Abstract
We recently showed that mucus from patients with ulcerative colitis, a chronic inflammatory disorder of the colon, is characterized by a low level of phosphatidylcholine (PC) while clinical studies reveal that therapeutic addition of PC using slow release preparations is beneficial. The positive role of PC in this disease is still elusive.
Here we tested the hypothesis that exogenous application of PC has anti-inflammatory properties using three model systems. First, human Caco-2 cells were treated with TNF-a? to induce a pro-inflammatory response via activation of NF-?B. Second, latex bead phagosomes were analyzed for their ability to assemble actin in vitro, a process linked to pro-inflammatory signaling and correlating with the growth versus killing of mycobacteria in macrophages. The third system used was the rapid assembly of plasma membrane actin in macrophages in response to sphingosine 1- phosphate (S1P).
TNF-a induced a pro-inflammatory response in Caco-2 cells including 1) assembly of plasma membrane actin; 2) activation of both MAP kinases ERK and p38; 3) transport of NF-?B subunits to the nucleus and 4) subsequent up-regulation of the synthesis of pro-inflammatory gene products. Exogenous addition of most PCs tested significantly inhibited these processes. Other phospholipids like sphingomyelin or phosphatidyl-ethanolamine showed no effects in these assays. PC also inhibited latex bead phagosome actin assembly, the killing of M. tuberculosis in macrophages and the S1P-induced actin assembly in macrophages.
TNF-a induces the activation of signaling molecules and the reorganization of the actin cytoskeleton in human intestinal cells. Exogenous application of PC blocks pro-inflammatory signaling in Caco-2 cells, in phagosomes in vitro and facilitates intracellular survival of mycobacteria. We provide further evidence that actin assembly by membranes is part of the pro-inflammatory response. Collectively, these results provide a molecular foundation for the clinical studies showing a beneficial effect of PC therapy in ulcerative colitis.
Introduction
Intestinal epithelial cells (IEC) are critical to the barrier and absorptive functions of the gastrointestinal tract. In addition, growing evidence suggests that these cells, which line the luminal surface of the gut, act as sentinels of the mucosal immune system (1). IEC express numerous receptors, adhesion molecules, and pro-inflammatory mediators that allow them to communicate with the immune system. IEC are protected against injurious contents of the intestinal lumen by a surface barrier, which consists in part of a continuous, hydrophobic and adherent mucus layer. This mucus represents a hydrated polymeric gel with a thickness of 50-500 μm (2,3). Phospholipids are an important part of the intestinal mucus. They are described as a continuous layer at the luminal side of the mucus gel, both within the mucus as liposome-like aggregates and as a monolayer at the surface of the mucosal cells (4,5).
Phosphatidylcholine (PC) (main species PC 16:0/18:1 and PC 16:0/ 18:2) and lysophosphatidylcholine (LPC) (main species PC 16:0) are the main phospholipid classes found in the mucus. Less abundant are the two glycerolipids phosphatidylethanolamine (PE) (<30%), phosphatidylinositol (<5%) and also sphingomyelin (<10%) (6-9). The function of phospholipids in the mucus is not yet clear, but there is growing evidence that phospholipids are largely responsible for establishing the hydrophobic surface and therefore play a key role in the barrier properties of the underlying tissue. A protective function of phospholipids in the gut has been suggested in the literature (3,5,10-12).
In animal studies exogenous phospholipids or phospholipids contained in food have been shown to prevent mucosal damage induced by acids, NSAIDs or bile salts in the stomach, duodenum and small intestine (13-23). Exogenous PC inhibits inflammation in trinitrobenzene sulfonic acid-(TNBS) or acetic acid-induced colitis and is described to be anti-fibrinogenetic (24,25). Recent studies have shown that a model organelle system, latex bead phagosomes (LBP), can assemble actin filaments de novo, in vitro. Many lipids were found to regulate this system. Some, such as arachidonic acid or phosphatidylethanolamine (PE), were positive regulators whereas others, such as eicosapentanoic acid (EPA) and, notably PC, inhibited the system (26, G. Griffiths and M. Kuehnel personal communication). The positive regulators tend to show pro-inflammatory properties. Adding them to macrophages infected with pathogenic mycobacteria M. tuberculosis (M. tb.) resulted in an induction of pathogen killing whereas in their absence M. tb. grow intracellularly. In contrast, the application of the anti-inflammatory lipid EPA facilitated a significant increase in pathogen growth. EPA is also known to have beneficial effects in ulcerative colitis (UC) (27,28). These data have led to the hypothesis that the membrane-catalyzed assembly of actin is one of the first reactions of the pro-inflammatory response (26,29). Here, we tested the effects of different PCs on the LBP actin assembly in vitro and examined natural phosphatidylcholine in macrophages infected with pathogenic mycobacteria M. tb.
A number of ligands for plasma membrane receptors, such as EGF induce within seconds a transient assembly of actin filaments underneath the plasma membrane (30,31). We recently showed that the sphingolipid sphingosine-1-phosphate induces a 5-15 sec transient peak of actin assembly in RAW and J774 macrophages (M. Kuehnel and G. Griffiths, personal communication). Since we hypothesized that this process represents an early stage in a pro-inflammatory response, we also tested the role of PC application to macrophages on this process.
We have previously shown that mucus from ulcerative colitis (UC) patients is characterized by a low level of the major phospholipid PC while a clinical study reveals that the therapeutic addition of a PC rich preparation to the colonic mucus is beneficial for UC patients (9,10). In a prospective, double-blind randomized, placebo-controlled study the benefit of an oral PC preparation with retarded release was evaluated in 60 patients with chronic active ulcerative colitis. All but one patient in the PC group experienced a decrease of the clinical activity index (CAI) by 70% compared to only three patients in the placebo group, and 53% even attained clinical remission (CAI ≤ 3) (10). Collectively, the available studies argue that phospholipids may have a potential for the therapy of human inflammatory diseases. However, little is known about the mechanisms by which PC shows beneficial properties.
In the main part of this study we addressed the anti-inflammatory role of PC in intestinal epithelial cells (Caco-2). An important regulator of epithelial function during inflammation is the cytokine tumor necrosis factor (TNF)-a. Its levels are elevated in both human inflammatory bowel disease (IBD) and animal models of intestinal inflammation. Importantly, TNF-a induces profound changes in epithelial function, including alterations in permeability (32), cell cycle progression (33), nutrient absorption (34) and gene expression (35). We provide evidence that exogenously added PC, applied to either the apical or the basolateral surfaces, was integrated into the cells and significantly inhibited TNF-a-induced inflammatory responses. Collectively, our data argue that PC is an important player in pro-inflammatory signaling cascades.
Experimental Procedures
Lipids and reagents
TNF-a (Promega, Mannheim) was dissolved in water containing 1% BSA (Sigma, Deisenhofen). The natural L-a-Phosphatidylcholine mixture (1, 2-diacyl-glycero-3-phosphocholine from bovine brain), 1, 2-didodecanoyl-sn-glycero-3-PC, 1, 2-tetra-decanoyl-sn-glycero-3-PC, 1, 2-dieicosanoyl-sn-glycero-3-PC and 1, 2-didocosanoyl-sn-glycero-3-PC, 1, 2-dioleyl-sn-glycero-3-phosphoethanol-amine and all other lipids described in this study were from Sigma (Deisenhofen). 1-pentadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine and 1-arachidoyl-2-hydroxy-sn-glycero-3-phosphocholine were from Avanti Polar Lipids (Alabaster, USA). 1-palmitoyl-glycero-3-phosphatidyl-[14C]-choline ([14C]LPC, 55mCi/1mM) and [3H]-methyl-choline-L-dipalmitoyl-phosphatidylcholine ([3H]-PC, 50 Ci/1mM) were purchased from New England Nulcear (Boston, MA, USA). Sphingosine-1-phosphate (S1P) was dissolved in ethanol (5mM). Solvents without lipids were routinely tested, referred to the Figures as “controls”.
