified on both strands, digested with EheI and XhoI, and ligated to a 2.5-kb SalI-XhoI fragment from the mVE-CadherinpBLCAT3 plasmid.38 The SalI site at the 5′ end of the mouse vascular endothelial (VE)-cadherin promoter was polished with T4 DNA polymerase before ligation. The resulting plasmid (pJM4) represents the mVE-Cadherin promoter-driven mammalian expression vector for 3XFLAG-tagged nmMLCK2.
Generation of transgenic mice
For microinjection, an 8985-bp DNA fragment that contains the mouse VE-Cadherin promoter, 3XFLAG-tagged nmMLCK2 ORF, SV40 splicing site, and polyA site was obtained free of vector sequences by AscI-BaeI-PvuI digestion of pJM4 and gel purification, followed by electroelution and ethanol precipitation. Transgenic B6 mice were produced by microinjection into the male pronuclei of B6/J mice39 and screened by PCR on tail DNA. Despite the high success rate of embryo survival in hybrid strains,39 we chose the B6 strain rather than a hybrid strain primarily because inbred strains eliminate ambiguity caused by different genetic backgrounds and segregating markers in the progeny, and secondarily because we are familiar with this strain in terms of their ALI/VALI responses.[40], [41] and [42] The transgene specific primers were J9 (forward; 5′-GTGGCTCCCCTCTCCCCTCCTG-3′) and J30 (reverse; 5′-CTTGGCGGTGGCTCCTTCTTTGAT-3′), which gave a 319-bp product. Mouse β-globin gene-specific primers J18 (forward; 5′-GGCAGCTCACAAGAAGAAGTTGGG-3′) and J19 (reverse; 5′-ATCAAAGTACCGCTGGGTCCAAGG- 3′) that amplify a 138-bp band were included in all reactions as positive control. All reactions were “hot-started” for 10′ at 93°C using the AmpliTaq Gold system (Applied Biosystems, Foster City, Calif), followed by 30 cycles of 30″ at 94°C, 30″ at 60°C, and 30″ at 72°C. The copy number of transgenes was determined using the same primer pair (J9/J30), but PCR conditions were reset according to the protocols suggested by the manufacturer of the quantitative PCR cycler and the corresponding SYBR green kit (BioRad, Hercules, Calif). Animals were housed under standard conditions (12-h light-dark cycle, 25-27°C, ~40% humidity) in ventilated racks fitted with automatic watering systems, and the animals were fed Teklad 2918 diet (Harlan Teklad, Madison, Wis) ad libitum.
Cell transfections and Western analysis
HPAECs (Lonza, Walkersville, Md) (5 × 105) grown in 100-mm diameter plates were transfected with 6 μg of pJM4 or pJM2.5 plasmids and polyethyleneimine conjugated to RGD peptide (jetPEI-RGD; Polyplus Transfection, Inc. New York) at a nitrogen:phosphate ratio of 10:1. HBECs[43] and [44] plated at the same density were transfected with Fugene HD (Roche) at a Fugene:DNA ratio of 3:2. Forty-eight hours after transfection, cells were harvested in radioimmunoprecipitation assay (RIPA) buffer45 that contained an antiprotease cocktail as per the manufacturer's recommendations (Roche). RIPA lysates were precipitated with 5 volumes of cold acetone to remove cationic detergents before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE and blotting of transfected cell lysates [30 μg for human pulmonary artery endothelial cell (HPAEC) lysate, 30 and 100 μg for human bronchial epithelial cell (HBEC) lysates] on polyvinylidene difluoride membranes (BioRad) were conducted as described.46 The blots were probed with anti-FLAG M2 mAb (Sigma, St. Louis, Mo) using an alkaline phosphatase conjugated secondary antibody and CDP-Star as the chemiluminescent substrate (Western Breeze; Invitrogen).
Detection of the MLCK transgene in murine lung tissues
Human nmMLCK2 protein concentrations in the lungs of transgenic mice were determined by Western blotting. The nmMLCK2 amino-terminal 3XFLAG epitope was detected by use of anti-FLAG M2 mouse monoclonal antibody (Sigma). Lung tissue (~100 mg) was homogenized with a Polytron (Brinkman, Cantiague, NY) in 1 mL of ice-cold RIPA buffer (containing 100-μg/mL phenylmethanesulfonyl fluoride and 300-μg/mL aprotinin in addition to a protease inhibitor cocktail), incubated on ice for 30 min, centrifuged at 15,000g for 20 min at 4°C, and the supernatant collected and recentrifuged as before. These samples were snap-frozen in liquid N2 and stored at -80°C until use. A range of lysate protein was precipitated with 5 volumes of acetone, suspended in 30 μL of 1 × SDS sample buffer, boiled for 5 min, and loaded on 1.5-mm thick, 4% to 12% SDS polyacrylamide gels (Invitrogen). Blotting and detection by Western analysis was performed similar to that for cell lysates, except that an alkaline phosphatase conjugated M2 antibody (Sigma) was used directly to avoid nonspecific reactions from a peroxidase conjugated anti-mouse secondary antibody.
Murine model of LPS-induced injury
All experiments were performed according to guidelines approved by the University of Chicago Institutional Animal Care and Use Committee for the humane treatment of experimental animals. The LPS-induced ALI model has been described previously.[40] and [41] Briefly, mice were anesthetized with intraperitoneal ketamine (150 mg/kg) and acetylpromazine (15 mg/kg) before the exposure of the trachea and the right internal jugular vein via neck incision. Sterile water (pH 7.0) or LPS (2.5 mg/kg in 50 μL of the same vehicle) were instilled intratracheally via a 20-gauge catheter. The animals were exposed for a total of 24 h before tissue harvest under deep anesthesia.