Cells and bacteria
Caco-2w cells are a sub-clone of the human Caco-2BBe that were selected because of their well-differentiated phenotype and provided from J.R. Turner (University of Chicago). Cells were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 1% non-essential amino acid and antibiotics (55IU/mL penicillin and 55μg/mL streptomycin). Vero cells (American Type Culture Collection; ATCC) were propagated in Dulbecco's Modified Eagle's Medium (DMEM) with 5% fetal calf serum and antibiotics (55IU/mL penicillin and 55μg/mL streptomycin). The J774A.1 mouse macrophage cell line as well as the strains M. smegmatis mc2 155 and M. tuberculosis H37Rv, were maintained as previously described (26,29). Macrophages RAW 264.7 and J774 A.1 were grown both as described in Anes et al. 2003 (26).
Mycobacterial infection
Infections with M. tuberculosis H37Rv were done as described in Anes et al. 2003 (26). After 2 days a J774 confluency of 80-90% was reached and cells were infected with bacteria at an OD600 of 0.1. To test the effect of PC on intracellular survival, a 10 μM preparation of a natural PC mixture from bovine brain (Sigma, p3811) was added to infected macrophages after 1 h of bacterial uptake. Every two days the old medium was replaced by fresh medium also containing a fresh PC preparation. At indicated times intracellular bacteria were recovered by lyses of infected macrophages using a 1% Igepal CA-630 (Sigma) solution in water and plated on 7H10 agar containing OADC. CFU counting was carried out as described before (26).
Gene expression analyses by Real-Time (RT)-PCR quantification
Caco-2 cells were grown under standard conditions in 6-wells for 48 h to 80% confluence, washed in Dulbecco's modified Eagle's medium without serum and incubated with 10 ng/mL recombinant human TNF-a (RnD Systems, Germany) together with either PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3-PC), LPC 16:0 (1-palmitoyl-glycero-3-PC) or PE (phosphatidylethanolamine) for various times at 37°C. Approximately 1 *106 cells were collected in 300 μl lysis buffer from the MagnaPure mRNA Isolation Kit I (RAS, Mannheim, Germany) and mRNA was isolated with the MagnaPure-LC device using the mRNA-I standard protocol. RNA was reverse transcribed using AMV-RT and oligo- (dT) as primer (First Strand cDNA synthesis kit, RAS) according to the manufacturer's protocol in a thermocycler. Primer sets specific for the sequences of selected genes (IL-8, IP-10, ICAM-1, MCP-1) optimized for the LightCycler (RAS) were developed and provided by SEARCH-LC GmbH, Heidelberg (www.search-lc.com). The PCR was performed with the LightCycler FastStart DNA Sybr GreenI kit (RAS) according to the pr otocol provided in the parameter specific kits. To correct for differences in the content of mRNA, the calculated copy numbers were normalized according to the average expression of two housekeeping genes (Cyclophilin B and ß-Actin). Values were thus given as input adjusted to copy number per μl of cDNA.
Immunoblotting
Caco-2 cells were seeded on collagen-coated, permeable polycarbonate filters (0.4μm pore size, Costar, Cambridge, MA) and grown for 2-3 weeks to get polarized. Compared to non-polarized cells, for the stimulation of the monolayer of polarized Caco-2 cells a higher amount of TNF-a (50 ng/mL) was necessary that was added to the basolateral buffer. Cells were pre-treated with a 200 μM preparation of PC 16:0/16:0 (1, 2-dipalmitoyl- glycero-3-PC) applied either to the basolateral or apical surface for 1 h before stimulation. Cells were lysed in protein loading buffer (50 mM Tris, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromphenol blue, 10% glycerol). Protein concentrations were measured and equal amounts of total cell lysates were separated by 12% SDS-PAGE, transferred to nitrocellulose and probed for various MAPKs. Membranes were blocked with 5% BSA for 1 h at room temperature and incubated over night with the primary antibodies. The antibodies were obtained from Cell Signaling Technology and used as follows: anti-phospho-MAPK (1:1000 in 5% BSA, rabbit), anti-phospho-p38 (1:1000 5% BSA, rabbit) and anti-phospho-JNK1/2 (1:1000 in 5% BSA, rabbit). Secondary staining was conducted with HRP-conjugated secondary antibody (1:2000 in 5% skimmed milk) followed by chemiluminescent detection with a commercial ECL reagent following the manufacturer's instructions (Amersham Biosciences). Comparisons were made only among samples isolated and transferred onto the same membrane. To confirm equal loading of protein, all Western blots using phospho-specific antibodies were stripped and re-probed with antibodies against the non-phosphorylated kinases.
Immunofluorescence Microscopy
Caco-2 cells were seeded on glass slides in 12-wells to 80% confluence, washed in Dulbecco's modified Eagle's medium without serum and incubated with TNF-a (10 ng/mL) and selected phospholipids (200 μM) for various times. Cells were fixed for 15 min with 4% paraformaldehyde, permeabilized for 5 min with 0.1% Triton X-100 and incubated in blocking solution (0.2% gelatin in PBS) for 30 min. Permeabilized cells were then incubated with a monoclonal mouse anti-NF-?B p65 Ab (Santa Cruz Biotechnology) for 1 h at room temperature. Cells were rinsed with PBS containing 0.2% gelatine, incubated with the appropriate Cy™3-conjugated anti-mouse secondary antibody (Jackson Immuno Research Laboratories) at room temperature for 1 h and nuclei were stained with Hoechst 33342 (Molecular Probes). Specimens were examined with an Olympus IX50 fluorescence microscope. For quantification, ten randomly chosen pictures were taken from one cover slip. An individual not involved in recording scored cells presented on the picture in three categories (1) strong activation (NF-?B found in the nucleus), (2) intermediate activation (NF-?B was equally distributed in nucleus and cytoplasm) and (3) no activation (NF-?B mainly in cytoplasm). The percentages of cells in each class of three independent experiments were expressed.
Transient Transfection and Reporter Assays
Caco-2 cells seeded in 12-wells were grown to a density of 1×105 cells/ml and transiently transfected with a NF-?B-dependent luciferase reporter plasmid according to the manufacturer's instructions (Stratagene). 20 h after transfection, cells were incubated in medium with or without TNF-a (10 ng/ml) and with different concentrations of a PC 18:2/18:2 (1, 2-dilinoleoyl-glycero-3-PC) preparation for 4 h at 37°C. Luciferase activity was assayed using the Luciferase Assay Kit (Stratagene) according to the manufacturer's directions and detected with a Fluorostar Optima (BMG Labtech). Each transfection was done in triplicate and repeated at least three times.
Electrophoretic Mobility Shift Assay (EMSA)
Caco-2 cells grown in 10 cm2 dishes were stimulated with TNF-a (10 ng/mL) for 1 h. The nuclear extracts were prepared using a standard protocol. Nuclear protein was incubated with 32P-labeled oligonucleotides (Amersham Pharmacia). Labeling of oligonucleotides NF-?B (sense and anti-sense) was performed using T4 polynucleotide kinase (New England Biolabs) following the manufacturer's instructions. Nuclear extracts were incubated at 37°C for 30 min with labeled oligonucleotides and separated by electrophoresis in a 6% TBE (Tris base, bor ic acid, EDTA) non-denaturating 5% acrylamide gel. Autoradiography was conducted at -70°C with Kodak X-OMAT film. Electrophoretic mobility shift assays (EMSAs) were also scanned with a Fujifilm BAS-1500 PhosphoImager. The specificity of the oligonucleotides was confirmed by competition with excess unlabeled probe.
Radioactive uptake assay using [3H]-PC and [14C]-LPC followed by TLC analyses
Caco-2 cells were grown to 80% confluence in 5 cm2-dishes and incubated for 30 min either with 1, 2-dipalmitoyl-3-phosphatidyl-[N-methyl-3H]-choline ([3H]PC, 81 Ci/1mM) and 1, 2-dipalmitoyl-glycero-3-phosphocholine (PC 16:0/16:0) or with 1-palmitoyl-glycero-3- phosphatidyl-[14C]-choline ([14C]LPC, 55mCi/ 1mM) and 1-dipalmitoyl-glycero-3-phosphocholine (LPC 16:0) in a ratio of 1:250. After washing cells were incubated with NaOH (1 M) for 10 min. Both the cell lysates and the supernatants were analyzed with counting solution in a scintillation counter (Beckman Coulter LS 6500). For thin-layer chromatography phospholipids were extracted from the cell lysates with methanol/chloroform using a standard method described in Folch et al. in 1956 and applied to Silica Gel 60 plates (Merck, Darmstadt, Germany). Plates were developed in chloroform/methanol/water (70/25/5, v/v) and phospholipids were visualized using Dittmer-Lester reagent. Phosphatidylcholine (PC) containing spots as well as equally sized regions of the silica plate above and below the PC spots were scraped off and the respective radioactivity was quantified.
Actin Assembly Assay
The preparation of LBP Phagosomes was done as described (26). The actin assembly assay was performed under standard conditions using 0.2mM ATP and rhodamine actin buffered with thymosin beta-4 (Defacque et al. (36)). Controls were performed in the presence of an equal amount of ethanol, which was used as a solvent for the different PC species.
Quantification of F-Actin
Caco-2 cells were seeded in 24-wells and grown to a density of 1 × 105 cells/ml. After 24 h cells were pre-treated with a 200 μM preparation of PC 18:2/18:2 (1, 2- dilinoleoyl-glycero-3-PC) in serum-free medium for 1 h at 37°C. After pre-treatment the cells were stimulated with TNF-a (50 ng/mL) for various times at 37°C. A 5μM preparation of Sphingosine-1-phosphate (S1P) in ethanol was used to stimulate RAW macrophages with a final concentration of 10 nM S1P. Cells were seeded as described above and stimulated with S1P (10nM) for 5 and 10 seconds after pre-treatment with a 200 μM preparation of either PC 18:2/18:2 (1, 2-dilinoleoyl-glycero-3-PC) or PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3-PC) in serum-free medium for 1 h at 37°C. Cells were fixed with 3% PFA for 15 min and permeabilized with 0.1% Triton X100 for 3 min. F-actin was stained with Phalloidin-TRITC (Sigma) for 30 min and extracted with methanol for 1 h (36,37). The supernatant was measured for fluorescence at excitation of 550 nm and emission of 570 nm to detect the relative amount of F-actin.
RESULTS
Effect of different PC species on actin assembly in vitro by latex bead phagosomes
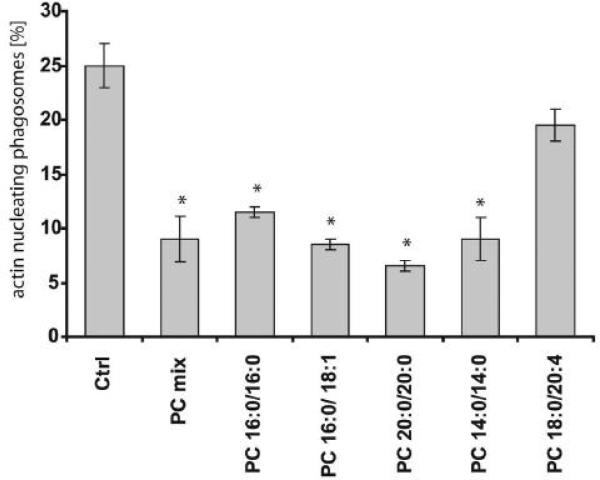
Recent studies using an in vitro phagosome actin assembly assay have led to the hypothesis that PC inhibits pro-inflammatory signaling networks in membranes (26). In the work presented here, we analyzed a variety of PC species in this system in which the polymerization of rhodamine actin filaments is monitored on the surface of latex bead phagosomes (26,36). Most tested PC species inhibited actin assembly in vitro (Figure 1). PCs with very long chain fatty acids were most effective, with the exception of PC 16:0/20:4 with an arachidonic fatty acid side chain. These data support the notion that PC is integrated into membranes and can influence a membrane-dependent process, the assembly of actin filaments.
Figure 1.
Effect of different PC species on actin assembly of isolated phagosomal membranes from J774 macrophages. Phagosomes containing latex beads were treated with a 10 μM preparation of different PC species. Polymerization of actin filaments was clearly inhibited by the different PC species tested in vitro. ATP alone activated the system (ctrl). Columns represent the mean percentages of phagosomes being positive for nucleating actin filaments. The percentages plus and minus standard deviation from three independent experiments are given. Asterisks indicate significant differences (p< 0.05).
PC inhibits S1P-receptor signaling to actin assembly in macrophages
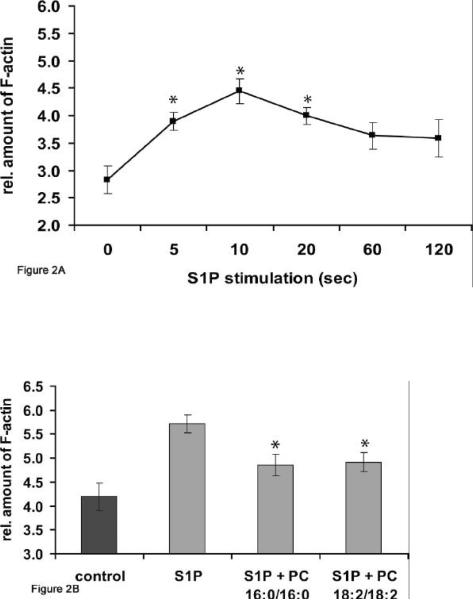
Based on the results from the in vitro phagosome assay we established another system to test the effect of PC on assembly of plasma membrane actin in mouse macrophages. In a recent study (M. Kuehnel and G. Griffiths, personal communication), it was shown that the addition of relative small concentrations (10nM) of sphingosine-1-phosphate induced a transient peak of actin assembly between 5 and 15 sec after stimulation in RAW macrophages (also shown in Figure 2A) and 10-15 sec in J774 cells. This observation provided us with a different system to test the effects of PC on a pro-inflammatory stimulus. For this, RAW macrophages were either pre-treated for 1 h with a 200μmol preparation of PC or left untreated. S1P (10nM) was then added and a fluorometric assay was applied to quantify the relative amount of F-actin (36). As shown in Figure 2B, pre-treatment with either PC 18:2/18:2 or PC 16:0/16:0 had a modest but statistically significant effect in reducing the S1P-induced actin assembly in RAW macrophages.
Figure 2.
Effect of PC 16:0/16:0 and PC 18:2/18:2 on S1P-induced actin assembly in RAW macrophages. The incubation of RAW macrophages with S1P (10nM) for either 5 or 10 seconds demonstrated a very quick F-actin-peak analyzed by a fluorometric assay after staining of F-actin with TRITC-phalloidin (A). RAW macrophages were pre-treated with a 200 μM preparation of either PC 18:2/18:2 (1, 2-dilinoleoyl-phosphatidylcholine) or PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3- phosphocholine) for 1 h at 37°C. The stimulation of RAW macrophages with S1P (10nM) with PC (S1P + PC 18:2/18:2 or S1P + PC 16:0/16:0) resulted in a significant reduction of actin assembly relative to the F-actin amount of cells stimulated with S1P alone (S1P) for 10 sec (B). Results were compared with relative F-actin amounts of untreated cells (control) within the same experiment using triplicate samples and are representative of three others carried out independently. Values are means ± SD (n=3) with a significance of *p<0.01.
PC-treatment of macrophages increases the intracellular growth of mycobacteria
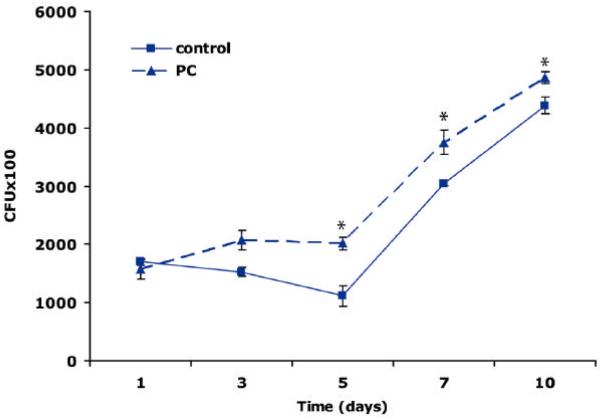
Killing of intracellular mycobacteria is a process that is dependent on actin assembly (36). We were therefore interested to confirm our previous inhibitory results with PC by testing the effect of PC on the intracellular growth of mycobacteria in macrophages. The pathogenic strain M. tuberculosis divides slowly (24 h doubling time in vitro) and can divide at a similar rate within phagosomes of J774 macrophages (26). We tested the addition of a 10 μM PC mixture (natural L-a-phosphatidylcholine from bovine brain) to J774 cells over the course of infection with this mycobacterium. As shown in Figure 3, in the presence of PC there was a significant increase in the survival of M. tuberculosis in macrophages relative to untreated controls. Inhibition of the inflammatory response results in an increase of pathogen growth. Therefore, also by this criterion, the addition of PC appears again to have anti-inflammatory properties as bacteria showed more survival at all times relative to the controls.