Murine mechanical ventilation model
Mice were anesthetized, and the trachea was exposed as in the LPS model as we have described previously.40 A 20-gauge catheter was inserted orally into the trachea, and it was ligated with silk sutures to prevent movement. Mice were connected to a Harvard Apparatus Inspira rodent ventilator (Harvard Apparatus, Cambridge, Mass) and ventilated with room air at 65 breaths per min, tidal volume of 30 mL/kg, and 0 PEEP for the first 2 h. For the next 2 h, the tidal volume was left unaltered, but the respiratory rate was adjusted to 75 breaths per min. Boluses of sterile saline (200 μL) were given at the onset and after 2 h of ventilation, to maintain a mean arterial pressure greater than 60 mm Hg. This ventilation strategy maintains the blood gas parameters within a physiologic range (arterial pH of 7.3-7.5, HCO3 11-16 mmol/L) at the end of the experiment.47
Bronchoalveolar lavage (BAL) fluid and leukocyte counts
At the termination of each experiment, animals were euthanized by exsanguination under anesthesia in accordance with institutional guidelines. Both lungs were lavaged with 1.0 mL of Hank's buffered saline solution with the fluid allowed to equilibrate for 10 s before withdrawing. The pulmonary vasculature was perfused clear via the pulmonary artery with sterile phosphate-buffered saline (PBS). Both lungs were excised, weighed, and snap-frozen in liquid N2 for subsequent analysis. The BAL fluid collected was centrifuged at 500 × g for 20 min at 4°C, and the supernatant was removed and recentrifuged at 12,000 × g before snap-freezing as above. Cell pellets were resuspended in 0.5 mL of red blood cell lysis buffer (ACK Lysing Buffer; BioSource International, Camarillo, Calif) for 20 min and then repelleted by centrifugation at 2500 rpm for 20 min at 4°C. The supernatant was decanted, and the cell pellet was resuspended in 0.2 mL of PBS for cellular analysis using a standard hemacytometer technique. A total of 300 BAL cells per slide were counted for cell differentials using a Diff-Quick-stained kit (Baxter Diagnostics, McGaw Park, III).47
Microvascular permeability assays
The protein concentration in BAL was determined by a colorimetric bicinchoninic acid assay. Albumin and IgG concentrations in the BAL at 1:1000 and 1:100 dilutions, respectively, was quantitated using sandwich enzyme-linked immunosorbent assay (ELISA; Bethyl Labs, Montgomery, Tex). Lung tissue homogenates were prepared in phosphate buffer (pH 6.0) that contained 0.1% hexadecyltrimethyl ammonium bromide (HTAB; Sigma) by homogenization on a Polytron fitted with a 7-mm probe. The homogenates were frozen, thawed, sonicated, and the supernatant recovered as described.48 Extravasation of albumin and IgG in the lung parenchyma was determined from the clear supernatant using the sandwich ELISA kits as above, at dilutions of 1:20,000 and 1:250, respectively. Neutrophils trapped in the lung parenchyma were monitored from the same tissue extract at 1:100 dilution using a sandwich ELISA kit specific for mouse myeloperoxidase (HyCult biotechnology bv, Uden, The Netherlands). Values read in concentration terms (ng/mL) were converted to mass antigen/wet weight of lungs by taking into account the weight of the tissue that was homogenized initially in 1 mL of phosphate-HTAB buffer.41
Statistical analysis
Values are reported as mean ± SD. Data were stored and analyzed with an SPSS package (SPSS Inc., Chicago, III) as described previously.41 To detect a phenotype, type I sums of squares (for fixed effects or model I analysis of variance) were computed for a 4-way factorial design in which genotype, treatment, age, and sex were considered as independent contributors (factors) to observed variances of means.49 However, as SPSS reports output of such multiway analysis of variance (ANOVA) in the form of multiple contrasts alone, the fixed factors (age, sex, genotype, and treatment) were collapsed into 24 permutations (wild-type untreated young female, wild-type LPS-treated young female, transgenic ventilated old male, etc) and analyzed in 1-way (single factor classification analysis of variance) ANOVA terms to facilitate visualization of significant differences between groups.
Results
Expression of the MLCK transgene in human lung endothelium
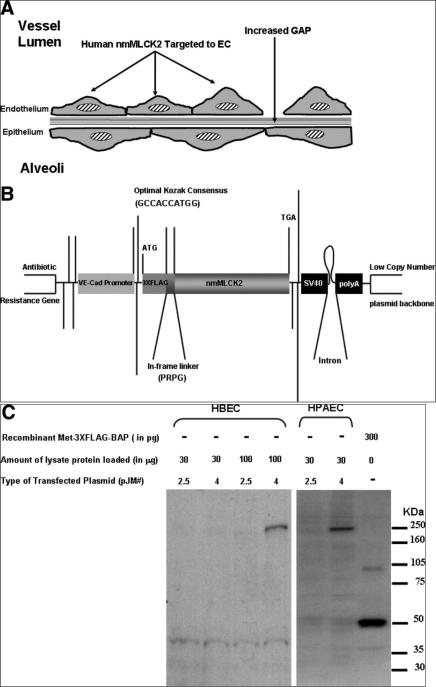
We used 2.5 kb of the mouse VE-cadherin promoter to construct a 3XFLAG-tagged MLCK2 transgene (Fig 1, B) for expression in mice. To verify MLCK transgenic expression in endothelium, we transfected the plasmid pJM4 in HPAECs. Western blotting of cell lysates transfected with this construct demonstrate a FLAG-reactive band of approximately 250 kDa (Fig 1, C). The transgenic construct is weakly expressible in HBECs but with lower efficiency (Fig 1, C). In contrast, a plasmid that bears the same ORF but lacks a promoter (pJM 2.5) is not expressible in either cell type.
Fig 1.
Concept, design, and verification of the endothelial targeted MLCK2 transgenic construct. (A) Depicted is the schematic underlying the generation of the transgenic mouse that exhibits increased expression of nmMLCK2 targeted to the vasculature; this finding will result in increased actomyosin contraction and vascular barrier disruption. (B) Schematic of the transgene construct, the 3XFLAG tag is 3 repeats of the octapeptide sequence DYKDDDDK, with the first octapeptide preceded by a methionine within a Kozak consensus sequence. The tag is separated from the nmMLCK2 ORF by a small proline-rich peptide linker to avoid any structural influence (of the tag) on the critical N-terminal of the MLCK2 protein; the SV40 polyA has the small T-antigen intron before the transcription termination signal to stabilize the mRNA. (C) Western blots of HBEC and HPAEC lysates that show a single FLAG-reactive band of larger-than-expected size when these cells were transfected with the transgene-containing plasmid. 3XFLAG-bacterial alkaline phosphatase (Sigma) was run as a positive control and cell lysates transfected with the promoter-less variant of the transgene as a negative control. HBEC express the transgene much less efficiently than HPAEC.
Transgenic mouse lines
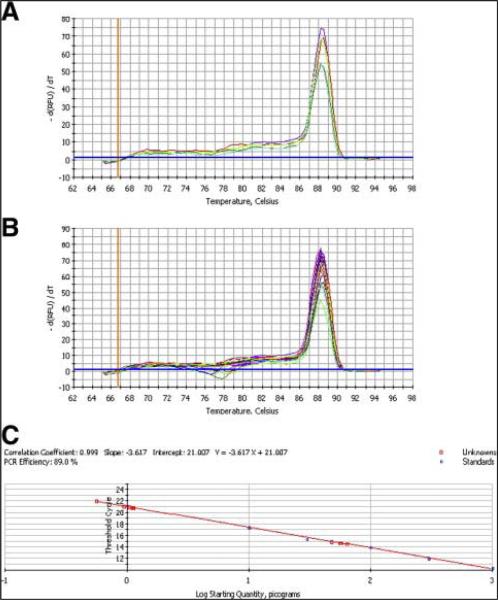
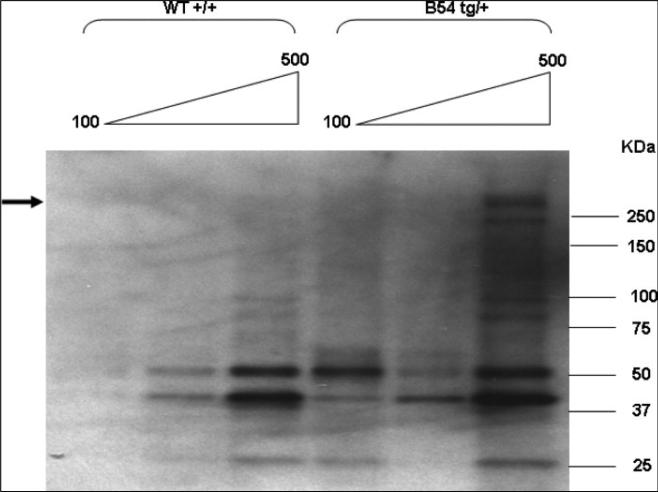
Two transgenic B6 founders were obtained from 62 live births. Tail DNA from 1 founder line (B54) showed an abundant signal in a Southern blot, whereas the B21 founder line could only be detected by PCR (data not shown). Copy number determination by quantitative PCR (Fig 2) showed that the B21 line incorporated ~10 copies of the transgene, whereas the B54 line incorporated ~100 copies. Both lines could transmit the transgene to the next generation, and expressed the correct size of the protein in lung tissue (Fig 3). The high copy B54 line is characterized in this article.
Fig 2.
Copy number determination of the MLCK2 transgene. (A) The melt curve of the plasmid pJM4 at different concentrations demonstrates the formation of a single product with a Tm of 89°C. (B) Depicted are the melt curves of the genomic DNA (blue and red curves) from transgenic mice imposed on top of the plasmid melt curve, which demonstrates fidelity of the transgene PCR product to the PCR product from the original plasmid and ability of the PCR system to determine copy number of the transgene when tested at different dilutions of genomic DNA. (C) Determination of the unknown concentration (copy number) of the transgene (squares) from a standard curve of picogram plasmid (circles) versus ΔCt. (Color version of figure is available online.)
Fig 3.
Expression of the transgene in lungs: 100, 300, and 500 μg of tissue lysates were concentrated by cold acetone before SDS-PAGE and Western blotting as described in the Methods section. The position of the FLAG-reactive transgene band is indicated with an arrow.
Low fecundity of transgenic mice
Hemizygous outcrosses had normal litter size (8 ± 2 pups) for the male parent and slightly diminished size (7 ± 2 pups) for the female parent. Their ability to hold the pups to weaning was normal (100% survival). Hemizygous incrosses, like the hemizygous female outcross, had diminished litter size (7 ± 2 pups) but 100% survival of the pups that were born. Thus, it was possible to obtain homozygous mice by F1 incrosses. Homozygosity was determined by backcrossing the F2 progeny to wild-type B6 mice. Both homozygous females and males of the high copy B54 line had reduced litter size whether outcrossed or incrossed (5 ± 3 pups), and difficulties in holding live births to weaning whether outcrossed (70% survival for outcrossed homozygous female parent, 60% survival for outcrossed homozygous male parent), or incrossed (40% survival). Gross pathologic examinations do not suggest any obvious reproductive defects in the female, but males demonstrated reduced sperm motility (50% to 65% compared with 80% in age-matched wild-type males) and a larger percentage of abnormal sperm counts (5% to 8% as opposed to 2% in age-matched wild-type males).