Figure 3.
Effect of PC on the intracellular survival of M. tuberculosis H37 RV in J774 macrophages. Cells were infected with bacteria and the effect of a 10 μM PC preparation (natural phosphatidylcholine from bovine brain) on the intracellular survival of bacteria was analyzed after 1 h of bacterial uptake. At indicated times intracellular bacteria were counted after lyses of infected macrophages. The figure clearly shows that the growths of M. tuberculosis was increased by PC-treatment (PC) compared to the intracellular survival of bacteria in untreated macrophages (control). Values are means ± SD (n=3) with a signific ance of *p<0.05.
Given these data in macrophages we decided to investigate the role of PC in more detail in a cell system that was closer to the clinical observations that initiated this study. For this, we selected the human intestinal cell line Caco-2 in combination with the well-studied pro-inflammatory stimulus TNF-a.
Quantification of PC uptake
Prior to testing PC in detail in Caco-2 cells, we asked how effectively these lipids could be incorporated into these cells. For this, we established a radioactive uptake assay using both 1, 2-dipalmitoyl-3- phosphatidyl-[N-methyl-3H]-choline ([3H]-PC) and 1-palmitoyl-glycero-3-phosphatidyl-[14C]-choline ([14C]-LPC) as tracers. Here it was clearly shown that [3H]-PC was indeed taken up by the cells in a time-dependent manner (data not shown). When cells were exposed to a 200μM [3H]-PC solution for 10 min, approximately 4% of the offered [3H]-PC was integrated into the cell. Uptake of LPC was 5 times higher than uptake of PC (data not shown). To determine the fate of LPC and PC within the cells, TLC analyses of phospholipids were performed with cell lysates after incubation with either radio-labeled [3H]-PC or [14C]-LPC for 30 min. The relative amounts of PC and LPC of the cell lysates were separated by TLC analysis and quantified by scintillation counting. We could determine that, when labeled PC was added, the species was stable over the incubation time tested. In contrast, when LPC was added the bulk of this lipid species was converted to PC (Table 1). In the subsequent experiments testing LPC we therefore assume that most of the effect of this lipid is due to its conversion to PC.
Table 1.
Incorporated amounts of radioactive-labeled [3H]-PC and [14C]-LPC in Caco-2 cell lysates were analyzed by TLC. The fate of PC 16:0/16:0 and LPC 16:0 within the cells was analyzed by TLC using cell lysates of Caco-2 cells that have been incubated with either radio-labeled [3H]-PC or [14C]-LPC for 30 min. The relative amounts of PC species were quantified by scintillation counting. The percentages plus and minus standard deviation from three independent experiments are given.
| Phospholipid species | [14C]-LPC | [3H]-PC |
|---|---|---|
| PC | 78.1 ± 12.9 % | 95.4 ± 2.2 % |
| LPC | 14.1 ± 11.1 % | 1.9 ± 1.0 % |
| PE | 3.4 ± 2.0 % | 1.9 ± 1.3 % |
PC inhibits TNF-a-induced F-actin assembly in Caco-2 cells
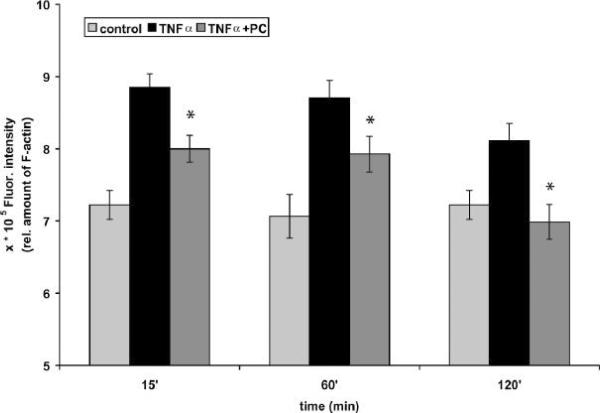
Based on the previous results we first looked at the effect of PC on F-actin assembly in Caco-2 cells. TNF-a-induced signaling events have been shown to interact with the actin cytoskeleton of cultured cells (38,39). Given the results with S1P in stimulating actin assembly in macrophages, we first asked whether TNF-a, a more classical pro-inflammatory ligand also induced actin assembly in Caco-2 cells. When we assayed this process in response to TNF-a there was no assembly of actin in the first seconds and minutes after addition of ligand (data not shown). However, a reproducible transient peak of actin was observed after 15 min, in agreement with an earlier study (40). Pre-treatment of cells with a 200 μM preparation of 1, 2-dilinoleoyl-glycero-3-PC (PC 18:2/18:2) for 1 h prior to the addition of TNF-a resulted in the inhibition of the extent of actin polymerization after 15 min (Figure 4). These data strengthen the hypothesis that the membrane-assembly of actin is part of the pro-inflammatory response and that exogenous addition of PC significantly leaves this response in epithelial cells.
Figure 4.
PC 18:2/18:2 inhibits the TNF-a-induced F-actin assembly in Caco-2 cells. The incubation of cells with TNF-a (50 ng/mL) for 15, 60 and 120 min demonstrated an increased formation of F-actin in Caco-2 cells. Cells were then pre-treated with a 200 μM preparation of PC 18:2/18:2 (1, 2-dilinoleoyl-phosphatidylcholine) for 1 h at 37°C. For fluorometric analyses F-actin was stained with TRITC-phalloidin. The stimulation of Caco-2 cells with TNF-a after pre-treatment with PC (TNF-a + PC) resulted in a decrease of the F-actin amount of cells stimulated with TNF-a alone (TNF-a) for the indicated times. Results were compared to F-actin amounts of untreated cells (control) within the same experiment using triplicate samples. Values are means ± SD (n=3) with a significance of p < 0.05 vs TNF-a alone.
TNF-a induces NF-?B activation in Caco-2 cells
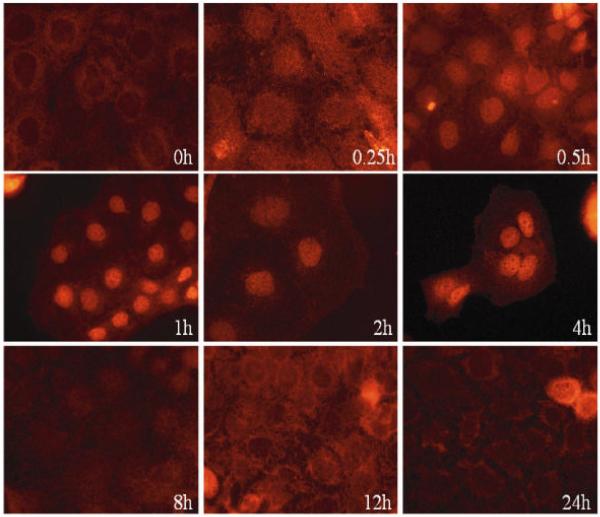
A major pathway in TNF-a signaling is the pro-inflammatory NF-?B pathway. Therefore we tested whether TNF-a exerts pro-inflammatory effects on the tumor cell line Caco-2 via the NF-?B pathway. In fully polarized Caco-2 cells grown on a permeable filter, the TNF-a-receptor is localized at the basolateral surface and is therefore not accessible to TNF-a applied to the apical surface (41,42). However, in subconfluent cells grown on plastic dishes, in which the cells are not fully polarized the receptor is still accessible to TNF-a applied to the medium, as becomes evident in the experiments reported below. This was the system we used for all but one subsequent experiment. When Caco-2 cells were stimulated with TNF-a (10 ng/mL) alone for various times, a time dependent nuclear translocation of the p65 subunit of NF-?B was induced and evaluated by fluorescence microscopy. The translocation was quite efficient as shown in Figure 5A.