Phenotyping of MLCK trangenic mice
Standard clinical chemistry analysis of serum samples from 2 males and 2 females at 6 months of age demonstrated mild anemia (hemoglobin 9-9.8 g/dL, hematocrit 29% to 31%; against reference ranges of 11-16 g/dL and 33% to 48%, respectively) in both males and females with more lymphocytes (88% to 96% against a reference range of 5% to 87%) but less neutrophils (3% to 11% against a reference range of 21% to 77%). Chloride and phosphorus levels were increased mildly (134-139 mmol/L and 9-13.8 mg/dL against reference ranges of 92-120 mmol/L and 6.1-10.1 mg/dL, respectively). Male trangenic mice demonstrated greater glucose 224-360 mg/dL against a reference range of 90-192 mg/dL), with an increase in alanine aminotransferase and aspartate aminotransferase levels (224-360 mg/dL, 190-288 U/L, and 296-475 U/L; against reference ranges of 90-192 mg/dL, 28-132 U/L, and 59-247 U/L, respectively). Standard urinalysis, however, was normal and without glucosuria. Both transgenic males and females had greater body weights at this age compared with their wild-type littermates. Gross pathology did not suggest any obvious defects, except a low frequency of polycystic kidney (4/88 dissections, 4.5%).
Mice with overexpressed endothelial MLCK have reduced vascular integrity
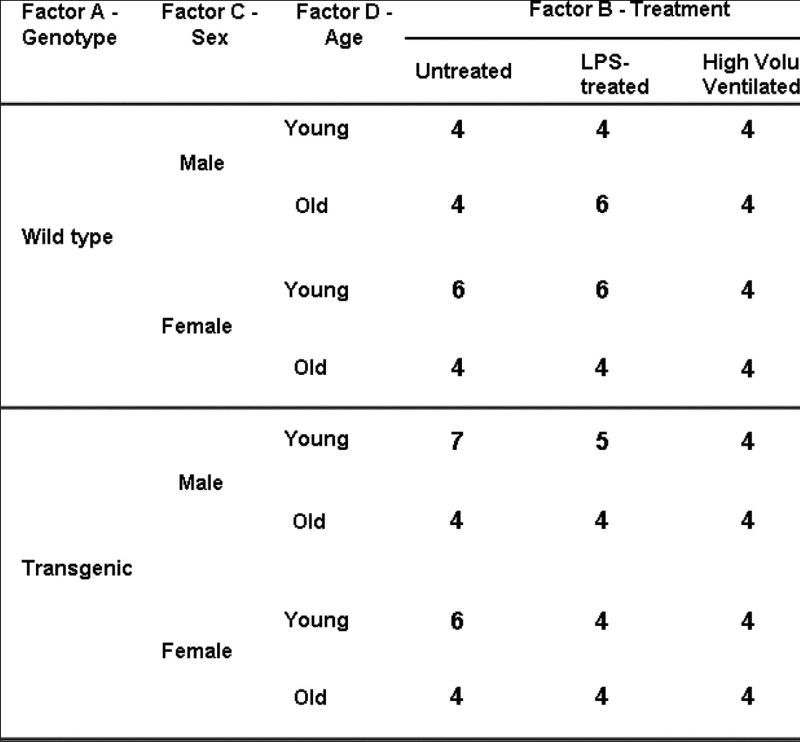
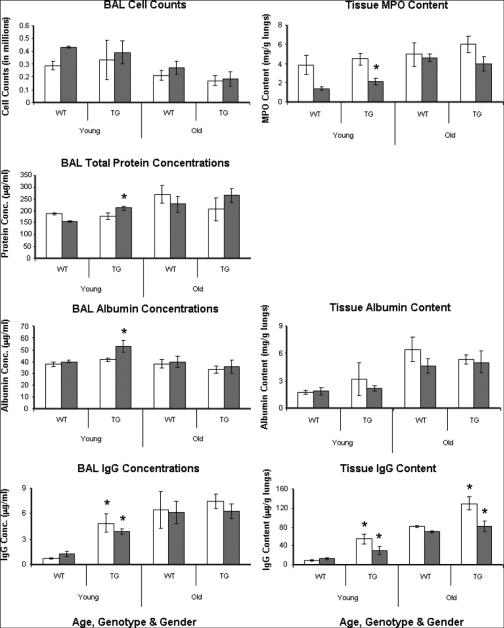
To examine potential differences in lung vascular barrier properties between transgenic mice and their wild-type counterparts, we took into consideration the potential comodifier effects of gender and age, and we designed our study accordingly (Fig 4). We measured major proteins in BAL as well as in the lung parenchyma. Figure 5 shows that compared with age-matched wild-type B6 mice, young MLCK transgenic males demonstrated modest but significant increases in total BAL protein (1.4-fold; P < 0.05), BAL albumin (1.3-fold; P < 0.05) and BAL IgG (3-fold; P < 0.05) levels as well as increased extravasation of IgG (2.2-fold; P < 0.05) and neutrophil infiltration (1.5-fold; P < 0.05) into the lung parenchyma. Although BAL cell counts and tissue albumin extravasation levels were not significantly different from control mice, young transgenic females demonstrated significantly increased IgG in both BAL and lung tissue (6.8-fold and 5.8-fold, respectively, P < 0.05). Older animals (whether wild type or transgenic) have significantly higher baseline values in each measured marker, although in terms of a discernible phenotype, only the old male mice exhibit a significantly increased IgG in lung tissue (1.6-fold; P < 0.01). These data suggest that aging masks basal genotypic differences that are clearly discernible in younger mice, and such masking allows only those phenotypic differences to be declared significant that are restrictive to the passage of IgG. We interpret this observation to indicate that the size of paracellular gap formed because of MLCK overexpression is at, or near, the size of IgG.
Fig 4.
Schematic of experimental design. Four-factor ANOVA design for detection of a phenotype. Young and old mice are 8-12 weeks and 30-36 weeks of age, respectively. Number of animals in each group (ANOVA cell) is indicated.
Fig 5.
Basal profiles of paracellular gap markers in transgenic mice lungs. Age, gender, and genotype-specific values (mean ± SD) of a panel of paracellular markers are shown. Open bars represent females, and closed bars represent males. A phenotype (genotype effect) is declared when a transgenic group is found significantly different from an age-matched and sex-matched wild-type group at P < 0.05 for type I error (marked with an asterisk above the transgenic group bar). Young males seem to be most affected in terms of lung injury markers, whereas young females have increased leak of IgG only.