Figure 5.
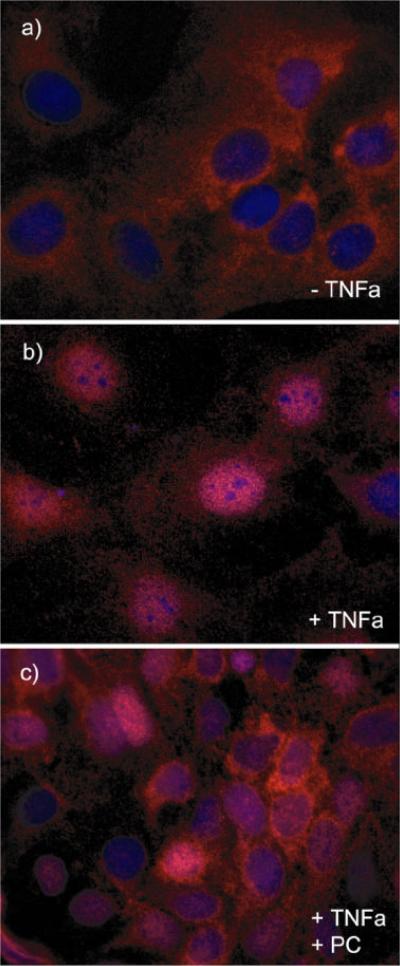
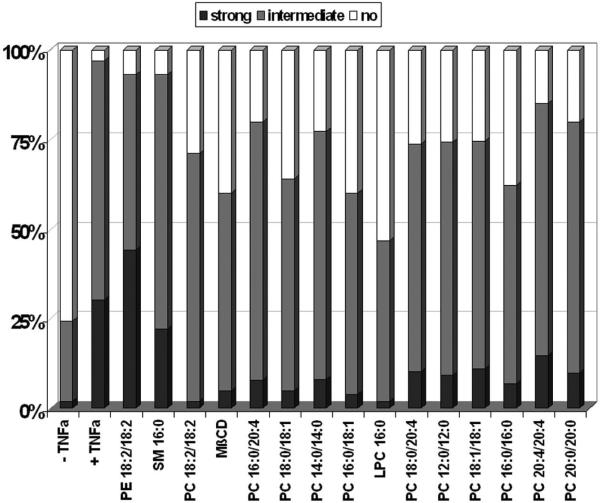
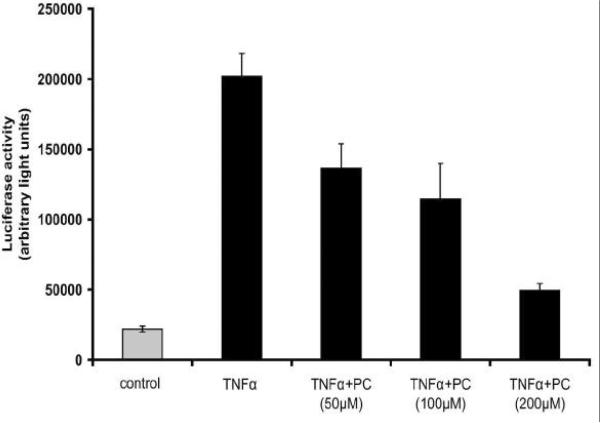
(A) TNF-a-induced NF-?B activation in Caco-2 cells. Cells were grown on glass slides and stimulated with TNF-a (10 ng/mL) at 37°C for various times (5 min to 24 h) to detect nuclear translocation of the p65 subunit of NF-?B by immunofluorescence microscopy with a specific p65 monoclonal antibody (in red). NF-?B activation could be detected already after a few minutes and stayed for at least 4 h. (B) PC 16:0/16:0 clearly inhibits TNF-a induced NF-?B activation. Caco-2 cells were incubated with 10 ng/mL TNF-a for 30 min either in the presence or in the absence of a 200 μM solution of 1, 2-dipalmitoyl-glycero-3-PC (PC16:0/16:0). a.) In absence of TNF-a NF-?B was predominately found in the cytoplasm. b.) After stimulation with TNF-a NF-?B (p65) was translocated into the nucleus. c.) Co-treatment of Caco-2 cells with both PC 16:0/16:0 and TNF-a resulted in a clear inhibition of the p65 translocation relative to the controls. (C) Effect of different phospholipid species on TNF-a induced NF-?B activation. Caco-2 cells were co-treated with both TNF-a (10 ng/mL) and a 200 μM preparation of selected phospholipids for 30 min at 37°C. The TNF-a-induced NF-?B activation was detected by immunofluorescence microscopy. Images of 10 randomly selected areas were taken and NF-?B activation was scored blinded into three groups: (1) strong activation, (2) intermediate activation and (3) no activation. The percentages of the amount of cells in each class of three independent experiments were expressed, respectively. All PC species tested inhibited the TNF-a-induced NF-?B activation in Caco-2 cells. Lysophosphatidylcholine (LPC 16:0) was the most effective one while phosphatidylethanolamine or sphingomyelin was non-effective. (D) PC 18:2/18:2 inhibits TNF-a-mediated NF-?B translocation in a dose dependent manner. Caco-2 cells were assayed for NF-?B activation by transient transfection of a NF-?B luciferase reporter plasmid. 20 h after transfection, cells were stimula ted with either 10 ng/ml human TNF-a alone (TNF-a), co-treated with both TNF-a (10 ng/mL) and PC 18:2/18:2 (1, 2-dilinoleoyl-phosphatidylcholine preparations in various concentrations (TNF-a + PC) or were cultured in TNF-a-free medium (control) for another 4 h. The lysates were assayed for luciferase activity. The differences of the expression levels of TNF-a-stimulated cells compared to cells co-treated with both TNF-a and PC showed a concentration dependent, anti-inflammatory effect. All stimulations were done in triplicates in =3 independent experiments.
PC inhibits TNF-a induced NF-?B activation in Caco-2 cells
We next tested the effect of several phospholipids in combination with TNF-a in Caco-2 cells on the translocation of the p65 subunit of NF-?B. Cells were either stimulated with TNF-a (10 ng/mL) for 30 min, a time when most cells were activated by TNF-a or stimulated with both TNF-a and selected phospholipids. The p65 nuclear translocation was detected by immunofluorescence microscopy and compared to the state of untreated cells (Figure 5B). A scoring of the activated cells was performed in a blind fashion (see methods). Several species of phosphatidylcholine were tested and the majority clearly inhibited the TNF-a-induced NF-?B activation in Caco-2 cells (Figure 5C). The strongest effect was seen with LPC 16:0 (1- palmitoyl-3-glycero-PC), while no effect could be detected with either phosphatidylethanolamine (PE) 18:1/18:1 (1, 2-dioleyl-glycero-3-phosphatidylethanolamine) or sphingomyelin (SM) 16:0 (n-palmitoyl-d-sphingomyelin). We confirmed these results both by electro-mobility shift assay (EMSA) to monitor the inhibition of the TNF-a-induced translocation of p65 (data not shown) and by the transient expression of a NF-?B luciferase reporter system. Importantly, the co-addition of TNF-a (10 ng/mL) and various concentrations of PC 18:2/18:2 (1, 2-dilinoleoyl-glycero-3-PC) inhibited NF-?B nuclear translocation in a dosedependent manner (Figure 5D).
Expression of pro-inflammatory genes is attenuated by PC-treatment of Caco-2 cells
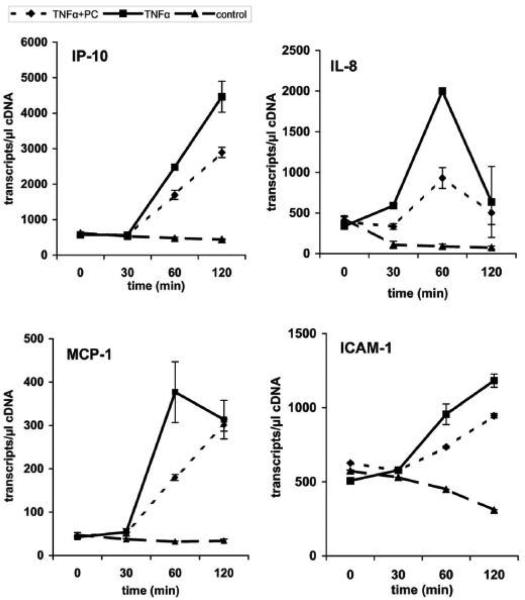
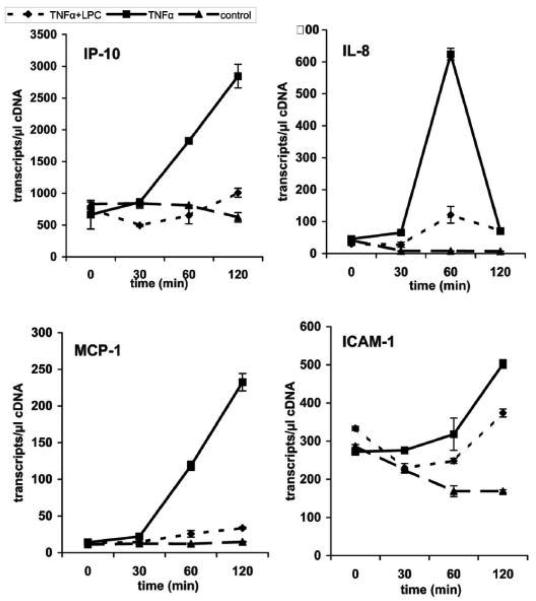
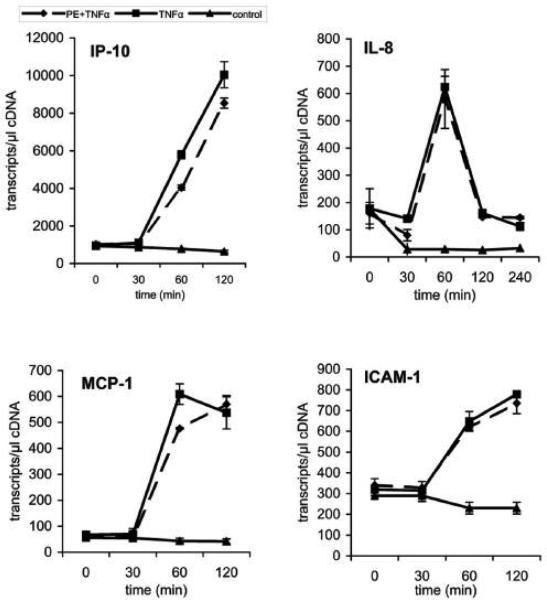
Having shown the inhibitory effect of PC on TNF-a-induced NF-?B activation in Caco-2 cells the effect of PC on cellular events downstream of NF-?B activation were analyzed by real-time-PCR (RT-PCR). We monitored the transcription levels of several oligonucleotides specific for selected pro-inflammatory marker genes (ICAM-1, MCP-1, IL-8 and IP-10). Sharing similar transcription factors (NF-kB, AP-1) it was expected that these genes would be regulated in a similar manner. Caco-2 cells were first stimulated with TNF-a alone for various times and mRNA was isolated to analyze several pro-inflammatory genes by quantitative real-time-PCR. After treatment of the cells with PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3-PC) for various times this TNF-a-induced up-regulation of selected genes was clearly delayed relative to the controls (Figure 6A). Similar and even stronger effects were found for the treatment with LPC 16:0 (1-palmitoyl-glycero-3-PC), as shown in Figure 6B. Again, co-treatment of cells with PE 18:1/18:1 (1, 2-dioleyl-glycero-3-phosphatidylethanolamine) and TNF-a did not show any change on the transcriptional level of the selected genes compared to cells stimulated with TNF-a alone (Figure 6C).
Figure 6.
Down-regulation of the TNF-a-induced gene expression in Caco-2 cells by PC 16:0/16:0 and LPC 16:0. The expression profiles of mRNAs for selected pro-inflammatory genes (ICAM-1, MCP-1, IL-8 and IP-10) are shown. Caco-2 cells were either stimulated with TNF-a (10 ng/ml) alone (TNF-a) or co-treated with TNF-a together with a 200 μM preparation of either (A) 1, 2- dipalmitoyl-glycero-3-phosphocholine (TNF-a + PC), (B) 1-palmitoyl-glycero-3-phosphocholine (TNF-a + LPC) or (C) phosphatidylethanolamine (TNF-a + PE). The expression profiles of mRNA of resting cells for the selected genes were analyzed in parallel (control). The up-regulation of selected pro-inflammatory genes by TNF-a was analyzed after different incubation times (30 to 120 min) and compared to the expression levels of either PC-treated (TNF-a + PC), LPC-treated (TNF-a + LPC) or PE-treated cells (TNF-a + PE). Within this period of time the mRNA expression of the selected genes was strongly delayed after treatment with PC or LPC while PE did not show any significant effect.
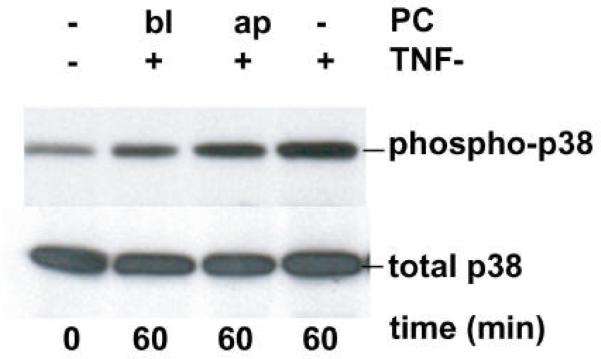
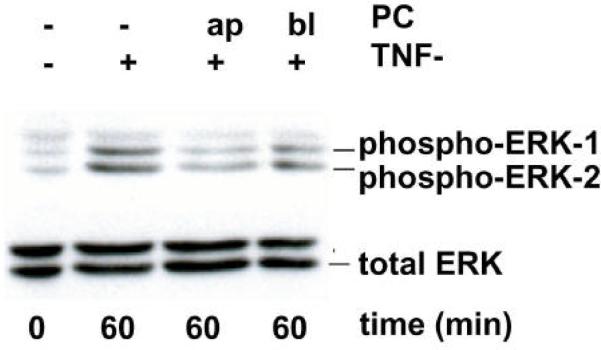
PC inhibits p38 and ERK1/2 phosphorylation in polarized Caco-2 cells
Activation of mitogen-activated protein kinases (MAPKs) is generally associated with TNF-a-receptor-mediated signaling in mammals. At least three distinct groups of MAPKs have been identified in mammals, including the extracellular signal-regulated kinases (ERKs), the c-Jun NH2-terminal kinases (JNKs) and p38. The activation of the ERK and p38 pathway was tested in response to TNF-a-stimulation for various incubation times in both polarized and non polarized Caco-2. The activation of ERK and p38 pathway as a consequence of TNF-a-stimulation for various incubation times in both polarized and non polarized Caco-2 cells was assayed by using antibodies specific for the phosphorylated, active forms of these kinases by Western blotting. Western blots with total cell lysates revealed that the phosphorylation of both p38 and ERK1/2 was inhibited after treatment with PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3-PC) (data not shown). Using highly polarized cells grown on a permeable filter system allowed the addition of TNF-a and PC to either the lower or upper chamber, corresponding to the basolateral and apical surface of the monolayer, respectively. These experiments revealed that PC added either apically or basolaterally 1 h before TNF-a-stimulation cells clearly inhibited both p38 and ERK1/2 phosphorylation (Figure 7A and 7B). Collectively, these data demonstrate that treatment with phosphatidylcholine inhibits both the p38 and ERK MAPK pathways and the transcriptional activation by NF-?B in response to TNF-a.
Figure 7.