LPS-induced phenotype in older male and young female MLCK transgenics
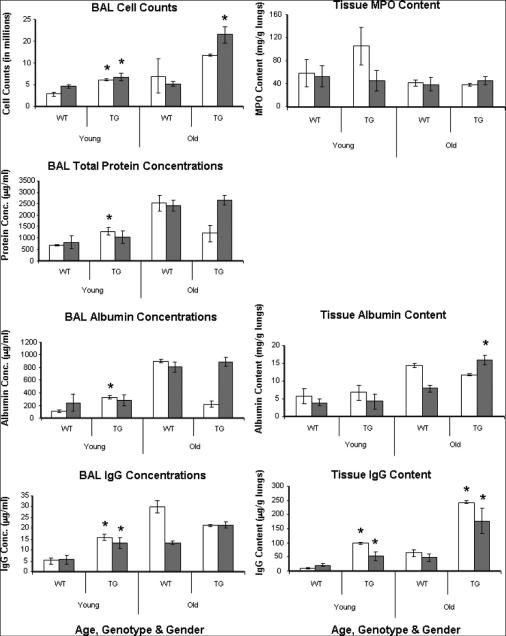
Consistent with our prior reports,22 intratracheal LPS significantly increases total BAL cell counts, total BAL protein and albumin, and BAL IgG in conjunction with significant neutrophil infiltration into the lung parenchyma in both young and old mice (Fig 5 and Fig 6). Similar enhancement in albumin and IgG tissue extravasation is observed in both young and old animals. Evaluation of an observable genotypic effect revealed transgenic young females with significantly increased protein transport in their lungs compared with age-matched wild-type females (1.9-fold total protein at P < 0.01 and 3-fold albumin as well as IgG both at P < 0.01 in the BAL, and 10-fold increased IgG in the lung tissue at P < 0.005), as well as increased BAL cell counts (2.2-fold; P < 0.01) (Fig 6). Transgenic males, irrespective of age, seem to have increased IgG levels (2.4-fold for young and 1.6-fold for older animals in the BAL, both at P < 0.01; 2.45-fold for young and 3.7-fold for older transgenic animals in the lung tissue, both at P < 0.01) (Fig 6). In addition, old transgenic males have increased total BAL cell counts (3.2-fold; P < 0.01) and have increased extravasation of albumin into lung tissues (2-fold, P < 0.01) compared with age-matched wild-type animals. Although the sexually dimorphic nature of the LPS response is apparent, the causality of nonuniform response may be referable to multifactorial technical issues (such as small sample size, high dose of LPS, or attenuation of LPS response by 24 h). Despite these limitations, these observations remain strongly suggestive of an exaggerated level of LPS-mediated injury in mice that express excess MLCK2 protein in the lung vasculature in a gender-specific and age-specific manner.
Fig 6.
Profile of paracellular gap markers in lungs of transgenic mice after LPS challenge. Age, gender, and genotype-specific values (mean ± SD) of a panel of paracellular markers are shown. Open and closed bars and asterisks are shown as in Fig 5. Young females are affected most severely in terms of lung injury parameters, whereas young or old males are less affected, which reveals the sexually dimorphic nature of the LPS injury.
High tidal volume ventilation-induced phenotype in MLCK transgenic males
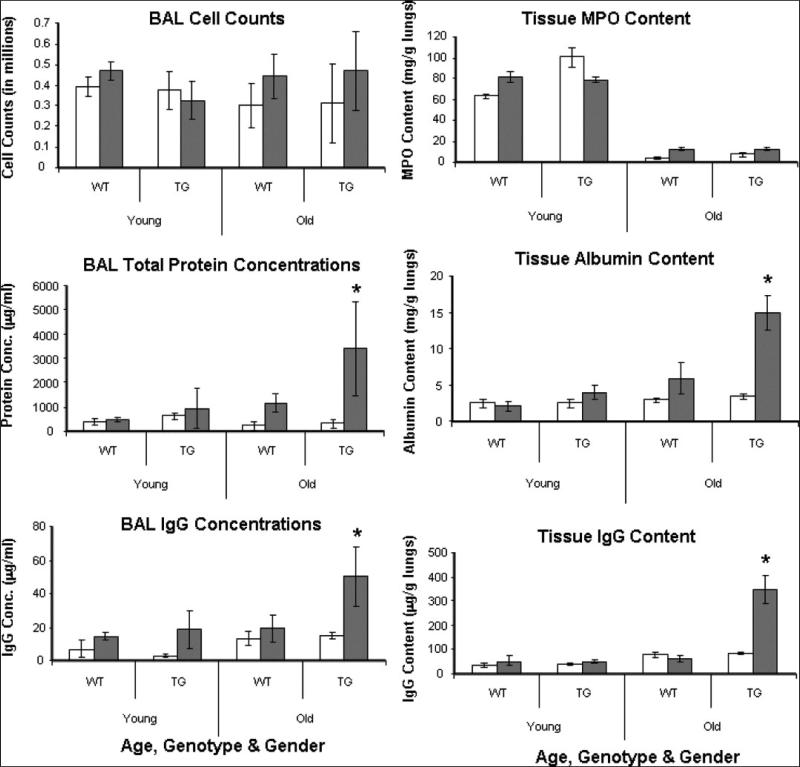
Similar to the LPS-challenged young mice, exposure of young mice to the levels of mechanical stress associated with high-volume ventilation significantly increases total protein and IgG levels in the BAL (but not cell counts), and it causes increased extravasation of albumin, IgG, and neutrophils (tracked as myeloperoxidase antigen) in the lung interstitium (Fig 5 and Fig 7). The only phenotypic effect, however, is observed in old transgenic males, which show remarkably increased lung leak consistently across 4 protein transport parameters: 1) total BAL protein (2.9-fold at P < 0.05) 2) BAL (IgG 2.6-fold; P < 0.05) 3) tissue albumin (2.5-fold; P < 0.05) and 4) tissue IgG (5.4-fold; P < 0.025) extravasation in the interstitial tissue. Interestingly, age-matched females, whether wild type or transgenic, displayed resistance to such injury. This result suggests that the basal “leaky” phenotype observed in transgenic males translates into a significant interaction effect (genotype × treatment × age × sex interaction; P < 0.01)—a severe susceptibility to ventilation injury as the mice age.