PC 16:0/16:0 (1, 2-dipalmitoyl-glycero-3-phosphocholine) inhibits the TNF-a-mediated activation of both p38 MAP kinase and ERK1/2 pathways in polarized Caco-2 cells. Filter-grown polarized Caco-2 cells were stimulated with TNF-a (50 ng/ml) from the basolateral (bl) surface to activate the MAP kinase pathways. To test the effect of PC on the MAP kinase pathway activation Caco-2 cells were pre-treated with a 200 μM preparation of PC 16:0/16:0 either from the upper (ap) or from the lower (bl) chamber before TNF-a-stimulation for the indicated times. Cell lysates were prepared and whole cell extracts were analyzed by Western blotting. Extracts were immunoblotted with antibodies specific for the phosphorylated (phospho) forms of either (A) p38 MAP kinase or (B) ERK. Caco-2 cells treated with PC showed reduced amounts of the activated forms of both ERK and p38. Equal protein loading was confirmed by protein assay and by control Western blots using antibodies specific for the non-phosphorylated (total) forms of p38 MAP kinase and ERK1/2. All gels were run at least two times and the representative blots of three independent experiments are shown.
DISCUSSION
The results in this study clearly show that the addition of PC to cells and to isolated phagosomes inhibits processes that are either pro-inflammatory or hypothesized to be so. Earlier work led to the hypothesis that the assembly of actin by phagosomal membranes in vitro is part of the pro-inflammatory response. A large number of effectors such as the pro-inflammatory lipid arachidonic acid, that stimulate this process in vitro, induce the killing of pathogenic mycobacteria in macrophages. In contrast, factors inhibiting actin assembly, including EPA that had beneficial properties against inflammatory bowel disease (IBD) (27,28) lead to increased intracellular survival of M. tuberculosis (26). In this respect, here, different species of PC inhibited LBP actin assembly, while a natural PC mixture led to a significant increase in the survival of M. tuberculosis in macrophages. Other phospholipids as phosphatidylethanolamine or sphingomyelin did not show any inhibitory effect.
In addition, in a separate signaling system in which S1P stimulates plasma membrane actin assembly in macrophages, a similar inhibitory role for PC was observed. We hypothesize that S1P-receptor signaling to actin assembly is part of a pro-inflammatory response of macrophages; a notion supported by our results with TNF-a (see below).
Having analyzed the inhibitory effects of PC on plasma membrane actin assembly we could also show within this study that PC inhibits TNF-a-induced nuclear factor-?B (NF-?B) transcription. TNF-a is a T helper (Th) 1 cytokine whose expression is increased in IBD (43,44). The importance of TNF-a in both IBD entities Crohn's disease (CD) and ulcerative colitis (UC) was shown by the significant beneficial action of its inhibition by specific monoclonal antibodies (45-47). TNF-a activates the nuclear factor-?B (NF-?B) transcription factor, which causes activation of a number of pro-inflammatory genes. It had been shown before that p38 and ERK MAPK signaling pathways constitute an additional level of gene regulation in the transcription by NF-?B, in particular of the p65 subunit, in response to TNF-a (48). PC inhibits all these processes and may therefore be involved in the fine tuning of its activation. The need for a fine balance between the pro- and anti-inflammatory responses has been dramatically demonstrated by the accumulating evidence that therapeutic intervention of the monoclonal antibody that blocks TNF-a or the TNF-receptors have undesirable side effects. For example, in a subset of patients carrying latent forms of M. tuberculosis, the bacteria can be activated and cause tuberculosis (49-51).
TNF-a-induced a transient peak of actin assembly in Caco-2 cells, but in contrast to S1P, EGF, thrombin and other ligands, which induce actin assembly in seconds, the process is significantly delayed in response to TNF-a becoming first significant after 15 min. Nevertheless, the addition of PC to Caco-2 cells before TNF-a stimulation significantly inhibited the actin response. However, while the physiological relevance of this receptor-mediated actin assembly process remains to be established, it seems to be a reliable marker of the pro-inflammatory response.
The idea that exogenously applied PC has anti-inflammatory properties was given support in particular by the further analysis of the pro-inflammatory response to TNF-a in Caco-2 cells. This complex pro-inflammatory response, triggered by activation of the TNF-receptors, is well-established in several cell lines (52-54). Within this study we monitored three processes known to be turned on by this system, namely activation of: 1.) NF-?B-transcription system; 2.) pro-inflammatory gene expression and; 3.) MAP kinase pathways. A significant inhibition of these pro-inflammatory processes was seen after PC-treatment. These results, in conjunction with our macrophage/phagosome experiments, argue strongly that PC has a broad ability to block many processes linked to the pro-inflammatory response.
PC is the most common phospholipid and is generally in a high concentration on the plasma membrane of most eukaryotic cells (55). Why should adding more of a lipid, presumed to have a `housekeeping' function in membranes, have such a striking effect in inhibiting many processes linked to a pro-inflammatory response? We speculate that PC in membranes may be present in a freely mobile pool and a second pool that is attached to membrane proteins. Proteins that bind selectively to PC have been described, such as the START, C1 or C2 domain structures, of which a large number are involved in signaling (56,57). Increasing the concentration of PC on the membrane might shift the balance between the free and the bound fraction. MAP kinases, PC-TP and F-actin are bound to the inner surface of the plasma membrane, so it is conceivable that an increase of PC to key signaling molecules in or on the membrane may initiate a negative signaling mode. Another possibility is that, upon delivering PC into cells, PC may distribute to either leaflet. Normally in cells, the bulk of PC is in the outer leaflet of the membrane. It is possible that added PC might induce a higher concentration of PC on the inner leaflet, where it could interfere with selected signaling proteins. The addition of PC to the bowel of UC patients is expected to elevate the levels of this lipid, that are naturally decreased in these patients in mucus and presumably in membranes. These differences in PC may be associated with changes in various species of PC, or in other lipids. Indeed, the fatty acid side chains in the mucosa of patients with inflammatory bowel disease have been shown to be shifted to less essential fatty acids and more arachidonic acid (58).
While our data show anti-inflammatory properties of PC they do not necessarily explain why retarded release PC preparations have beneficial effects in ulcerative colitis patients (10). The situation is of course more complex in humans, where epithelial cells in the body have the mucus layer on their apical surfaces. In general UC is characterized by an uncontrolled intestinal inflammatory response. While the exact pathogenesis of this disease is not yet understood, it is likely that the initiation of the immune response is triggered by luminal factors. The nature of these initiating agents is unclear, but both orally ingested nutrients and microbial agents have been implicated. An impaired barrier function, and in particular a defect of the mucus layer, leads to an increased exposure of the mucosal immune system to luminal antigens, which in genetically susceptible individuals may induce an inappropriate and unrestrained inflammatory response (59-62).
From our results it seems likely that oral administration of PC that is directly integrated in the mucus layer as well as in membranes of enterocytes will change the barrier properties of the mucosa and thus alleviate the inflammatory response e.g. to bacterial antigens. This is also in agreement with animal studies of acetic acid- and TNBS-induced colitis, where it has been shown that PC reduces the inflammatory response of the gut [3, 20, 24-25].
Finally, our results provide the first molecular description of the anti-inflammatory properties of PC and also LPC, which our TLC data reveal to be largely converted to PC. Our results provide a foundation for experiments leading to a more mechanistic explanation of how PC (LPC) can reduce inflammation in chronic inflammatory diseases like ulcerative colitis.
Acknowledgments
We thank Sabine Tuma (Department of Gastroenterology, Heidelberg) and Daniela Holzer (EMBL Heidelberg) for excellent technical assistance. Markus Meiβner and his group (Department of Parasitology, Heidelberg) are thanked for their help with analyzing samples using their luminometer. I. T. was financed by the Y.I.A. of the Medical Faculty of Heidelberg. E. A. work was supported by a grant of FCT, POCI/BIA-BCM/55327/2004 with co-participation of the FEDER programme POCI2010. J. F. and R. E. were supported by a grant of the “Stiftung Nephrologie”.