Fig 7.
Profile of paracellular gap markers in lungs of transgenic mice after high-volume ventilation. Age, gender, and genotype-specific values (mean ± SD) of a panel of paracellular markers are shown. Open and closed bars and asterisks are as in Fig 5. The sex-dependent and age-dependent injury effect is most prominent in 30-36-week-old males.
Discussion
A cardinal pathophysiologic feature of all inflammatory disorders is the disruption of the continuous, semipermeable endothelial barrier in intact blood vessels that results in increased vascular permeability and the consequent leakage of luminal contents into the interstitium. When inflammation involves hollow organs such as the lungs, such leakage may produce profound alveolar flooding (pulmonary edema) that results in hypoxemia and multiorgan dysfunction and, in extreme cases, death.50 Indeed, mortality rates in ALI range from 34% to 58% and constitute a major health-care burden.[51] and [52]
The semiselective endothelial monolayer is modulated by mechanical forces that control the formation of paracellular gaps with a lesser contribution by receptor-mediated transcellular pathways.53 During inflammatory states, an increase in paracellular gaps occur that are governed predominantly by the dynamic contractile function of the actin-based EC cytoskeleton (reviewed in Ref.54), which together regulate EC shape changes.[3] and [8] A variety of actin-binding proteins is critical to both tensile force generation and linkage of the actin cytoskeleton to adhesive membrane components.
Numerous in vitro studies in cell culture-based models have demonstrated a pivotal role for the actin-binding protein, MLCK, in regulating vascular permeability.54 Studies in ex vivo models that use MLCK-specific inhibitors also demonstrated the essential role of MLCK in regulating pulmonary edema.[55] and [56] The elegant knockout of the nonmuscle MLCK (MLCK210) isoform from all murine tissues resulted in protection from a combined injury induced by LPS challenge and mechanical ventilation.12 As the exact site of the major effects of nmMLCK modulation were unknown, we attempted to use a genetically engineered transgenic murine approach to assess whether overexpression of a nonmuscle MLCK isoform in the vascular endothelium, a semi-selective cellular barrier, would result in increased transport of proteins, fluids, and cells into the alveolar space. Because of our expectation of an endothelial leak phenotype, we focused our attention on blood proteins as markers of increased transport (reviewed in57) as the most relevant physiologic determinants of increased nmMLCK expression. We have assumed that an increase in blood proteins per unit mass of lung parenchyma is a more useful indicator of the existence of a paracellular gap, an idea similar to the popular Evans Blue dye leakage assay.41 An advantage of this approach is the evaluation of endothelial but not epithelial gap formation—the size of the gap is approximated by the size of the trapped protein regardless of the presence/absence of these proteins in the BAL fluid.
We attempted to understand the contribution of increased but restricted nmMLCK2 expression in a relevant clinical model (intratracheal LPS injury). We found age and gender to serve as comodifiers in LPS injury that leads to an exacerbated protein transport in older transgenic males compared with age-matched transgenic females. The contribution of vascular nmMLCK2 overexpression is thus synergistic (significant genotype × treatment × age × sex interaction; P < 0.01) to a basal leakiness for proteins that exists in older male animals (Fig 3). In contrast, young transgenic females, but not age-matched transgenic males, exhibit increased lung protein transport compared with age-matched wild-type animals (significant genotype × treatment × age × sex interaction; P < 0.01). Older transgenic females do not seem to be protected from LPS-mediated injury per se (compare Y-axes in Fig 5 and Fig 6), but our small sample size did not allow us to establish significant differences across permeability markers between the wild-type and the transgenic female groups at this age. Thus, transgenic females are hyper-responsive to LPS when they are young but hypo-responsive when they are old. The converse is true for transgenic males.
It is possible that the higher basal level of protein trapped in the interstitium of older mice (Fig 5) masks the LPS-mediated enhancement of protein flux in old transgenic females. However, if it were the only reason, older transgenic males would be similarly unaffected, but they are not. Several studies have suggested that the female sex hormone estrogen has a protective role in several pulmonary disease models in mice[58], [59] and [60] and in humans,[61] and [62] and it is possible that estrogen or 17β-estradiol are responsible for the masking effect observed in old transgenic females after LPS treatment. Although additional studies are clearly needed, taken together with the data from young mice, these observations suggest that the effect of LPS-mediated lung injury in mice is complex, with age and gender both being important determinants.
To extend our understanding of the contribution of excess nmMLCK2 in another clinically relevant model, we subjected mice to mechanical stress induced by exposure to high-volume mechanical ventilation. Such mechanical ventilation is often combined together with an infectious injury to mimic clinical sepsis-induced ALI,[12], [40], [42], [63] and [64] although the direct effect of each challenge is difficult to dissociate. We assumed that high-volume ventilation will cause mechanical stress-related injury and hypothesized that the resulting barrier dysfunction[65] and [66] will be exacerbated by increases in EC paracellular gap formation in nmMLCK2 transgenic mice. Our data demonstrate that high-volume mechanical ventilation for 4 h is sufficient to produce a significant marker protein leak in old transgenic males but not in females at either age. Like the LPS-mediated synergistic injury in old males, the contribution of excess vascular nmMLCK2 in mechanical ventilation-mediated injury is also synergistic to a basal leakiness for proteins that exist in old male animals (Fig 3).
In summary, our studies illuminate the critical role of gender and age in detecting a pulmonary phenotype because of overexpression of nmMLCK2 in the murine vasculature with enhanced inflammatory processes, including barrier dysfunction. Age increases collagen but decreases elastin content of lung parenchyma in mice35—developmentally determined remodeling events that are expected to result in loss of lung integrity and recoil properties. Such an occurrence may explain the enhanced protein transport in older mice and the increased susceptibility to high-volume ventilation injury. Sex hormones influence a broad range of genes in the liver, kidney, and brain,[67] and [68] although a set of genes in the lungs that are regulated by such hormones, and that may specifically influence a lung phenotype such as fluid and protein transport, remain to be elucidated. In the absence of such evidence, it is tempting to speculate that LPS-mediated enhanced injury of young transgenic females is different in nature from the basal, LPS-induced, or ventilation-induced injury in transgenic males in terms of the mechanisms of protein transport involved. Although the injuries in males may be governed largely by the paracellular pathway, injuries in females may be dominated by transcellular pathways.69 Thus, sex and age have decisive influences on inflammatory lung injury and highlight the role for nmMLCK2 in pulmonary edema in a uniquely subtle manner.
Acknowledgments
Supported by Grant P50 HL 73994 and PPG HL 50864 from the NIH/NHLBI (to J.G.N.G.), and DK61931 from the NIH/NIDDK (to J.R.T).
Abbreviations
- ALI
acute lung injury
- ANOVA
analysis of variance
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- EC
endothelial cell
- ELISA
enzyme-linked immunosorbent assay
- HBEC
human bronchial epithelial cell
- HPAEC
human pulmonary artery endothelial cell
- HTAB
hexadecyltrimethyl ammonium bromide
- KRP
kinase-related protein
- LPS
lipopolysaccharide
- MLCK
myosin light chain kinase
- nmMLCK
nonmuscle myosin light chain kinase
- ORF
open reading frame
- PBS
phosphate buffered saline
- RIPA
radioimmunoprecipitation assay
- RT-PCR
reverse transcription-polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- smMLCK
smooth muscle myosin light chain kinase
- VE
vascular endothelial
References
- 1.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 2.Wysolmerski RF, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA. 1990;87:16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia JGN, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J Investig Med. 1995;43:117–126. [PubMed] [Google Scholar]
- 4.Garcia JGN, Lazar V, Gilbert-McClain LI, Gallagher P, Verin A. Myosin light chain kinase in the endothelium: molecular cloning and regulation. Am J Repir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 5.Giorgi D, Brand-Arpon V, Rouquier S. The functional myosin light chain kinase (MYLK) gene localizes with marker D3S3552 on human chromosome 3q21 on a >5-Mb yeast artificial chromosome region and is not linked to olfactory receptor genes. Cytogenet Cell Genet. 2001;92:85–88. doi: 10.1159/000056874. [DOI] [PubMed] [Google Scholar]
- 6.Lazar V, Garcia JGN. A single human myosin light chain kinase gene (MLCK; MYLK) transcribes multiple nonmuscle isoforms. Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 7.Shirinsky VP, Vorotnikov AV, Birukov KG, et al. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J Biol Chem. 1993;268:16578–16583. [PubMed] [Google Scholar]
- 8.Garcia JG, Schaphorst KL, Shi S, et al. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. Am J Physiol. 1997:273, L172–84. doi: 10.1152/ajplung.1997.273.1.L172. [DOI] [PubMed] [Google Scholar]
- 9.Kudryashov DS, Stepanova OV, Vilitkevich EL, et al. Myosin light chain kinase (210 kDa) is a potential cytoskeleton integrator through its unique N-terminal domain. Exp Cell Res. 2004;298:407–417. doi: 10.1016/j.yexcr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Clayburgh DR, Rosen S, Witkowski ED, et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birukov KG, Csortos C, Marzili L, et al. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60Src. J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright MS, Rossi J, Schavocky J, et al. Protein kinase involved in lung injury susceptibility: Evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aube H, Milan C, Blettery B. Risk factors for septic shock in the early management of bacteremia. Am J Med. 1992;93:283–288. doi: 10.1016/0002-9343(92)90234-3. [DOI] [PubMed] [Google Scholar]
- 14.Schröder J, Kahlke V, Staubach K-H, Zabel P, Stöber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 15.Majetschak M, Christensen B, Obertacke U, et al. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma. 2000;48:832–839. doi: 10.1097/00005373-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bindl L, Buderus S, Dahlem P, et al. Gender-based differences in children with sepsis and ARDS: the ESPNIC ARDS Database Group. Intens Care Med. 2003;29:1770–1773. doi: 10.1007/s00134-003-1948-z. [DOI] [PubMed] [Google Scholar]
- 17.Imahara SD, Jelacic S, Junker CE, O'Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138:275–282. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Trawick DR, Holm C, Wirth J. Influence of gender on rates of hospitalization, hospital course, and hypercapnea in high-risk patients admitted for asthma: a 10-year retrospective study at Yale-New Haven hospital. Chest. 2001;119:115–119. doi: 10.1378/chest.119.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Caracta CF. Gender differences in pulmonary disease. Mt. Sinai J Med. 2003;70:215–224. [PubMed] [Google Scholar]
- 20.Machado ML, Krishnan JA, Buist SA, et al. Sex differences in survival of oxygen-dependent patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:524–529. doi: 10.1164/rccm.200507-1057OC. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Pan L, Phillips N, Parry R, Kurina L. Sex-specific genetic architecture of asthma-associated quantitative trait loci in a founder population. Curr Allergy Asthm R. 2006;6:241–246. doi: 10.1007/s11882-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 22.Weiss L, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- 23.Suchyta MR, Clemmer TP, Elliott CG, et al. Increased mortality of older patients with acute respiratory distress syndrome. Chest. 1997;111:1334–1339. doi: 10.1378/chest.111.5.1334. [DOI] [PubMed] [Google Scholar]