REFERENCES
- 1.Maaser C, Kagnoff MF. Z Gastroenterol. 2002;40:525–529. doi: 10.1055/s-2002-32808. [DOI] [PubMed] [Google Scholar]
- 2.Wallace JL, Granger DN. Faseb J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenberger LM. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 4.Kao YC, Lichtenberger LM. Gastroenterology. 1991;101:7–21. doi: 10.1016/0016-5085(91)90454-s. [DOI] [PubMed] [Google Scholar]
- 5.Bengmark S, Jeppsson B. JPEN J Parenter Enteral Nutr. 1995;19:410–415. doi: 10.1177/0148607195019005410. [DOI] [PubMed] [Google Scholar]
- 6.DeSchryver-Kecskemeti K, Eliakim R, Carroll S, Stenson WF, Moxley MA, Alpers DH. J Clin Invest. 1989;84:1355–1361. doi: 10.1172/JCI114306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler BD, Lichtenberger LM, Hills BA. Am J Physiol. 1983;244:G645–651. doi: 10.1152/ajpgi.1983.244.6.G645. [DOI] [PubMed] [Google Scholar]
- 8.Bernhard W, Postle AD, Linck M, Sewing KF. Biochim Biophys Acta. 1995;1255:99–104. doi: 10.1016/0005-2760(94)00221-j. [DOI] [PubMed] [Google Scholar]
- 9.Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U, Stremmel W. Scand J Gastroenterol. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- 10.Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Gut. 2005;54:966–971. doi: 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manjari V, Das UN. Prostaglandins Leukot Essent Fatty Acids. 2000;62:85–96. doi: 10.1054/plef.1999.0125. [DOI] [PubMed] [Google Scholar]
- 12.Nervi F. Gastroenterology. 2000;118:1265–1267. doi: 10.1016/s0016-5085(00)70380-5. [DOI] [PubMed] [Google Scholar]
- 13.Barrios JM, Lichtenberger LM. Gastroenterology. 2000;118:1179–1186. doi: 10.1016/s0016-5085(00)70371-4. [DOI] [PubMed] [Google Scholar]
- 14.Dial EJ, Lichtenberger LM. Gastroenterology. 1984;87:379–385. [PubMed] [Google Scholar]
- 15.Dunjic BS, Axelson J, Ar'Rajab A, Larsson K, Bengmark S. Scand J Gastroenterol. 1993;28:89–94. doi: 10.3109/00365529309096051. [DOI] [PubMed] [Google Scholar]
- 16.el-Hariri LM, Marriott C, Martin GP. J Pharm Pharmacol. 1992;44:651–654. doi: 10.1111/j.2042-7158.1992.tb05487.x. [DOI] [PubMed] [Google Scholar]
- 17.Hills BA, Kirwood CA. Gastroenterology. 1989;97:294–303. doi: 10.1016/0016-5085(89)90064-4. [DOI] [PubMed] [Google Scholar]
- 18.Kiviluoto T, Paimela H, Mustonen H, Kivilaakso E. Gastroenterology. 1991;100:38–46. doi: 10.1016/0016-5085(91)90580-e. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenberger LM, Graziani LA, Dial EJ, Butler BD, Hills BA. Science. 1983;219:1327–1329. doi: 10.1126/science.6828859. [DOI] [PubMed] [Google Scholar]
- 20.Lugea A, Mourelle M, Guarner F, Domingo A, Salas A, Malagelada JR. Gastroenterology. 1994;107:720–727. doi: 10.1016/0016-5085(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 21.Martin GP, Marriott C. J Pharm Pharmacol. 1981;33:754–759. doi: 10.1111/j.2042-7158.1981.tb13926.x. [DOI] [PubMed] [Google Scholar]
- 22.Puglielli L, Amigo L, Arrese M, Nunez L, Rigotti A, Garrido J, Gonzalez S, Mingrone G, Greco AV, Accatino L, et al. Gastroenterology. 1994;107:244–254. doi: 10.1016/0016-5085(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 23.Swarm RA, Ashley SW, Soybel DI, Ordway FS, Cheung LY. Am J Surg. 1987;153:48–53. doi: 10.1016/0002-9610(87)90200-5. [DOI] [PubMed] [Google Scholar]
- 24.Mourelle M, Guarner F, Malagelada JR. Gastroenterology. 1996;110:1093–1097. doi: 10.1053/gast.1996.v110.pm8612998. [DOI] [PubMed] [Google Scholar]
- 25.Fabia R, Ar'Rajab A, Willen R, Andersson R, Ahren B, Larsson K, Bengmark S. Digestion. 1992;53:35–44. doi: 10.1159/000200969. [DOI] [PubMed] [Google Scholar]
- 26.Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. Nat Cell Biol. 2003;5:793–802. doi: 10.1038/ncb1036. [DOI] [PubMed] [Google Scholar]
- 27.Aslan A, Triadafilopoulos G. Am J Gastroenterol. 1992;87:432–437. [PubMed] [Google Scholar]
- 28.Belluzzi A. Proc Nutr Soc. 2002;61:391–395. doi: 10.1079/pns2002171. [DOI] [PubMed] [Google Scholar]
- 29.Anes E, Peyron P, Staali L, Jordao L, Gutierrez MG, Kress H, Hagedorn M, Maridonneau-Parini I, Skinner MA, Wildeman AG, Kalamidas SA, Kuehnel M, Griffiths G. Cell Microbiol. 2006;8:939–960. doi: 10.1111/j.1462-5822.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 30.Omann GM, Allen RA, Bokoch GM, Painter RG, Traynor AE, Sklar LA. Physiol Rev. 1987;67:285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- 31.Peppelenbosch MP, Tertoolen LG, Hage WJ, de Laat SW. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- 32.Marano CW, Lewis SA, Garulacan LA, Soler AP, Mullin JM. J Membr Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser GC, Polk DB. Gastroenterology. 1997;112:1231–1240. doi: 10.1016/s0016-5085(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 34.Mehran M, Seidman E, Marchand R, Gurbindo C, Levy E. Am J Physiol. 1995;269:G953–960. doi: 10.1152/ajpgi.1995.269.6.G953. [DOI] [PubMed] [Google Scholar]
- 35.Yang SK, Eckmann L, Panja A, Kagnoff MF. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 36.Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, Mangeat P, Voelter W, Marriott G, Pfannstiel J, Faulstich H, Griffiths G. Embo J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard TH, Oresajo CO. J Cell Biol. 1985;101:1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koukouritaki SB, Vardaki EA, Papakonstanti EA, Lianos E, Stournaras C, Emmanouel DS. Mol Med. 1999;5:382–392. [PMC free article] [PubMed] [Google Scholar]
- 39.Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. J Immunol. 2006;176:1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 40.Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. J Cell Sci. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- 41.Fish SM, Proujansky R, Reenstra WW. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallee S, Laforest S, Fouchier F, Montero MP, Penel C, Champion S. Exp Cell Res. 2004;297:165–185. doi: 10.1016/j.yexcr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Van Deventer SJ. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 45.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura K, Honda K, Mizutani T, Akiho H, Harada N. World J Gastroenterol. 2006;12:4628–4635. doi: 10.3748/wjg.v12.i29.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 48.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 49.Hanauer SB, Present DH. Rev Gastroenterol Disord. 2003;3:81–92. [PubMed] [Google Scholar]
- 50.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, Pritchard ML, Sandborn WJ. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 52.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. J Biol Chem. 2005;280:17435–17448. doi: 10.1074/jbc.M500437200. [DOI] [PubMed] [Google Scholar]
- 54.Pedron T, Thibault C, Sansonetti PJ. J Biol Chem. 2003;278:33878–33886. doi: 10.1074/jbc.M303749200. [DOI] [PubMed] [Google Scholar]
- 55.Simons K, van Meer G. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 56.Hurley JH, Tsujishita Y, Pearson MA. Curr Opin Struct Biol. 2000;10:737–743. doi: 10.1016/s0959-440x(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 57.Alpy F, Tomasetto C. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 58.Pacheco S, Hillier K, Smith C. Clin Sci (Lond) 1987;73:361–364. doi: 10.1042/cs0730361. [DOI] [PubMed] [Google Scholar]
- 59.Fiocchi C. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 60.Patel B, Schutte R, Sporns P, Doyle J, Jewel L, Fedorak RN. Inflamm Bowel Dis. 2002;8:340–346. doi: 10.1097/00054725-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Podolsky DK. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 62.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr., Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]