- 24.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157:1159–1164. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- 25.Savage-Brown A, Mannino DM, Redd SC. Lung disease and asthma severity in adults with asthma: data from the Third National Health and Nutrition Examination. J Asthma. 2005;42:519–523. doi: 10.1081/JAS-67605. [DOI] [PubMed] [Google Scholar]
- 26.Teramoto S. COPD pathogenesis from the viewpoint of risk factors. Internal Med. 2007;46:77–79. doi: 10.2169/internalmedicine.46.1775. [DOI] [PubMed] [Google Scholar]
- 27.Bont J, Hak E, Hoes AW, Schipper M, Schellevis FG, Verheij TJM. A prediction rule for elderly primary-care patients with lower respiratory tract infections. Eur Respir J. 2007;29:969–975. doi: 10.1183/09031936.00129706. [DOI] [PubMed] [Google Scholar]
- 28.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple-cause mortality data (1979-1996) Crit Care Med. 2002;30:1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 30.Adrie C, Azoulay E, Francais A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 31.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mammalian Genome. 2002;13:429–437. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 32.Schulz H, Johner C, Eder G, et al. Respiratory mechanics in mice: strain and sex specific differences. Acta Physiol Scand. 2002;174:367–375. doi: 10.1046/j.1365-201x.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 33.Flandre TD, Leroy PL, Desmecht DJ-M. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J Appl Physiol. 2003;94:1129–1136. doi: 10.1152/japplphysiol.00561.2002. [DOI] [PubMed] [Google Scholar]
- 34.Bozanich EM, Collins RA, Thamrin C, Hantos Z, Sly PD, Turner DJ. Developmental changes in airway and tissue mechanics in mice. J Appl Physiol. 2005;99:108–113. doi: 10.1152/japplphysiol.01111.2004. [DOI] [PubMed] [Google Scholar]
- 35.Huang K, Rabold R, Schofield B, Mitzner W, Tankersley CG. Age-dependent changes in airway and lung parenchyma in C57BL/6J mice. J Appl Physiol. 2007;102:200–206. doi: 10.1152/japplphysiol.00400.2006. [DOI] [PubMed] [Google Scholar]
- 36.Fitzerald SM, Gan L, Wickman A, Bergstrom G. Cardiovascular and renal phenotyping of genetically modified mice: a challenge for traditional physiology. Clin Exp Pharmacol Physiol. 2003;30:207–216. doi: 10.1046/j.1440-1681.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown SDM, Hancock JM, Gates H. Understanding mammalian genetic systems: The challenge of phenotyping in the mouse. PLoS Genetics. 2006;2:1131–1137. doi: 10.1371/journal.pgen.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelialcadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- 39.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2004. [Google Scholar]
- 40.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 41.Moitra J, Sammani S, Garcia JGN. Re-evaluation of Evans Blue Dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res J Lab Clin Med. 2007;150:253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 42.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JGN. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 43.Bernacki S, Nelson AI, Abdullah I, et al. Mucin gene expression during differentiation of human airway epithelia in vitro: MUC4 and MUC5B Are Strongly Induced. Am J Respir Cell Mol Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Cummings R, Zhao Y, et al. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor in human bronchial epithelial cells. J Biol Chem. 2003;278:39931–39940. doi: 10.1074/jbc.M302896200. [DOI] [PubMed] [Google Scholar]
- 45.Harlow E, Lane D. Using antibodies: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [Google Scholar]
- 46.Verin A, Lazar V, Torry RA, Laberre CA, Patterson CE, Garcia JGN. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Repir Cell Mol Biol. 1998;19:758–766. doi: 10.1165/ajrcmb.19.5.3125. [DOI] [PubMed] [Google Scholar]
- 47.Nonas SA, Moreno Vinasco L, et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L292–L302. doi: 10.1152/ajplung.00481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 49.Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research. W. H. Freeman and Co; New York, NY: 1995. [Google Scholar]
- 50.Staub NC, Taylor AE. Edema. Raven Press; New York, NY: 1984. [Google Scholar]
- 51.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 52.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcome of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 53.Stevens T, Garcia JGN, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–L422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 54.Dudek SM, Garcia JGN. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 55.Khimenko PL, Moore TM, Wilson PS, Taylor AE. Role of calmodulin and myosin light chain kinase in lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 1996;271:L121–L125. doi: 10.1152/ajplung.1996.271.1.L121. [DOI] [PubMed] [Google Scholar]
- 56.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol. 2000;89:2241–2248. doi: 10.1152/jappl.2000.89.6.2241. [DOI] [PubMed] [Google Scholar]
- 57.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 58.Card JW, Carey MA, Bradbury JA, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:612–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tesfaigzi Y, Rudolph K, Fischer MJ, Conn CA. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness. J Appl Physiol. 2001;91:2182–2189. doi: 10.1152/jappl.2001.91.5.2182. [DOI] [PubMed] [Google Scholar]
- 60.Speyer CL, Rancilio NJ, McClintock SD, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol. 2005;288:C881–C890. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- 61.Dubey RK, Jackson EK. Cardiovascular protective effects of 17β-estradiol metabolites. J Appl Physiol. 2001;91:1868–1883. doi: 10.1152/jappl.2001.91.4.1868. [DOI] [PubMed] [Google Scholar]
- 62.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 63.Dolinay T, Kaminski N, Felgendreher M, et al. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics. 2006;26:68–75. doi: 10.1152/physiolgenomics.00110.2005. [DOI] [PubMed] [Google Scholar]
- 64.Dhanireddy S, Altemeier WA, Matute-Bello G, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86:790–799. doi: 10.1038/labinvest.3700440. [DOI] [PubMed] [Google Scholar]
- 65.West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries: role in lung and heart disease. Lancet. 1992;340:762–767. doi: 10.1016/0140-6736(92)92301-u. [DOI] [PubMed] [Google Scholar]
- 66.Parker JC, Hernandez LA, Peevy KJ. Mechanism of ventilator-induced lung injury. Crit Care Med. 1993;21:131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 67.Rinn JM, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]